Abstract
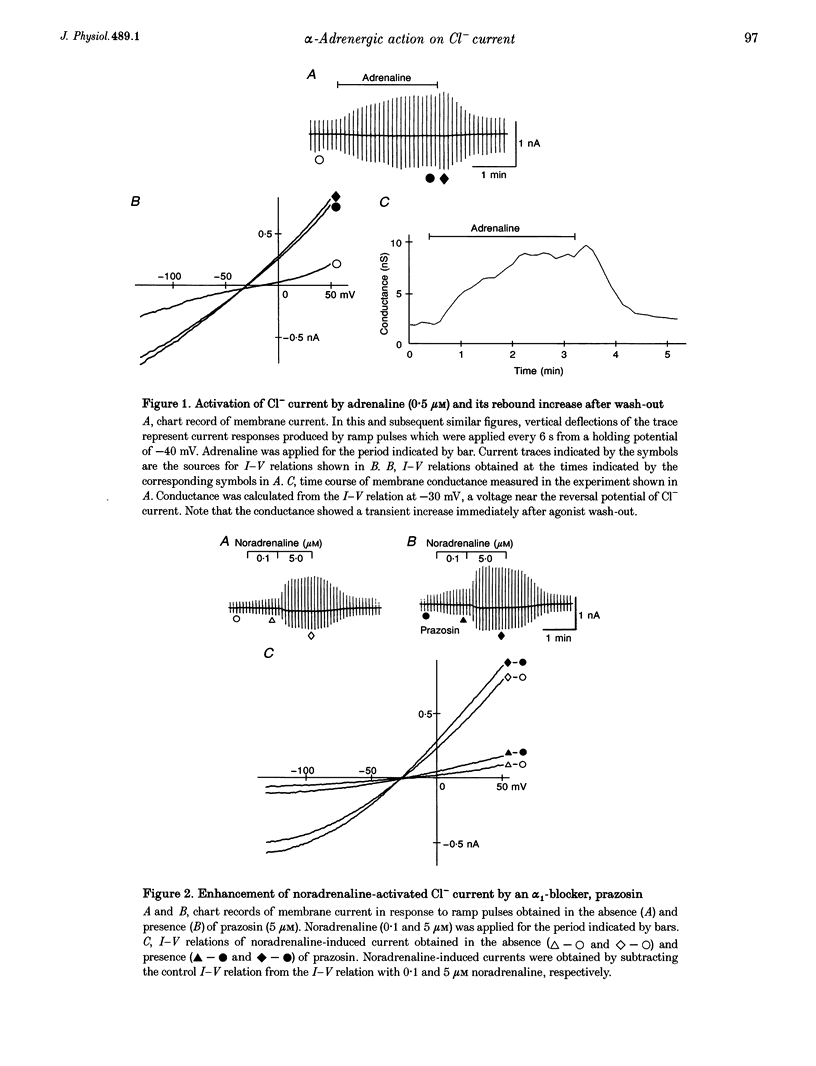
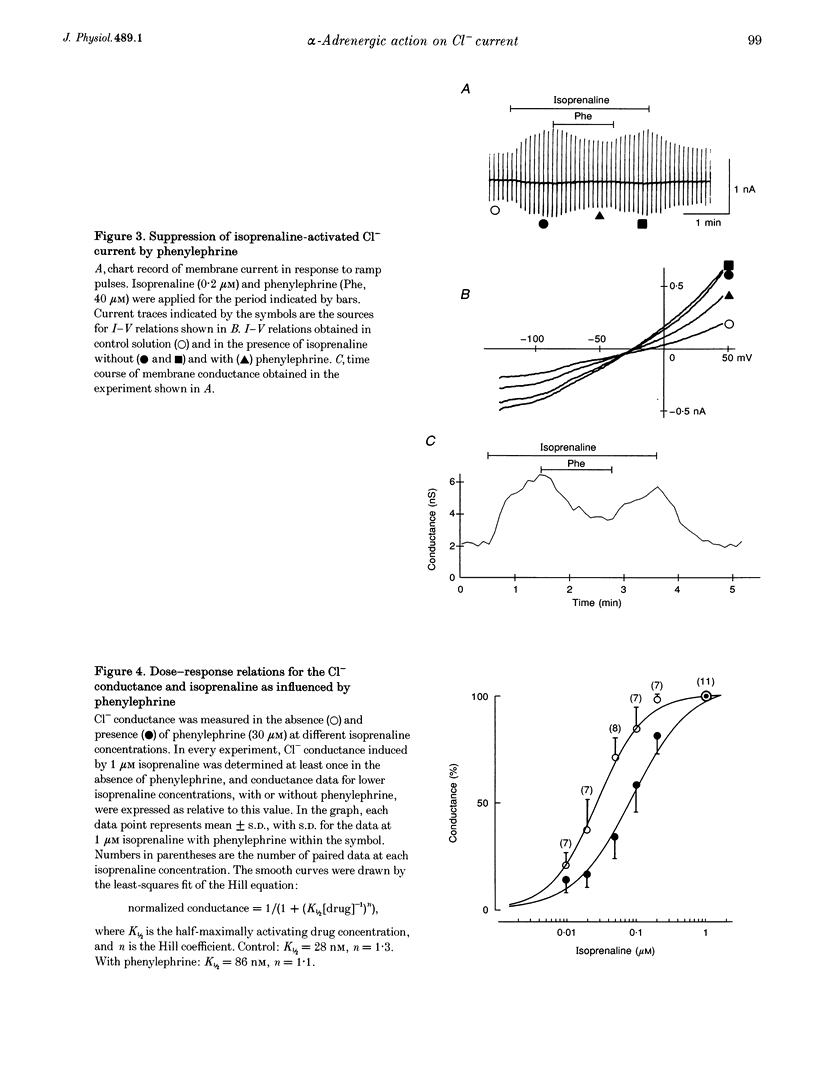
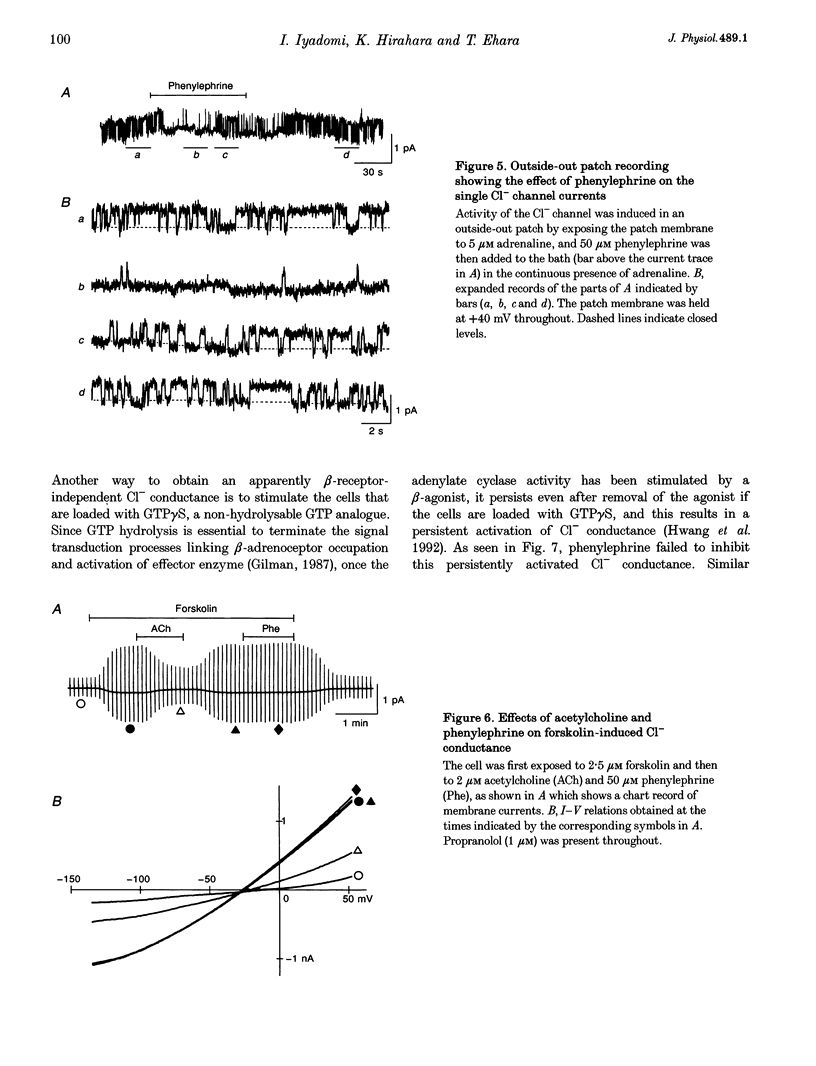
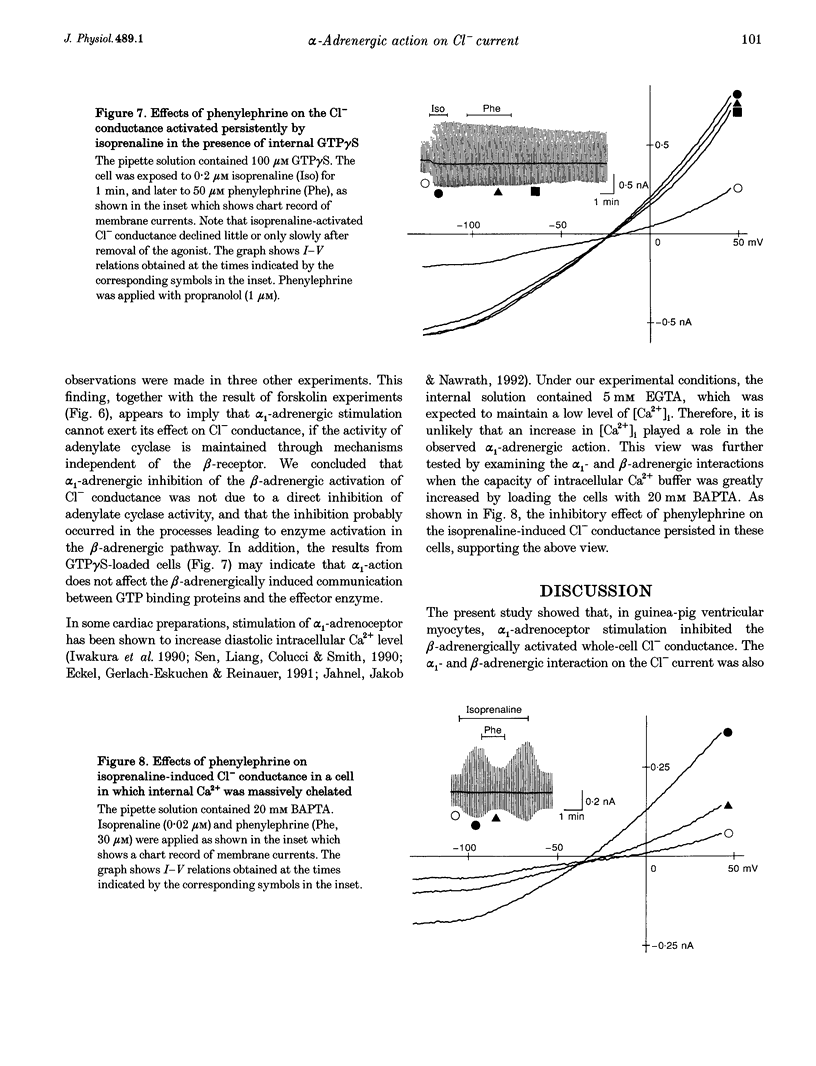
1. alpha 1-Adrenoceptor-mediated inhibition of the beta-adrenoceptor-dependent Cl- current was investigated in guinea-pig ventricular myocytes using the patch clamp technique. The Cl- conductance activated by noradrenaline (0.1-10 microM) with an alpha 1-blocker (prazosin, 5 microM) was significantly greater than that activated by noradrenaline alone. Phenylephrine and methoxamine, alpha 1-agonists, exerted an inhibitory effect on the Cl- conductance activated by isoprenaline. The dose-response relationship for isoprenaline and the Cl- current activation was shifted to higher doses in the presence of phenylephrine (30 microM). 2. The interaction of alpha 1- and beta-agonists on Cl- current was also observed on the single channel level; in some of the outside-out membrane patches, phenylephrine (50 microM) depressed the activity of the single Cl- channel which was induced by 5 microM adrenaline. 3. Phenylephrine had no effect on the Cl- conductance induced by forskolin (0.5-5 microM), an activator of adenylate cyclase. The Cl- conductance activated persistently by isoprenaline in GTP gamma S-loaded cells was also insensitive to phenylephrine. The results suggest that the observed alpha 1-adrenergic attenuation of the beta-adrenergic response is not primarily due to inhibition of adenylate cyclase activity. The alpha 1-adrenergic action may interfere with the processes leading to enzyme activation in the beta-adrenergic pathway.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Burch R. M., Jelsema C. L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988 Mar;11(3):117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Barrett S., Honbo N., Karliner J. S. Alpha 1-adrenoceptor-mediated inhibition of cellular cAMP accumulation in neonatal rat ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1993 Apr;347(4):384–393. doi: 10.1007/BF00165388. [DOI] [PubMed] [Google Scholar]
- Bordoni A., Biagi P. L., Rossi C. A., Hrelia S. Alpha-1-stimulated phosphoinositide breakdown in cultured cardiomyocytes: diacylglycerol production and composition in docosahexaenoic acid supplemented cells. Biochem Biophys Res Commun. 1991 Jan 31;174(2):869–877. doi: 10.1016/0006-291x(91)91498-2. [DOI] [PubMed] [Google Scholar]
- Boutjdir M., Restivo M., Wei Y., el-Sherif N. Alpha 1- and beta-adrenergic interactions on L-type calcium current in cardiac myocytes. Pflugers Arch. 1992 Jul;421(4):397–399. doi: 10.1007/BF00374231. [DOI] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Action of the cardiac alpha 1-adrenergic receptor. Activation of cyclic AMP degradation. J Biol Chem. 1985 Jun 10;260(11):6733–6737. [PubMed] [Google Scholar]
- Collier M. L., Hume J. R. Unitary chloride channels activated by protein kinase C in guinea pig ventricular myocytes. Circ Res. 1995 Feb;76(2):317–324. doi: 10.1161/01.res.76.2.317. [DOI] [PubMed] [Google Scholar]
- Eckel J., Gerlach-Eskuchen E., Reinauer H. Alpha-adrenoceptor-mediated increase in cytosolic free calcium in isolated cardiac myocytes. J Mol Cell Cardiol. 1991 May;23(5):617–625. doi: 10.1016/0022-2828(91)90053-o. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Noble D., Noble S. J., Powell T., Twist V. W. An isoprenaline activated sodium-dependent inward current in ventricular myocytes. Nature. 1987 Aug 13;328(6131):634–637. doi: 10.1038/328634a0. [DOI] [PubMed] [Google Scholar]
- Ehara T., Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990 Sep 20;347(6290):284–286. doi: 10.1038/347284a0. [DOI] [PubMed] [Google Scholar]
- Ehara T., Matsuura H. Single-channel study of the cyclic AMP-regulated chloride current in guinea-pig ventricular myocytes. J Physiol. 1993 May;464:307–320. doi: 10.1113/jphysiol.1993.sp019636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Hume J. R. Autonomic regulation of a chloride current in heart. Science. 1989 May 26;244(4907):983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Harvey R. D. Chloride conductance pathways in heart. Am J Physiol. 1991 Sep;261(3 Pt 1):C399–C412. doi: 10.1152/ajpcell.1991.261.3.C399. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Nairn A. C., Gadsby D. C. Role of GTP-binding proteins in the regulation of mammalian cardiac chloride conductance. J Gen Physiol. 1992 Apr;99(4):465–489. doi: 10.1085/jgp.99.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Iwakura K., Hori M., Watanabe Y., Kitabatake A., Cragoe E. J., Jr, Yoshida H., Kamada T. Alpha 1-adrenoceptor stimulation increases intracellular pH and Ca2+ in cardiomyocytes through Na+/H+ and Na+/Ca2+ exchange. Eur J Pharmacol. 1990 Sep 4;186(1):29–40. doi: 10.1016/0014-2999(90)94057-5. [DOI] [PubMed] [Google Scholar]
- Jahnel U., Jakob H., Nawrath H. Electrophysiologic and inotropic effects of alpha-adrenoceptor stimulation in human isolated atrial heart muscle. Naunyn Schmiedebergs Arch Pharmacol. 1992 Jul;346(1):82–87. doi: 10.1007/BF00167575. [DOI] [PubMed] [Google Scholar]
- Keung E. C., Karliner J. S. Complex regulation of calcium current in cardiac cells. Dependence on a pertussis toxin-sensitive substrate, adenosine triphosphate, and an alpha 1-adrenoceptor. J Clin Invest. 1990 Mar;85(3):950–954. doi: 10.1172/JCI114524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka S., Ehara T., Noma A. Chloride-sensitive nature of the adrenaline-induced current in guinea-pig cardiac myocytes. J Physiol. 1990 Jun;425:579–598. doi: 10.1113/jphysiol.1990.sp018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Hwang T. C., Nastiuk K. L., Nairn A. C., Gadsby D. C. The protein kinase A-regulated cardiac Cl- channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992 Nov 5;360(6399):81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- Okumura K., Kawai T., Hashimoto H., Ito T., Ogawa K., Satake T. Sustained diacylglycerol formation in norepinephrine-stimulated rat heart is associated with alpha 1-adrenergic receptor. J Cardiovasc Pharmacol. 1988 Jun;11(6):651–656. doi: 10.1097/00005344-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Otani H., Otani H., Das D. K. Alpha 1-adrenoceptor-mediated phosphoinositide breakdown and inotropic response in rat left ventricular papillary muscles. Circ Res. 1988 Jan;62(1):8–17. doi: 10.1161/01.res.62.1.8. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Sen L., Liang B. T., Colucci W. S., Smith T. W. Enhanced alpha 1-adrenergic responsiveness in cardiomyopathic hamster cardiac myocytes. Relation to the expression of pertussis toxin-sensitive G protein and alpha 1-adrenergic receptors. Circ Res. 1990 Nov;67(5):1182–1192. doi: 10.1161/01.res.67.5.1182. [DOI] [PubMed] [Google Scholar]
- Tareen F. M., Ono K., Noma A., Ehara T. Beta-adrenergic and muscarinic regulation of the chloride current in guinea-pig ventricular cells. J Physiol. 1991;440:225–241. doi: 10.1113/jphysiol.1991.sp018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A., Pucéat M., Vassort G., Vogel S. M. Cardiac alpha 1-adrenoceptors: an overview. Pharmacol Rev. 1993 Jun;45(2):147–175. [PubMed] [Google Scholar]
- Tohse N., Nakaya H., Kanno M. Alpha 1-adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circ Res. 1992 Dec;71(6):1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Walsh K. B. Activation of a heart chloride current during stimulation of protein kinase C. Mol Pharmacol. 1991 Sep;40(3):342–346. [PubMed] [Google Scholar]
- Walsh K. B., Long K. J. Properties of a protein kinase C-activated chloride current in guinea pig ventricular myocytes. Circ Res. 1994 Jan;74(1):121–129. doi: 10.1161/01.res.74.1.121. [DOI] [PubMed] [Google Scholar]
- Zhang K., Barrington P. L., Martin R. L., Ten Eick R. E. Protein kinase-dependent Cl- currents in feline ventricular myocytes. Circ Res. 1994 Jul;75(1):133–143. doi: 10.1161/01.res.75.1.133. [DOI] [PubMed] [Google Scholar]


