Abstract
INTRODUCTION
Brain magnetic resonance imaging (MRI) and inflammatory biomarkers are crucial for investigating preclinical neurocognitive disorders. Current investigations focus on a few inflammatory markers. The study aims to investigate the associations between inflammatory biomarkers and MRI measures and to examine sex differences among the associations in the Framingham Heart Study.
METHODS
Dementia and stroke‐free participants underwent OLINK Proteomics profiling and MRI measurements within 5 years. Pairwise cross‐sectional analysis assessed 68 biomarkers with 13 brain MRI volumes, adjusting for covariates and familial correlations.
RESULTS
Elevated CDCP1, IL6, OPG, and 4E.BP1 were related to smaller total cerebral brain volume (TCBV), whereas higher HGF, IL8, and MMP10 were associated with smaller TCBV, total and frontal white matter volumes. Higher SCF and TWEAK were associated with larger TCBV. In sex‐stratified analyses, associations were observed exclusively among males.
DISCUSSION
We report several associations between inflammatory biomarkers and brain volumes, highlighting different associations within sex subgroups.
Highlights
Higher CDCP1, IL6, OPG, and 4E.BP1 levels were associated with smaller TCBV.
Higher levels of HGF, IL8 and MMP10 were associated with smaller TCBV, CWV and FWV.
Higher levels of SCF and TWEAK, were associated with larger TCBV.
Significance diminished in models adjusting for CVD risk factors.
Associations were observed exclusively in males.
Keywords: aging, Alzheimer's disease, biomarkers, cognitive aging, dementia, MRI
1. INTRODUCTION
Alzheimer's disease (AD) and AD related dementias (ADRD) pose a significant public health challenge. It is estimated that by 2050, approximately 153 million people worldwide will be suffering from these diseases. 1 The escalating prevalence will exert immense strain on healthcare systems worldwide, resulting in substantial economic implications. It is estimated that by 2050, the cost of dementia care alone will exceed 17 trillion USD. 2 AD is the most common subtype of dementia, accounting for approximately 70%–80% of all dementia cases.
It is crucial to detect AD at an early stage to enhance the likelihood of more effective management strategies prior to substantial cognitive decline. Brain magnetic resonance imaging (MRI) measures of total cerebral brain volume (TCBV), white matter hyperintensity volume (WMH), hippocampal volume, and regional gray and white matter volumes, have been used as important endophenotypes of AD/ADRD, as they indicate abnormal changes before the onset of the disease. 3 , 4 , 5 , 6 For example, higher TCBV is hypothesized to be a buffer, providing the brain with an increased ability to adapt in the presence of neurodegenerative processes, while diverse patterns of regional gray matter atrophy associate with dementia onset and cognitive function. 7
Neuroinflammation as one of the major pillars of aging contributes to the pathogenesis of dementia and chronic peripheral inflammation has been related to an increased risk for AD. 8 , 9 Inflammatory biomarkers are a promising candidate to detect patterns of neurodegeneration that can lead to higher risk for ADRD. Therefore, investigating the relationship between circulating inflammatory biomarkers and neuroimaging biomarkers using total and regional brain volume measures could provide a better understanding of the complex relationship between neuroinflammation and neurodegenerative pathophysiology, and potentially identify individuals earlier in the disease course.
Several population‐based studies have observed associations between C‐reactive protein (CRP) and/or interleukin 6 (IL6) with TCBV, WMH, hippocampal volume, and total gray and white matter volumes. Research conducted in the population‐based Rotterdam Study and the Framingham Heart Study (FHS) have reported an association between higher levels of CRP and smaller TCBV along with greater WMH volume. 10 , 11 , 12 In contrast, a report from the Study of Health in Pomerania (SHIP) did not find an association between CRP and TCBV, but did observe an association with gray matter volume. 13 Larger panels of inflammatory biomarkers have demonstrated associations of osteoprotegerin (OPG), IL6, and tumor necrosis factor alpha (TNFα) with smaller TCBV. 14 In a recent report from our group, higher CD5L and CD14 levels were significantly associated with smaller TCBV, whereas higher levels of sRAGE were linked to larger TCBV. 15 Most studies to date have focused on a select number of circulating biomarkers, focusing almost exclusively on pro‐inflammatory biomarkers. Additionally, no potential sex differences have been investigated in these associations. Furthermore, the majority of studies have not investigated specific regional gray and white matter volume measurements. Exploring brain atrophy patterns at the regional level as outcomes can potentially unveil more granular information on the processes leading to neurodegenerative disorders. The exploration of a comprehensive panel of inflammatory markers has not been examined in relation to global and regional brain volumes in a carefully characterized community‐based cohort. Therefore, the aim of this study is to investigate the association of multiple circulating inflammatory biomarkers, measured with the OLINK inflammatory panel, with various brain MRI markers of AD/ADRD in the FHS Offspring cohort, while also examining potential sex differences in these associations.
2. METHODS
2.1. Study sample
The Framingham Offspring Study recruited 5214 offspring of the FHS Original cohort and Offspring spouses in 1971. 16 The FHS offspring cohort has been examined every 4–8 years since enrollment and has had brain MRI assessed every 2–6 years beginning with Exam 7 in 1999. 17 Sample selection for brain MRI outcomes is provided in the flow chart in Figure S1. Overall, 879 dementia‐free Offspring participants at least 40 years old at Exam 7 had stored plasma for inflammatory biomarker profiling. We excluded participants with incomplete biomarker profiles that did not pass the quality control procedures (n = 2), without apolipoprotein E (APOE) genotypes (n = 8), missing a brain MRI within 5 years before or after Exam 7 (n = 211), and those with prevalent stroke (n = 12), leaving a final sample of 646 participants for analyses. Participants provided written informed consent before attending each examination. The Institutional Review Board at Boston University Medical Center reviewed and approved the study protocol and examinations.
2.2. OLINK inflammatory panel
Stored EDTA plasma samples from Exam 7 were sent to OLINK (Waltham, MA, USA) for proteomics using their OLINK Target 96 inflammatory panel, which provides measurements of 92 protein biomarkers. The approach is based on high‐throughput and multiplexing Proximity Extension Assay (PEA) technology. Protein expression levels were quantified using Normalized Protein eXpression (NPX) units, which is a relative measurement on scale, where one NPX difference corresponds to a doubling of protein concentrations. 18 Details can be found at: https://olink.com/. To assist in the quality control of plasma samples, phantoms and OLINK plate controls were included as part of the sample for calculations of the limit of detection (LOD) for OLINK panels. 18 The samples were randomly distributed in 11 plates and then processed together in a single batch at OLINK. The coefficient of variation for all proteins across the plates was less than 5%, indicating the absence of any plate‐related effects. A selection of 68 proteins with <50% of participant values below LOD were selected for downstream analyses as suggested by OLINK and other investigations. 19 , 20 The full list of protein biomarkers in the OLINK inflammatory panel and the percentage of samples missing or with values below LOD for each of them are provided in Table S1, while the list of 68 proteins for analysis is shown in Table S2a. The actual data value below LOD was used in accordance with the OLINK guidelines for the subset of proteins that fell below LOD. 20 This approach was adopted due to the conservative nature of LOD measurements in large multi‐plate studies, and statistical power could be improved using observed values. We also conducted rank‐based inverse normal transformations on raw protein values to standardize and reduce skewness. 21
RESEARCH IN CONTEXT
Systematic review: Magnetic resonance imaging (MRI) and circulating inflammatory biomarkers play vital roles in the exploration of preclinical neurocognitive disorders. Nevertheless, existing literature tends to concentrate on a limited selection of inflammatory markers and lack an exploration of sex differences.
Interpretation: We report links between several circulating inflammatory biomarkers and brain MRI measures, suggesting their roles in neurodegeneration. Elevated CDCP1, IL6, OPG, 4E.BP1, HGF, IL8, and MMP10 associate with smaller brain MRI volume, while SCF and TWEAK correlate with bigger MRI brain volume. Sex‐specific differences are evident, with certain biomarkers affecting brain volumes exclusively in males.
Future directions: The associations observed between various circulating inflammatory biomarkers and brain MRI, particularly in males, warrant further exploration using a longitudinal approach in a larger, more diverse sample. This could enhance understanding of the pathophysiological pathways underlying neurodegeneration, facilitating the development of early detection strategies and therapeutic targets.
2.3. Brain MRI measures
FHS Offspring cohort participants attending Exam 7 were approached for an opportunity to receive brain MRI and participate in a brain aging study beginning in 1999. 17 Details about FHS brain MRI acquisition and quantification have been described previously. 17 , 22 The majority of MRI machines were provided by Siemens and General Electric, with a few machines being supplied by Philips. 17 Participants underwent brain MRI in a machines ranging from 1 T to 1.5 T field strength, and brain MRI was obtained either at Framingham, MA, or offsite by other centers in the United States. No substantial differences were observed across sites according to analyses of MRI measures. 17 All images were centrally read and blind to participants' personal identification information. Image evaluation involves the removal of non‐brain elements through semiautomatic segmentation analyses, and the resulting total cranial volume (TCV) is used as a proxy of head size. 17 , 23 Protocols for quantifying and segmenting total, regional brain volumes, and WMH volumes have been previously reported. 17 , 24 Brain MRI measures of interest included TCBV, hippocampal brain volume (HPV), and WMH. In brief, TCBV is calculated as the ratio of total brain parenchymal volume to TCV. HPV and WMH were also computed as proportions of TCV to account for the head size. Five measures of gray matter volume were included due to different potential associations of regional gray matter in the disease progression of cognitive aging. 25 , 26 Similarly, the same regional volumes for white matter were also considered since they are linked to cognition and brain activity. 27 , 28 , 29 These ten measures were also regarded as outcomes, which were also calculated as proportions of TCV: cerebral gray matter volume (CGV), frontal gray matter volume (FGV), temporal gray matter volume (TGV), parietal gray matter volume (PGV), and occipital gray matter volume (OGV), cerebral white matter volume (CWV), frontal white matter volume (FWV), temporal white matter volume (TWV), parietal white matter volume (PWV), and occipital white matter volume (OWV).
All 13 brain MRI measures except TCV were included as outcomes of interest and were rank‐based inverse‐normal transformed to attain a distribution with a mean of 0 and SD of 1. Some participants received brain MRIs multiple times. We identified a sample of 646 participants for whom MRI outcomes were measured within 5 years of the Exam 7 core visit, at which the blood sample for the biomarker panel was drawn. If multiple MRIs were available within 5 years of Exam 7, the MRI with the date closest to Exam 7 was selected.
2.4. Pairwise association analyses
Pairwise associations were investigated between each of the 68 protein biomarkers and all 13 brain MRI outcomes. For each pair, a combined analysis for the full sample and stratified analyses within sex subgroups (male and female) were conducted. Two statistical models were considered for the analyses. The primary model (Model 1) included the covariates of age, age2, sex, a sex × age interaction term, the time difference between the blood draw and the MRI measurement, and the additive coding for the number of APOE ε2 and ε4 alleles, as these are key variables associated with brain volumes and cognitive outcomes. Age and sex information were ascertained at Exam 7, and APOE genotypes were identified from polymerase chain reaction and restriction isotyping. 30 The terms age 2 and sex × age interaction were included due to the non‐linear and sex‐specific effects of age on the MRI measures. 17
A secondary model (Model 2) was used to test for the robustness of the associations accounting for prevalent cardiovascular disease (CVD) and CVD risk factors, which included all covariates in Model 1 as well as: (1) indicators for prevalent CVD and prevalent atrial fibrillation (AF) at Exam 7 and (2) CVD risk factors including systolic and diastolic blood pressures (SBP, DBP, mmHg), diabetes status, treatment for hypertension, body‐mass index (BMI, kg/m2), current smoking status, total cholesterol level (TC, mg/dL), high‐density lipoprotein cholesterol levels (HDL, mg/dL), and use of lipid‐lowering agents at Exam 7. The presence of CVD was established by diagnoses made before Exam 7 and confirmed by a panel of senior investigators including coronary heart disease (myocardial infarction, angina pectoris, coronary insufficiency), transient ischemic attack, intermittent claudication, and congestive heart failure. 31 Diabetes status was defined by any of the conditions: use of antidiabetic medications, fasting blood glucose level ≥126 mg/dL, or random blood glucose level ≥ 198 mg/dL. The inclusion of additional prevalent CVD and CVD risk factors in Model 2 accounted for the mediation effects through the CVD pathways on biomarkers.
In stratified analyses, we performed sex‐stratified analyses following the same analytical strategy and model covariates (excluding sex), and all pairwise analyses used separate linear mixed‐effects models to test the associations between each of the 68 proteins (predictor) with each of the 13 brain MRI volumes (outcome), accounting for familial relationships via the kinship coefficient matrix. Effect sizes and 95% confidence intervals (CIs) of proteins were reported and interpreted as effects in standard deviation units due to the standardization of both predictors and outcomes. False rejections of the true null hypotheses for the 68 × 13 = 884 correlated association tests were controlled by the false discovery rate (FDR). 32 FDR ≤0.05 was set to declare significant associations. FDRs were computed within each stratum separately. All the statistical analyses were implemented by R‐4.2.1 software 33 and the linear mixed‐effect models were conducted by the lmekin function in the coxme package. 34
2.5. Sensitivity analyses
Two additional sensitivity analyses were conducted to further assess the robustness of the pairwise association analyses. First, we tested the associations between proteins and brain MRI outcomes using Model 1 in the subsample that excluded participants who underwent brain MRI more than 2 years before or after Exam 7 (subsample n = 603). Second, to examine if a specific subgroup of participants drove the significant associations, we performed analyses using Model 1 excluding participants who had prevalent chronic leukemia or lymphoma, who reported use of glucocorticoids at Exam 7, or who were identified as outliers by principal component analysis (PCA) using the 68 rank‐normalized proteins (36 individuals excluded, subsample n = 610). We also performed another sensitivity analysis to examine potential effect modification by the APOE genotype. This was conducted by including and assessing the interaction term between each protein and the indicator for being APOE ε4 carriers using Model 1 (excluding the number of APOE ε2 and ε4 alleles, and instead using an indicator of APOE ε4 carriers) in the full sample (n = 646).
2.6. Network analysis
Besides the marginal pairwise associations we have assessed, we also investigated the conditional associations of the 68 proteins and 13 brain MRI measures represented by Gaussian Graphical Models (GGMs). In GGMs, two nodes (variables) are connected by an edge if and only if this pair of nodes is conditionally dependent given all the other nodes in the network, and each edge weight corresponds to the conditional dependency between the node pair. 35 We used the Fused graphical lasso (FGL) to jointly estimate multiple sparse GGMs regarding related but distinct stratum groups, where the conditional dependency networks across groups are expected to share similar structures but also hold differential edges. 36 Based on the full data including 81 nodes (68 proteins and 13 outcomes), we identified the differential edges across sex groups, mainly focusing on protein—MRI outcome pairs to add insights into our sex‐stratified pairwise analyses. Differential edges were defined as those pairs that have precision matrix entry differences between the male and female larger than 0.001, and those non‐zero edges with differences across sex less than 0.001 were considered as similar edges. The FGL was conducted by function JGL in the JGL R package.
3. RESULTS
3.1. Participants characteristics
Table 1 presents the demographic characteristics of the participants in the brain MRI sample. The average age at Exam 7 was 61 years, approximately 52% of the participants were female, the median MMSE score was 29, and the proportion of APOE ε2 and ε4 carriers was roughly 16% and 22%, respectively. The brain MRI outcomes were measured on average 0.75 years (SD = 0.77 years) after Exam 7, 3% had MRIs > 3 years after Exam 7, and 0 had MRIs > 3 years before Exam 7. The mean and SD of the 68 proteins are shown in Table S2a. Table S2b presents the mean (SD) of brain MRI outcomes.
TABLE 1.
Participant characteristics at Exam 7, time of plasma sample for inflammatory biomarker measurement.
| Brain MRI sample | Full sample | Female | Male |
|---|---|---|---|
| Sample size, n, n (%) | 646 | 338 | 308 |
| Female, n (%) | 338 (52.3 %) | —— | —— |
| Age, mean(range) | 61 (40, 88) | 61 (41, 85) | 60 (40, 88) |
| APOE ε2 carriers, n (%) | 101 (15.6%) | 56 (16.6%) | 45 (14.6%) |
| APOE ε4 carriers, n (%) | 142 (22.0%) | 78 (23.1%) | 64 (20.8%) |
| Attended college, n (%) | 507 (78.5%) | 254 (75.1%) | 253 (82.1%) |
| Current smoker, n (%) | 78 (12.1%) | 50 (14.8%) | 28 (9.1%) |
| BMI Kg/m2, mean (sd) | 28 (5) | 27 (5) | 28 (4) |
| SBP mmHg, mean (sd) | 125 (18) | 124 (19) | 126 (17) |
| DBP mmHg, mean (sd) | 74 (9) | 72 (9) | 75 (9) |
| Hypertension Rx, n (%) | 197 (30.5%) | 91 (26.9%) | 106 (34.4%) |
| Total cholesterol mg/dL, mean (sd) | 200 (37) | 207 (37) | 192 (35) |
| Triglycerides mg/dL, mean (sd) | 132 (76) | 129 (71) | 136 (82) |
| Lipid Rx, n (%) | 128 (19.8%) | 50 (14.8%) | 78 (25.3%) |
| Fasting blood glucose mg/dL, mean (sd) | 104 (27) | 99 (22) | 109 (30) |
| Type 2 diabetes Rx, n (%) | 32 (5.0%) | 13 (3.8%) | 19 (6.2%) |
| MMSE, median (IQR) | 29 (2) | 29 (1) | 29 (2) |
| parent with dementia Dx, n (%) | 145 (22.4%) | 71 (21.0%) | 74 (24.0%) |
| Incident dementia through 2022, n (%) | 65 (10.1%) | 34 (10.1%) | 31 (10.1%) |
| Blood cancer prevalent Exam 7, n (%) | 6 (0.9%) | 5 (1.5%) | 1 (0.3%) |
| Blood cancer through 2019, n (%) | 27 (4.2%) | 12 (3.6%) | 15 (4.9%) |
| Time difference between brain MRI and Exam 7, mean (sd) | 0.75 (0.77) | 0.74 (0.81) | 0.76 (0.74) |
Abbreviations: APOE, Apolipoprotein E; BMI, body mass index; DBP, diastolic blood pressure; IQR, interquartile range; MMSE, mini‐mental state examination; MRI, magnetic resonance imaging; Rx, prescription; SBP, systolic blood pressure; sd, standard deviation.
3.2. Association analysis results
Figure 1 shows the forest plot of significant associations between the proteins and the 13 brain MRI measures in the full sample and in male and female strata. Figure 2 shows the same associations as well as any newly discovered significant associations after accounting for the Model 2 CVD and risk factor covariates. Most of the significant protein associations were observed with TCBV and regional white matter volumes. In the combined analysis, higher levels of CDCP1, HGF, IL6, IL8, MMP10, OPG, and 4E.BP1 were significantly associated with smaller TCBV, with effect sizes ranging from −0.15 to −0.11 SD units per SD unit increase in protein levels. Higher levels of SCF (, SE = 0.029, FDR = 0.004, see Figure 1) and TWEAK (, SE = 0.030, FDR = 0.004, see Figure 1) were associated with larger TCBV. HGF, IL8, and MMP10 were also found to be significantly associated with CWV and FWV, with higher protein levels relating to smaller CWV and FWV. Effect sizes for HGF, IL8, and MMP10 on CWV were similar (, see Figure 1), as were their effect sizes on FWV (, see Figure 1).
FIGURE 1.
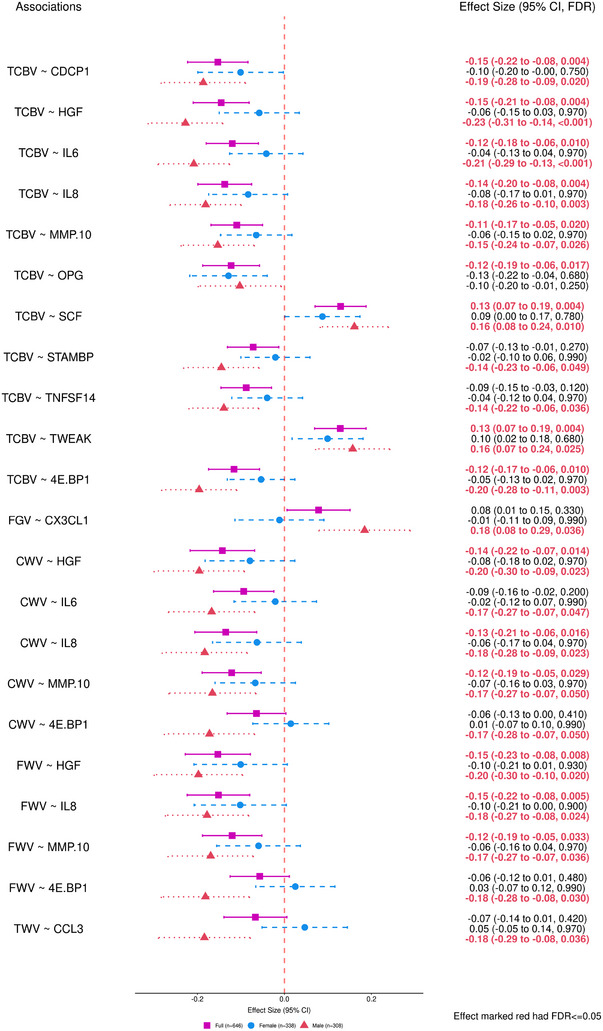
Forest plots of effect size for significant associations with the 13 brain MRI outcomes using Model 1 (FDR ≤ 0.05). Model 1 covariates included: age, age2, sex, an interaction term of sex × age, the time difference between the blood draw (Exam 7) and the MRI measurement, and APOE genotype. The pink square represents the combined sample (n = 646), the blue circle represents the Female stratum (n = 338), and the red triangle represented the Male stratum (n = 308). APOE, apolipoprotein E; CI, confidence interval; CWV, cerebral white matter volume; FDR, false discovery rate; FWV, frontal white matter volume; MRI, magnetic resonance imaging; PGV, parietal gray matter volume; TCBV, total cerebral brain volume; TWV, temporal white matter volume.
FIGURE 2.
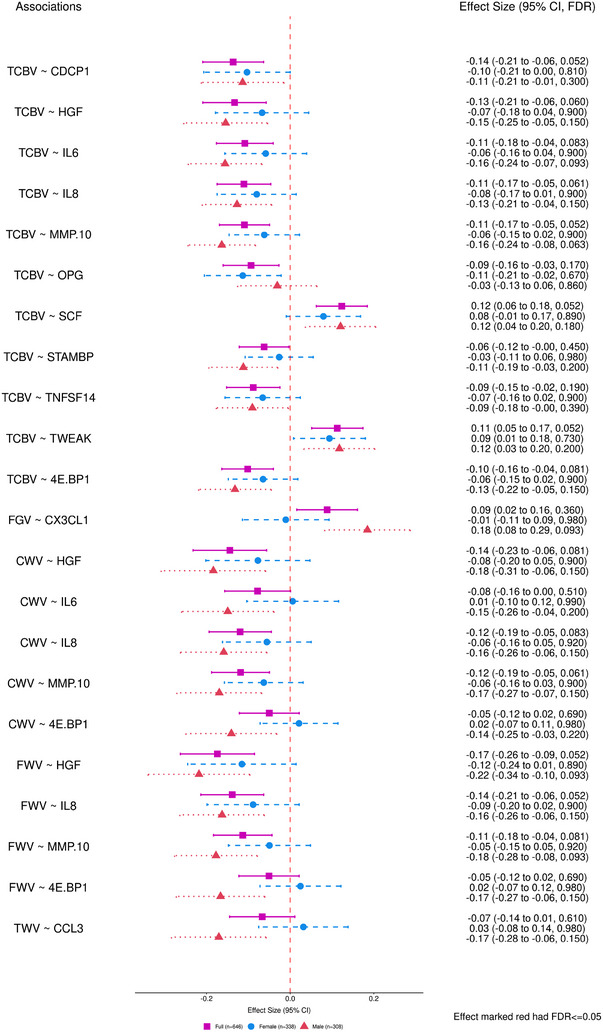
Forest plots of effect size for significant associations with the 13 brain MRI outcomes using Model 2 (FDR ≤ 0.05). Model 2 covariates included: age, age2, sex, an interaction term of sex × age, the time difference between the blood draw (Exam 7) and the MRI measurement, APOE genotype, prevalent CVD, prevalent AF at Exam 7, and CVD risk factors such as systolic and diastolic blood pressures (mmHg), diabetes status, treatment for hypertension, body‐mass index (kg/m2), current smoking status, total cholesterol level (mg/dL), high‐density lipoprotein cholesterol levels (HDL, measured in mg/dL), and use of lipid‐lowering agents at Exam 7. The pink square represented the combined sample (n = 646), the blue circle represented the Female (n = 338), and the red triangle represented the male (n = 308). AF, atrial fibrillation; APOE, Apolipoprotein E; CI, confidence interval; CVD; cardiovascular disease; CWV, cerebral white matter volume; FDR, false discovery rate; FWV, frontal white matter volume; MRI, magnetic resonance imaging; PGV, parietal gray matter volume; TCBV, total cerebral brain volume; TWV, temporal white matter volume.
In the sex‐stratified analyses, we observed significant associations only in males (n = 308), where the effects were in the same directions, but of larger size compared with the combined analysis. For example, higher levels of CDCP1, HGF, IL6, IL8, MMP10, and 4E.BP1 were still significantly associated with smaller TCBV in the male stratum, with increased effect sizes ranging from −0.23 to −0.15 SD units per SD unit increase in protein levels. SCF (, SE = 0.039, FDR = 0.010, see Figure 1) and TWEAK (, SE = 0.043, FDR = 0.025, see Figure 1) were associated with larger TCBV, with increased effect sizes in the male stratum. Significant results found only in the male stratum include negative associations of STAMBP and TNFSF14 with TCBV (, see Figure 1), IL6 and 4E.BP1 with CWV (, see Figure 1), CCL3 with TWV (, SE = 0.053, FDR = 0.036, see Figure 1), and 4E.BP1 with FWV (, SE = 0.051, FDR = 0.030, see Figure 1), and a positive association of CX3CL1 with FGV (, SE = 0.053, FDR = 0.036, see Figure 1). In females (n = 338), all effects were closer to 0 than in the full sample, and none were significant, suggesting strong evidence for differential associations across sex subgroups.
As shown in Figure 2, after adjusting for the additional CVD‐risk Model 2 covariates, all proteins significant in Model 1 showed consistent direction of effects, while displaying reduced effect sizes and lower levels of association significance. None remained significantly associated with the outcomes of interest at FDR ≤ 0.05. Results for analyses of all proteins with all brain MRI measures, using both Model 1 and Model 2 adjustments are provided in supporting information file 3.
3.3. Sensitivity analysis results
The subsample of participants who underwent brain MRI within 2 years before or after Exam 7 excluded 43 individuals (remaining n = 603). The subsample excluding participants with prevalent chronic leukemia or lymphoma, participants who reported use of glucocorticoids at Exam 7, and participants who were identified as outliers by PCA using the 68 rank‐normalized proteins excluded 36 individuals (remaining n = 610). Most of the significant associations between brain MRI measures and proteins using Model 1 in the full sample and male subgroups were still significant for these two sensitivity analyses, including CDCP1, HGF, IL6, IL8, MMP10, SCF, TWEAK, and 4E.BP1 with TCBV (Figure S2), as well as HGF, IL8, and MMP10 with CWV and FWV (Figure S3), with most changes in the absolute effect ranging within 0.02. The direction of effect and effect size were consistent with the primary analysis, suggesting that the observed associations are robust, even when considering the time lapse between protein measurement and brain MRI, as well as the potential inclusion of outliers (Figures S2 and S3). Still, no significant associations were observed in the females for both sensitivity analyses. Complete results for these two sensitivity analyses are provided in supporting information file 3. An additional sensitivity analysis was conducted to investigate potential effect modification by the APOE genotype. This involved including and assessing the interaction term between each protein and the indicator for being APOE ε4 carriers using Model 1 (excluding the number of APOE ε2 and ε4 alleles, and instead using an indicator of APOE ε4 carriers) on the full sample (n = 646). Among all pairwise associations, none of the interactions were significant (supporting information file 3), and we conclude that the APOE genotype did not modify the association between proteins and brain MRI measures in our sample.
3.4. Network analysis results
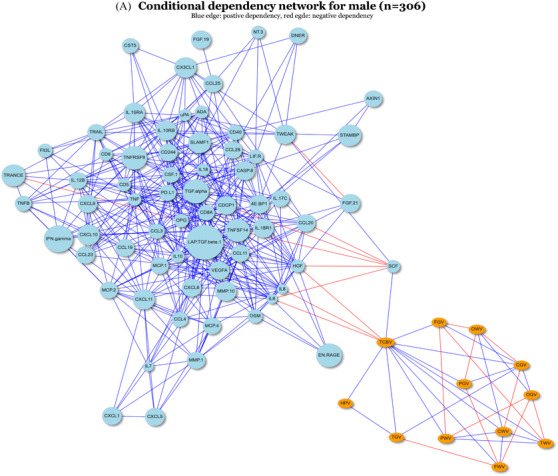
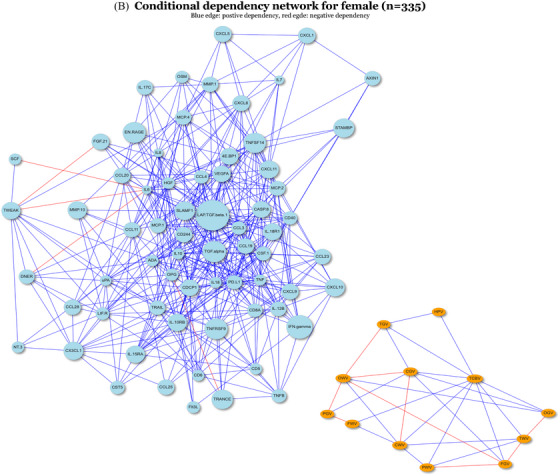
To assess the differential associations observed between males and females from another perspective, we applied FGL to the complete cases of the full sample including 68 proteins and 13 brain MRI measures stratified by sex (male n = 306, female n = 335) to identify differential conditional dependencies across sex subgroups. As shown by the unweighted conditional dependency networks in Figure 3, there were a total of 320 differential edges and 242 similar edges inferred by FGL. We focused on the edges between proteins and outcomes only. In the male subgroup, we observed edges between pairs between IL8, IL6, SCF, and HGF, with TCBV, respectively. The proteins and outcomes were clustered into two parts separately in the network (Figure 3A), with the only path linking the two parts through TCBV. In the female subgroup, no edges existed between proteins and outcomes (Figure 3B). Thus, the four edges between IL8, IL6, SCF, and HGF, with TCBV were concluded to have conditional dependencies only in the male, consistent with the stratified pairwise associations observed in Figure 1. Directions of conditional dependencies (not shown) were consistent with the stratified pairwise analysis as well. These observations suggest that the other marginal pairwise associations observed in the male subgroup may be associated through TCBV due to the nature of conditional dependency.
FIGURE 3.
Unweighted conditional dependency networks inferred by FGL for male and female. For generating the networks, we include a total of 81 nodes, consisted of 68 proteins and 13 brain MRI measures. Nodes that have only degree of 1 will be excluded from being shown in the networks above. The sample size for the male (A) and female (B) is 306 and 335, respectively. FGL, fused graphical lasso; MRI, magnetic resonance imaging.
4. DISCUSSION
We examined the association of 68 circulating inflammatory biomarkers with 13 total and regional brain MRI volumetric measures in 646 participants from the FHS Offspring cohort. Our findings are several folds. First, higher CDCP1, IL6, OPG, and 4E.BP1 levels were associated with smaller TCBV, and higher levels of HGF, IL8, and MMP10 were associated with smaller TCBV, CWV, and FWV. Further, higher levels of two proteins, SCF and TWEAK, were associated with larger TCBV. However, associations were no longer significant in models adjusting for CVD risk factors, although the direction of effects were preserved. Further exploration in stratified analyses revealed that these associations were only present in males, where the effects were in the same direction and larger than observed in the full sample. Additional associations found only in males include 4E.BP1 levels with FWV and CWV, STAMBP and TNSF14 with TCBV, CX3CL1 with FGV, IL6 with CWV, and CCL3 with TWV. Using GGMs, we observed a positive association between TCBV and SCF, and a negative association between TCBV and IL6, IL8, and HGF in males, whereas no associations were seen in females. These results underline differential associations between circulating inflammatory markers and brain imaging measures by sex. Our findings add to the knowledge base of the complex association of inflammation with dementia as we identify both protective and detrimental proteins related to early neuroimaging endophenotypes of dementia and AD.
A clear link has been established between intracranial brain volume and brain aging. Royle et al. showed that both the current and prior size of the brain serve as important indicators of current cognitive ability. 38 Wolf et al. also showed that intracranial volume could distinguish between cognitive normalcy and mild cognitive impairment (MCI) and between MCI and dementia. 39 It is hypothesized that a larger brain volume has a greater neural reserve capacity, potentially contributing to a more extensive functional reserve. However, some studies showed no significant association between intracranial volume and risk of dementia. 40 , 41 , 42 It is important to note that those studies had relatively small sample sizes and showed a similar direction of effect as the studies in favor of the hypothesis. Age‐related alterations in the brain progress more rapidly in the presence of systemic inflammation. While inflammation is a nonspecific biomarker of dementia and AD, inflammatory markers play a significant role in the early stages of neurodegeneration. This role includes inhibiting angiogenic and neuroprotective mechanisms, leading to neuron loss and injury to myelin and a higher risk for cognitive impairment. 43 Our report showed an association between TCBV and circulating biomarkers related to different inflammatory cascades. For instance, IL6 and OPG are cardiovascular‐related pro‐inflammatory cytokines that have been associated with a higher risk for cardiovascular and neurodegenerative conditions. 44 , 45 , 46 Moreover, CDCP1(CD318) is an immunoinflammatory marker enhancing T‐cell activation and infiltration, leading to increased production of proinflammatory cytokines. 47 Furthermore, 4E.BP1, part of the mTOR pathway, plays a role in regulating synaptic remodeling and regulating autophagy in neurons. 48 Higher levels of SCF and TWEAK were associated with larger TCBV, particularly in men. Consistent with our findings, research suggests SCF has neuroprotective properties in animal models. 49 Lower SCF plasma levels are associated with a faster cognitive decline in patients with AD. 50 Additionally, a small study of patients with AD receiving treatment with donepezil suggested that the treatment was associated with significantly higher SCF plasma levels and SCF levels correlated with improved cognitive scores. 51 TWEAK, also known as tumor necrosis factor (ligand) superfamily member 12, participates in a wide range of cellular activities, including proliferation, migration, apoptosis, inflammation, and angiogenesis. Importantly, previous work from our group identified an association between TWEAK and a lower risk of incident AD dementia, 52 further supporting a potential protective role of TWEAK against neurodegeneration.
Age‐related white matter changes are characterized by partial loss and pallor of myelin, loss of myelinated fibers, sparsely distributed macrophages, and malformation of myelin. 53 WMH is one of the major age‐related white matter changes on MRI, partly due to small vessel disease related to aging and/or cardiovascular disease; however, the causes are multifactorial and non‐specific. Another contributing factor is blood‐brain barrier dysfunction, where small vessel alterations can lead to chronic fluid and macromolecule leakage in the white matter. 54 Cardiovascular risk factors such as hypertension and diabetes have been associated with WMH. 55 , 56 Recent reports indicate that conventional cardiovascular risk factors contribute to the effect on white matter as significantly as age does. 57 This is consistent with our data showing attenuated associations when adjusting for traditional CVD risk factors.
White matter atrophy has also been investigated as part of age‐related changes. The Rotterdam study showed that age and cardiovascular risk factors are associated with smaller white matter volume. 58 The same cohort showed that white matter atrophy happened simultaneously with the formation of white matter lesions and not independently. 59
We report an association between CWV and FWV with three circulating biomarkers, namely HGF, IL8, and MMP10. We observed the association of these vascular related biomarkers in the frontal lobe which is the biggest and the most vulnerable cerebral lobe for vascular pathological insult. 60 Previous cross‐sectional studies reported an association between high serum and CSF‐HGF concentration and larger WMH in AD patients. 61 , 62 HGF is a plasminogen‐like growth factor with angiogenesis activity and plays a role in axon outgrowth, neuronal survival, and synaptic function. 63 IL8, a proinflammatory cytokine, has been associated with a higher risk for AD, but only in the presence of WMH. 64 Lastly, MMP‐10 is a zinc‐dependent enzyme. MMP‐10 levels in CSF were associated with AD+ MCI and AD‐dementia, while plasma MMP10 levels were not associated, despite a relatively high correlation between the two (r = 0.312, FDR = 1 × 10E‐20). 65
In sex‐stratified models, several biomarkers were linked to reduced TCBV in males, while SCF and TWEAK were associated with larger TCBV. Two specific biomarkers, namely STAMP and TNFSF14, showed significant association with TCBV in male only. Both proteins have been reported to be associated with AD in human and mouse studies. 66 , 67 Additionally, higher levels of CX3CL1 were associated with higher FGV. CX3CL1 is a CNS transmembrane chemokine which has been extensively investigated in the last few decades. Soluble form of CX3CL1 is suggested to be neuroprotective. Overexpression of the soluble CX3CL1 isoform reduced the effect of Tau, neuronal loss, and microglia activation in a mouse model. 68 Furthermore, an association between CCL3 and TWV was observed in the male subgroup. Reports suggested that CCL3 is involved in controlling glial activation and impacting cognitive functions. 69 In a transgenic mouse model of tauopathy called THY‐Tau22, there was an observed increase in the levels of CCL3 along with the infiltration of T lymphocytes into the hippocampus. 70 CCL3 plays important role in trafficking of inflammatory cells across the blood brain barrier during inflammatory conditions. 71
Despite a higher proportion of white matter changes in females, the impact of vascular risk factors such as hypertension and diabetes on the increased risk of developing WMH seems to be more pronounced in men. 72 , 73 Midlife vascular risk factors have an impact on the rate of progression of vascular brain injury. 74
Sexual dimorphism in circulating protein levels has been reported in large‐scale studies on healthy participants from the FHS and Dallas Heart Study. 14 , 75 In our study sample males were observed to have a more adverse cardiovascular risk profile compared to females, and the associations between proteins and MRI outcomes were attenuated in models adjusting for cardiovascular covariates. This indicates the involvement of cardiovascular pathways such as inflammation, adiposity, and fibrosis as mediators for these associations, which may explain the significant results observed in the male subgroup. Additionally, we do not discount the potential confounding effect influencing the observations.
Our study has several strengths, including the well‐characterized, community‐based cohort under continuous surveillance for dementia outcomes and with extensive, long‐term data collection protocols for preclinical dementia phenotypes including neuroimaging. The inclusion of a wide array of inflammatory markers, alongside numerous total, and regional brain measures, significantly enriches our analytical scope, enhancing the exploration of the link between inflammation and structural brain changes. The inclusion of a dementia‐free sample ensures a cross‐sectional baseline measurement of the association between inflammatory biomarkers and brain changes.
Several limitations should be noted. First, the majority of FHS participants self‐report as non‐Hispanic White, potentially limiting the generalizability of our findings to other racial and ethnic populations. Second, the inflammatory biomarker data collection was conducted at a single time point, restricting our ability to capture longitudinal fluctuations in relation to brain volumes. Declines in inflammation over time have been observed to be associated with MRI indicators of brain health whereas transition to higher levels over time portend worsening white matter changes. 53 As our study is observational and cross‐sectional, it cannot establish causal relationships. Additionally, the sample size may be a limitation, potentially obscuring some associations due to its scale.
To conclude, our analysis has revealed complex connections involving inflammatory biomarkers and brain volumes, offering insights into their potential roles in neurodegeneration. The initial model highlighted significant associations between increased levels of CDCP1, IL6, OPG, 4E.BP1, HGF, IL8, and MMP10, and adverse brain volume outcomes. Moreover, favorable brain volume changes were associated with SCF and TWEAK. Although some associations were moderated in the fully adjusted model, their trends remained. Furthermore, CX3CL1 demonstrated a significant connection with FGV, while HGF, IL6, IL8, MMP10, and 4E.BP1 displayed adverse associations with white matter volumes. Notably, CCL3 exhibited an adverse association with TWV. In males, certain biomarkers were associated with brain volume measures, unlike in females. Taken together, these findings underscore the complex interplay of specific inflammatory protein biomarkers in association with changes in brain structure, providing deeper insights into potential mechanisms underlying neurodegeneration.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest related to this study. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Each participant provided written informed consent during each examination attended as part of the Framingham Heart Study (FHS), a procedure evaluated and approved by the Institutional Review Board (IRB) at Boston University Medical Center (BUMC). The IRB number for the ongoing FHS at BUMC is H‐32132, whereas for the present study, it is H‐39876.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the Framingham Heart Study participants, as well as the study team for their contributions. This study was supported by funding from the National Institute on Aging including the collection of data from the OLINK Inflammatory Panel funded by grant number R01 AG067457. The gathering of MRI data was supported by multiple grants from the National Institute on Aging: R01 AG08122, R01 AG054076, R01 AG049607, R01 AG033193, and R01 NS017950. Additionally, The National Heart, Lung, and Blood Institute, provided support for the Framingham examinations under contract number 75N92019D00031.
Chen J, Ragab AAY, Doyle MF, et al. Inflammatory protein associations with brain MRI measures: Framingham Offspring Cohort. Alzheimer's Dement. 2024;20:7465–7478. 10.1002/alz.14147
Jiachen Chen and Ahmed A. Y. Ragab contributed equally to this study.
REFERENCES
- 1. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105‐e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nandi A, Counts N, Chen S, et al. Global and regional projections of the economic burden of Alzheimer's disease and related dementias from 2019 to 2050: a value of statistical life approach. eClinicalMedicine. 2022;51:101580. doi: 10.1016/j.eclinm.2022.101580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Márquez F, Yassa MA. Neuroimaging biomarkers for Alzheimer's disease. Mol Neurodegener. 2019;14(1):21. doi: 10.1186/s13024-019-0325-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging. 2010;31(3):378‐386. doi: 10.1016/j.neurobiolaging.2008.04.008 [DOI] [PubMed] [Google Scholar]
- 5. Sabuncu MR, Desikan RS, Sepulcre J, et al. The dynamics of cortical and hippocampal atrophy in Alzheimer disease. Arch Neurol. 2011;68(8):1040‐1048. doi: 10.1001/archneurol.2011.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu H, Ou Y, Shen X, et al. White matter hyperintensities and risks of cognitive impairment and dementia: a systematic review and meta‐analysis of 36 prospective studies. Neurosci Biobehav Rev. 2021;120:16‐27. doi: 10.1016/j.neubiorev.2020.11.007 [DOI] [PubMed] [Google Scholar]
- 7. Pettigrew C, Soldan A. Defining cognitive reserve and implications for cognitive aging. Curr Neurol Neurosci Rep. 2019;19(1):1. doi: 10.1007/s11910-019-0917-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. 2018;4:575‐590. doi: 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam study. Arch Neurol. 2004;61(5):668‐672. doi: 10.1001/archneur.61.5.668 [DOI] [PubMed] [Google Scholar]
- 10. van Dijk EJ, Prins ND, Vermeer SE, et al. C‐reactive protein and cerebral small‐vessel disease: the Rotterdam Scan Study. Circulation. 2005;112(6):900‐905. doi: 10.1161/CIRCULATIONAHA.104.506337 [DOI] [PubMed] [Google Scholar]
- 11. Hilal S, Ikram MA, Verbeek MM, et al. C‐reactive protein, plasma amyloid‐β levels, and their interaction with magnetic resonance imaging markers. Stroke. 2018;49(11):2692‐2698. doi: 10.1161/STROKEAHA.118.022317 [DOI] [PubMed] [Google Scholar]
- 12. Pikula A, Beiser AS, DeCarli C, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury. Circulation. 2012;125(17):2100‐2107. doi: 10.1161/CIRCULATIONAHA.110.989145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janowitz D, Habes M, Toledo JB, et al. Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav. 2020;14(4):1108‐1117. doi: 10.1007/s11682-019-00058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jefferson AL, Massaro JM, Wolf PA, et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032‐1038. doi: 10.1212/01.wnl.0000257815.20548.df [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fang Y, Doyle MF, Chen J, et al. Association between inflammatory biomarkers and cognitive aging. PLoS One. 2022;17(9):e0274350. doi: 10.1371/journal.pone.0274350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham offspring study. Design and preliminary data. Prev Med. 1975;4(4):518‐525. doi: 10.1016/0091-7435(75)90037-7 [DOI] [PubMed] [Google Scholar]
- 17. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491‐510. doi: 10.1016/j.neurobiolaging.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 18. Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195‐204. doi: 10.1016/j.bbi.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drake I, Hindy G, Almgren P, et al. Methodological considerations for identifying multiple plasma proteins associated with all‐cause mortality in a population‐based prospective cohort. Sci Rep. 2021;11(1):6734. doi: 10.1038/s41598-021-85991-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harlid S, Harbs J, Myte R, et al. A two‐tiered targeted proteomics approach to identify pre‐diagnostic biomarkers of colorectal cancer risk. Sci Rep. 2021;11(1):5151. doi: 10.1038/s41598-021-83968-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Folkersen L, Gustafsson S, Wang Q, et al. Genomic and drug target evaluation of 90 cardiovascular proteins in 30,931 individuals. Nature Metabolism. 2020;2(10):1135‐1148. doi: 10.1038/s42255-020-00287-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Debette S, Wolf PA, Beiser A, et al. Association of parental dementia with cognitive and brain MRI measures in middle‐aged adults. Neurology. 2009;73(24):2071‐2078. doi: 10.1212/WNL.0b013e3181c67833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16(2):274‐284. doi: 10.1097/00004728-199203000-00018 [DOI] [PubMed] [Google Scholar]
- 24. Murabito JM, Beiser AS, Decarli C, Seshadri S, Wolf PA, Au R. Parental longevity is associated with cognition and brain ageing in middle‐aged offspring. Age Ageing. 2014;43(3):358‐363. doi: 10.1093/ageing/aft175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer's disease: unbiased analysis of fluid‐registered serial MRI. Proc Natl Acad Sci USA. 2002;99(7):4703‐4707. doi: 10.1073/pnas.052587399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baron JC, Chételat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel‐based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14(2):298‐309. doi: 10.1006/nimg.2001.0848 [DOI] [PubMed] [Google Scholar]
- 27. Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19(4):415‐435. doi: 10.1007/s11065-009-9113-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109‐117. doi: 10.1002/gps.2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Olesen PJ, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto‐parietal network. Cognitive Brain Research. 2003;18(1):48‐57. doi: 10.1016/j.cogbrainres.2003.09.003 [DOI] [PubMed] [Google Scholar]
- 30. Lahoz C, Schaefer EJ, Cupples LA, et al. Apolipoprotein E genotype and cardiovascular disease in the Framingham Heart Study. Atherosclerosis. 2001;154(3):529‐537. doi: 10.1016/s0021-9150(00)00570-0 [DOI] [PubMed] [Google Scholar]
- 31. Lloyd‐Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791‐798. doi: 10.1161/CIRCULATIONAHA.105.548206 [DOI] [PubMed] [Google Scholar]
- 32. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Behav Brain Res. 1995;57(1):289‐300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 33. Team R. R: A language and environment for statistical computing. MSOR Connections. 2014;1. https://api.semanticscholar.org/CorpusID:215755663 [Google Scholar]
- 34. Therneau TM. coxme: Mixed Effects Cox Models. R package version 2.2‐20. 2024. https://CRAN.R-project.org/package=coxme
- 35. Shutta KH, De Vito R, Scholtens DM, Balasubramanian R. Gaussian graphical models with applications to omics analyses. Stat Med. 2022;41(25):5150‐5187. doi: 10.1002/sim.9546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Danaher P, Wang P, Witten DM. The joint graphical lasso for inverse covariance estimation across multiple classes. J R Stat Soc Ser B Stat Methodol. 2014;76(2):373‐397. doi: 10.1111/rssb.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Danaher P. JGL: Performs the Joint Graphical Lasso for Sparse Inverse Covariance Estimation on Multiple Classes. R package version 2.3.2. 2023. https://CRAN.R-project.org/package=JGL
- 38. Royle NA, Booth T, Valdés Hernández MC, et al. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol Aging. 2013;34(12):2726‐2733. doi: 10.1016/j.neurobiolaging.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wolf H, Julin P, Gertz H, Winblad B, Wahlund L. Intracranial volume in mild cognitive impairment, Alzheimer's disease and vascular dementia: evidence for brain reserve? Int J Geriatr Psychiatry. 2004;19(10):995‐1007. doi: 10.1002/gps.1205 [DOI] [PubMed] [Google Scholar]
- 40. Tate DF, Neeley ES, Norton MC, et al. Intracranial volume and dementia: some evidence in support of the cerebral reserve hypothesis. Brain Res. 2011;1385:151‐162. doi: 10.1016/j.brainres.2010.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Edland SD, Xu Y, Plevak M, et al. Total intracranial volume: normative values and lack of association with Alzheimer's disease. Neurology. 2002;59(2):272‐274. doi: 10.1212/wnl.59.2.272 [DOI] [PubMed] [Google Scholar]
- 42. Jenkins R, Fox NC, Rossor AM, Harvey RJ, Rossor MN. Intracranial volume and Alzheimer disease: evidence against the cerebral reserve hypothesis. Arch Neurol. 2000;57(2):220‐224. doi: 10.1001/archneur.57.2.220 [DOI] [PubMed] [Google Scholar]
- 43. Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8(1):267. doi: 10.1038/s41392-023-01486-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Blum‐Degena D, Müller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin‐1β and interleukin‐6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202(1):17‐20. doi: 10.1016/0304-3940(95)12192-7 [DOI] [PubMed] [Google Scholar]
- 45. Capogna E, Watne LO, Sørensen Ø, et al. Associations of neuroinflammatory IL‐6 and IL‐8 with brain atrophy, memory decline, and core AD biomarkers – in cognitively unimpaired older adults. Brain Behav Immun. 2023;113:56‐65. doi: 10.1016/j.bbi.2023.06.027 [DOI] [PubMed] [Google Scholar]
- 46. Emanuele E, Peros E, Scioli GA, et al. Plasma osteoprotegerin as a biochemical marker for vascular dementia and Alzheimer's disease. Int J Mol Med. 2004;13(6):849‐853. [PubMed] [Google Scholar]
- 47. Enyindah‐Asonye G, Li Y, Ruth JH, et al. CD318 is a ligand for CD6. Proc Natl Acad Sci. 2017;114(33):E6912‐E6921. doi: 10.1073/pnas.1704008114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li X, Alafuzoff I, Soininen H, Winblad B, Pei J. Levels of mTOR and its downstream targets 4E‐BP1, eEF2, and eEF2 kinase in relationships with tau in Alzheimer's disease brain. FEBS J. 2005;272(16):4211‐4220. doi: 10.1111/j.1742-4658.2005.04833.x [DOI] [PubMed] [Google Scholar]
- 49. Sun L, Lee J, Fine HA. Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest. 2004;113(9):1364‐1374. doi: 10.1172/JCI20001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Laske C, Sopova K, Hoffmann N, et al. Stem cell factor plasma levels are decreased in Alzheimer's disease patients with fast cognitive decline after one‐year follow‐up period: the Pythia‐study. J Alzheimers Dis. 2011;26(1):39‐45. doi: 10.3233/JAD-2011-110008 [DOI] [PubMed] [Google Scholar]
- 51. Leyhe T, Hoffmann N, Stransky E, Laske C. Increase of SCF plasma concentration during donepezil treatment of patients with early Alzheimer's disease. Int J Neuropsychopharmacol. 2009;12(10):1319‐1326. doi: 10.1017/S1461145709990216 [DOI] [PubMed] [Google Scholar]
- 52. Chen J, Doyle MF, Fang Y, et al. Peripheral inflammatory biomarkers are associated with cognitive function and dementia: Framingham Heart Study Offspring cohort. Aging Cell. 2023;22(10):e13955. doi: 10.1111/acel.13955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xiong YY, Mok V. Age‐related white matter changes. J Aging Res. 2011;2011:617927. doi: 10.4061/2011/617927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wardlaw JM, Valdés Hernández MC, Muñoz‐Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J Am Heart Assoc. 2015;4(6):001140. doi: 10.1161/JAHA.114.001140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brundel M, Kappelle LJ, Biessels GJ. Brain imaging in type 2 diabetes. Eur Neuropsychopharmacol. 2014;24(12):1967‐1981. doi: 10.1016/j.euroneuro.2014.01.023 [DOI] [PubMed] [Google Scholar]
- 56. van Dijk EJ, Breteler MMB, Schmidt R, et al. The association between blood pressure, hypertension, and cerebral white matter lesions. Hypertension. 2004;44(5):625‐630. doi: 10.1161/01.HYP.0000145857.98904.20 [DOI] [PubMed] [Google Scholar]
- 57. Koohi F, Harshfield EL, Markus HS. Contribution of conventional cardiovascular risk factors to brain white matter hyperintensities. J Am Heart Assoc. 2023;12(14):e030676. doi: 10.1161/JAHA.123.030676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population: the Rotterdam Scan Study. Neurobiol Aging. 2008;29(6):882‐890. doi: 10.1016/j.neurobiolaging.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 59. Vernooij MW, de Groot M, van der Lugt A, et al. White matter atrophy and lesion formation explain the loss of structural integrity of white matter in aging. Neuroimage. 2008;43(3):470‐477. doi: 10.1016/j.neuroimage.2008.07.052 [DOI] [PubMed] [Google Scholar]
- 60. Sira CS, Mateer CA. Frontal lobes. In: Aminoff MJ, Daroff RB, eds. Encyclopedia of the Neurological Sciences. 2nd ed. Academic Press; 2014:358‐365. [Google Scholar]
- 61. Zhu Y, Hilal S, Chai YL, et al. Serum hepatocyte growth factor is associated with small vessel disease in Alzheimer's dementia. Front Aging Neurosci. 2018;10:8. doi: 10.3389/fnagi.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsuboi Y, Kakimoto K, Nakajima M, et al. Increased hepatocyte growth factor level in cerebrospinal fluid in Alzheimer's disease. Acta Neurol Scand. 2003;107(2):81‐86. doi: 10.1034/j.1600-0404.2003.02089.x [DOI] [PubMed] [Google Scholar]
- 63. Nakamura T, Mizuno S. The discovery of Hepatocyte Growth Factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Japan Acad, Ser B. 2010;86(6):588‐610. doi: 10.2183/pjab.86.588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhu Y, Chai YL, Hilal S, et al. Serum IL‐8 is a marker of white‐matter hyperintensities in patients with Alzheimer's disease. Alzheimers Dement. 2017;7:41‐47. doi: 10.1016/j.dadm.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whelan CD, Mattsson N, Nagle MW, et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer's disease. Acta Neuropathologica Communications. 2019;7(1):169. doi: 10.1186/s40478-019-0795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Whelan CD, Mattsson N, Nagle MW, et al. Multiplex proteomics identifies novel CSF and plasma biomarkers of early Alzheimer's disease. Acta Neuropathol Commun. 2019;7(1):169. doi: 10.1186/s40478-019-0795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cucos CA, Milanesi E, Dobre M, Musat IA, Manda G, Cuadrado A. Altered blood and brain expression of inflammation and redox genes in Alzheimer's disease, common to APPV717I × TAUP301L mice and patients. Int J Mol Sci. 2022;23(10):5799. doi: 10.3390/ijms23105799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nash KR, Lee DC, Hunt JBJ, et al. Fractalkine overexpression suppresses tau pathology in a mouse model of tauopathy. Neurobiol Aging. 2013;34(6):1540‐1548. doi: 10.1016/j.neurobiolaging.2012.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marciniak E, Faivre E, Dutar P, et al. The Chemokine MIP‐1α/CCL3 impairs mouse hippocampal synaptic transmission, plasticity and memory. Sci Rep. 2015;5:15862. doi: 10.1038/srep15862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Laurent C, Dorothée G, Hunot S, et al. Hippocampal T cell infiltration promotes neuroinflammation and cognitive decline in a mouse model of tauopathy. Brain. 2017;140(1):184‐200. doi: 10.1093/brain/aww270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chui R, Dorovini‐Zis K. Regulation of CCL2 and CCL3 expression in human brain endothelial cells by cytokines and lipopolysaccharide. J Neuroinflammation. 2010;7:1‐1. doi: 10.1186/1742-2094-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jongen C, van der Grond J, Kappelle LJ, et al. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50(7):1509‐1516. doi: 10.1007/s00125-007-0688-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Filomena J, Riba‐Llena I, Vinyoles E, et al. Short‐Term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66(3):634‐640. doi: 10.1161/HYPERTENSIONAHA.115.05440 [DOI] [PubMed] [Google Scholar]
- 74. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461‐468. doi: 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lew J, Sanghavi M, Ayers CR, et al. Sex‐based differences in cardiometabolic biomarkers. Circulation. 2017;135(6):544‐555. doi: 10.1161/CIRCULATIONAHA.116.023005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information


