Abstract
Background
The present study investigated the function of G protein-coupled receptor kinase 2 (GRK2) in acute liver injury (ALI) by cisplatin, and investigated the protective effect of pharmacological inhibition of GRK2.
Methods
ALI models were generated in global adult hemizygous (ALI-Grk2±) mice and wild-type (WT) mice. Liver biochemistry parameters and histopathology were used to evaluate the severity of ALI and the protective effect of pharmacological inhibition of GRK2. GRK2-siRNA was used to knock down the expression of GRK2 in AML12 cells in vitro.
Results
ALI model mice exhibited increased blood levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels and abnormal liver pathology accompanied by imbalanced L-glutathione (GSH) levels. Cisplatin administration upregulated GKR2, p-GRK2 and NADPH oxidase 4 (NOX4) expression in the liver tissues of ALI model mice. Compared to WT mice injected with cisplatin, Grk2± mice that received cisplatin showed significant improvements in liver function and pathological performance, decreased NOX4 levels, reduced endoplasmic reticulum (ER) stress, and diminished liver cell apoptosis. In vitro, the transfection of AML12 cells with siRNA significantly reduced NOX4 expression and inhibited cisplatin-induced reactive oxygen species production, ER stress (increased levels of GRP94, GRP78, p-elF2α and CHOP) and apoptotic death. Moreover, pharmacological treatment with drugs that inhibit GRK2 (CP-25 or paroxetine) significantly ameliorated cisplatin-induced ALI by improving liver pathological manifestations, inhibiting oxidative stress and ER stress, and reducing liver cell apoptosis. Similar results were observed in vitro.
Conclusions
GRK2 mediates the development of cisplatin-induced ALI by modulating NOX4 and ER stress. Pharmacological inhibition of GRK2 with CP-25 or paroxetine effectively alleviated ALI. GRK2 can be used as a potential target for the prevention and treatment of liver injury.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10565-024-09940-y.
Keywords: Cisplatin, Liver injury, GRK2, NOX4, Endoplasmic reticulum stress
Background
Cisplatin is a classical antineoplastic drug with good antitumor effects on a variety of tumors, including lung cancer, neck cancer, head-and-neck cancer, testicular cancer, and ovarian cancer (Ghosh 2019). However, cisplatin can cause severe adverse reactions, such as ototoxicity, nephrotoxicity, hepatotoxicity and neurotoxicity, seriously limiting its clinical application (Qi et al. 2019; Suk and Kim 2012). The main metabolic organ of the human body is the liver, and liver injury can lead to unpredictable results and can even be life-threatening (Oun et al. 2018). Until recently, the specific molecular mechanism of cisplatin-induced acute liver injury (ALI) has been poorly understood, resulting in a lack of effective approaches for its prevention and treatment (Qi et al. 2019; Alrashed and El-Kordy 2019).
Oxidative stress is an important factor in inducing ALI. Oxidative stress was caused by the disruption of the balance between reactive oxygen species (ROS) and antioxidants. The O2 was catalyzed by NADPH oxidase (NOX) family enzymes to reduce to reactive oxygen species, superoxides, or hydrogen peroxide. Increased NOX4 expression is an important factor that promotes the release of ROS during the development of ALI (Piera-Velazquez and Jimenez 2021). Moreover, increased ROS lead to endoplasmic reticulum (ER) stress, which further promotes cell apoptosis and causes tissue damage (Chen et al. 2019; Verfaillie et al. 2012). But, the role of abnormal NOX4 expression and the function of NOX4 in ALI remain unclear.
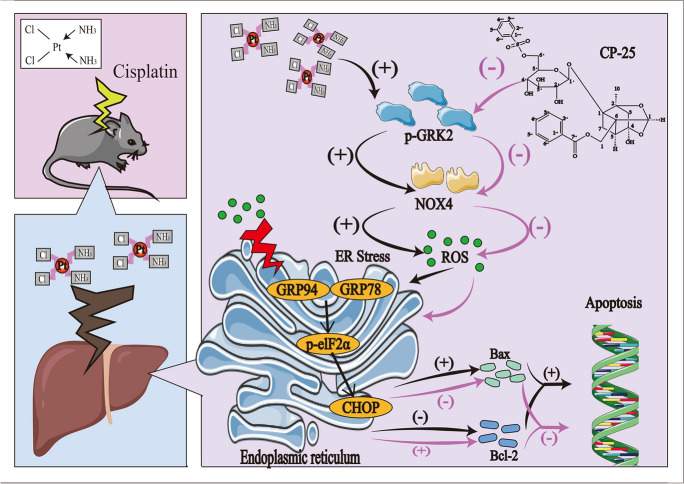
G protein-coupled receptor kinase 2 (GRK2) is the multifunctional hub of non-G protein-coupled receptor (non-GPCR) and GPCR channels and various signaling networks. GRK2 plays an important role in the occurrence and development of multiple organ diseases. Previous studies have shown that GRK2 expression is significantly elevated in heart failure, hypertension, pulmonary fibrosis, nonalcoholic fatty liver disease and other diseases (Cannavo et al. 2013; Piao et al. 2012; Cruces-Sande et al. 2018; Su et al. 2021). Interestingly, recent studies have shown that increased GRK2 expression in cardiomyocytes promotes an increase in NOX4 activity and that GRK2 in cardiomyocytes directly acts on NOX4 (Theccanat et al. 2016; Ammone et al. 2021). All information suggests that GRK2 may be an effective target for preventing ALI. The main objective of this study was to determine the function of GRK2 in cisplatin-induced ALI, and to evaluate the protective effect of pharmacological inhibition of GRK2.
Materials and methods
Drugs
Cisplatin was purchased from MedChemExpress Co., Ltd. (purity > 98%, lot number: #115,583; New Jersey, USA). CP-25 (paeoniflorin-6'-O-benzene sulfonate; purity > 98%) was obtained from the Chemistry Lab of the Institute of Clinical Pharmacology of Anhui Medical University (Hefei, China). Paroxetine was purchased from Aladdin (pharmacological inhibitor of GRK2; purity > 98%, lot number: #F1510065; Shanghai, China). Total glucoside of paeony (TGP) was purchased from Ningbo Li Hua Pharmaceutical Company (Ningbo, China). Both GSK180736A (a pharmacological inhibitor of GRK2; purity > 98%; lot number: GC19177) and GLX351322 (a pharmacological inhibitor of NOX4; purity > 98%; lot number: GC31367) were obtained from GLPBIO Technology, Inc. (Montclair, CA, USA).
Animals and experimental design
C57BL/6 mice (female and male, 7–8 weeks old, weighing 20 ± 2 g) were purchased from SPF (Beijing) Biotechnology Co., Ltd. (license number: SCXK (Beijing) 2019–0010). Hemizygous whole-body Grk2 knockout (Grk2±) in adult male mice with weights of 20 ± 4 g were generated by GemPharmatech Co., Ltd. (Nanjing, China). The genotypes of the genetically engineered mice were determined via standard PCR method (see Additional file 1). All mice were fed in the specific-pathogen-free (SPF) animal room, Institute of Clinical Pharmacology, Anhui Medical University. All of the mice were allowed free access to a standard commercial diet and water and maintained a light and dark cycle for 12 h.
The animal experiment was divided into two parts. In Part I, Grk2± mice (genetic background of C57BL/6) were divided into normal and ALI groups, and wild-type (WT) littermate C57BL/6 mice were used as control groups. On Day 0, cisplatin was injected intraperitoneally (20 mg/kg, i.p.) into the ALI group, and all of the mice were sacrificed 72 h later. In Part II, C57BL/6 mice were randomly divided into 7 groups: Normal group, ALI group, paroxetine (5 mg/kg, i.p.) group, TGP (280 mg/kg, i.g.) group, CP-25 (35 mg/kg, intragastric administration, i.g.) group, CP-25 (70 mg/kg, i.g.) group, and CP-25 (140 mg/kg, i.g.) group. The normal and ALI groups were orally administered 0.5% CMC-Na solution, whereas the other groups were treated with the corresponding drugs for one week. On the 4th day postadministration, the animals in all of the groups (except the normal group) were injected with cisplatin (20 mg/kg, i.p.), and all mice were sacrificed on the 7th day. This study was approved by the Subcommittee of the Institute of Clinical Pharmacology, Animal Research Ethics Committee, Anhui Medical University (No. PZ-2021–015; Date: May 8th, 2021). All animal experiments are carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986, and our agency guidelines for the care and use of animals.
Hepatic biochemical analysis
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were measured by commercial kits (Jiancheng Bioengineering, Nanjing, China) according to the instructions provided with the kits.
Pathological examination
Performed according to existing published histopathological examination methods (Wang et al. 2022; Gong et al. 2021). In short, liver tissue was fixed with 4% paraformaldehyde and then embedded with paraffin wax. Finally, 5-μM-thick slices were prepared and stained with hematoxylin and eosin (H&E). Pathological sections were examined and graded under blinded conditions based on the degree of inflammatory cell infiltration, liver cell spot necrosis and vacuoles, and hepatic cord disorders under blinded conditions. Pathology scores range from 0 to 3 for each index (0 is defined as normal; 3 is defined as severe).
Glutathione (GSH) and malondialdehyde (MDA) assays
The 100 mg liver tissue was mixed with 900μL normal saline, the tissue homogenate was prepared via a tissue homogenizer, and the supernatant was obtained by centrifugation. The contents of MDA and GSH in liver tissue were detected by commercial kits (Jiancheng Bioengineering, Nanjing, China).
TUNEL staining
Fresh liver tissue was immediately fixed with 4% paraformaldehyde, and then paraffin embedded to make 5 μm sections. Liver tissue sections were stained according to the experimental protocol of A terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) one-step staining kit (Elabscience, Wuhan, China). Images of each slide were taken with the Leica TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany) at a magnification of × 400 on the basis of 6 random fields. The percentage of nucleo-staining positive cells was calculated by image software (Image-Pro Plus 6.0, Media Cybernetics, Inc., Rockville, MD, USA), and apoptotic cells were quantified.
Cell culture and treatment
Alpha mouse liver 12 (AML12) cells (Procell Life Science & Technology Co., Ltd., Wu Han, China) were cultured in DMEM/F12 medium containing a mixture of 1% penicillin/streptomycin and 10% fetal bovine serum and maintained at 37 °C in a 5% CO2 incubator. To knock down GRK2, the cells were transfected with GRK2-siRNA or control siRNA (GP-Mate) in accordance with the instructions provided by GenePharma (Shanghai, China). The Additional file 1 provides detailed protocols for siRNA transfection (see Additional file 1). Moreover, to evaluate the effects of pharmacological inhibition of GRK2 and NOX4 on cisplatin-induced injury in cells, GSK180736A (2 µM) and GLX351322 (10 µM) were added to the plates 10 min and 3 h before cisplatin treatment, respectively, whereas CP-25 was added to the medium simultaneously with cisplatin.
CCK-8 assay
Add the pretreated cell suspension (5 × 104 cells/mL) to the 96-well plate (100 µL per well). After cell stabilization, the cells were divided into 5 groups and stimulated with different concentrations of cisplatin (0 µM, 5 µM, 10 µM, 20 µM, or 40 µM) for 24 h. Afterwards, 10 µL of CCK-8 solution was added to each well and left for 1.5 h in the incubator. The absorbance at 450 nm was measured by a microplate reader. Moreover, Viability of the cells recevived CP-25 was measured by CCK8 assay. Briefly, after the cells were divided into 4 groups, and each group of cells was added with different concentrations of CP-25 (0 μM, 0.1 μM, 1 μM, or 10 μM) for 24 h.
Apoptotic cell detection
Cell apoptosis was detected with Annexin V-FITC/PI double staining (BestBio Co., Ltd., Shanghai, China). In brief, the 400µL of binding buffer was added to the pretreated cells, followed by 5µL of Annexin V-FITC and 5µL of PI to further incubate the cells. The CytoFLEX LX flow cytometry (Beckman Coulter, Brea, CA, USA) was used to determine the apoptosis rate of all groups. The number of cells per assay was controlled at 1 × 104. The percentage of apoptotic cells was calculated by FlowJo software.
Detection of ROS fluorescence
Add the pretreated cell suspension (6 × 104 cells/mL) to the 24-well plate (500 µL per well). After the corresponding stimulation and drug intervention, 500 µL of DCFH-DA fluorescent probe (10 μM/L) was mixed into each well, and the culture was continued for 20 min. Finally, the cell slides were removed, and an anti-fluorescence quenching agent was added. A fluorescence microscope was used for observation, and images were obtained at a wavelength of 488 nm.
Western blot analysis
Total protein was prepared by grinding liver tissue with a tissue homogenizer. Briefly, 50 mg of liver tissue was added to the cell lysate, which was fully ground and centrifuged. The protein concentration was determined by a DS-11 Spectrophotometer (DeNovix, Wilmington, USA). Protein samples were separated by SDS‒PAGE electrophoresis and then transferred to nitrocellulose membranes. After blocking with skim milk, the membranes were incubated with primary antibodies against GRK2 (A4443, ABclonal, Wuhan,China), p-GRK2 (PA567507, Invitrogen, NM, USA), NOX4 (ab112414, Abcam, Cambridge, UK), B-cell lymphoma-2-associated X protein (Bax; ab32503, Abcam, Cambridge, UK), B-cell lymphoma-2 (Bcl-2; AF6139, Affinity, OH, USA), glucose-regulated protein 94 (GRP94; AF5287, Affinity, OH, USA), GRP78 (AF5366, Affinity, OH, USA), p-elF2α (AF3087, Affinity, OH, USA), C/EBP-homologous protein (CHOP; AF5280, Affinity, OH, USA), β-actin (AF7018, Affinity, OH, USA), and β-tubulin (Q7M3J9, Abmart, Shanghai, China) overnight at 4 °C and then incubated with a secondary antibody (HRP-conjugated AffiniPure Goat Anti-Rabbit IgG, 15,015, Proteintech, Chicago, USA) at room temperature for 2 h. Finally, Visualization results were obtained by Tanon 5200 automatic chemiluminescence image analysis system (Tanon, Shanghai, China). The gray intensity ratio of the target band to the internal reference band was analyzed by Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All data were statistically analyzed by GraphPad Prism software (8.0.2). One-way analysis of variance (ANOVA) and the least significant difference (LSD) post-hoc tests were used for comparison between groups. All data are expressed as mean ± SD. P values < 0.05 were considered to indicate statistical significance.
Results
Hemizygous Grk2 knockout protected against cisplatin-induced ALI in vivo
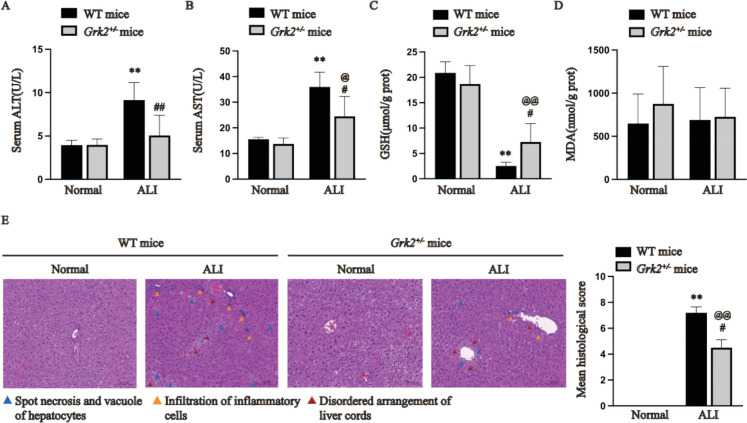
Male Grk2±mice and WT mice received cisplatin, and the differences in liver injury severity were compared. As shown in Fig. 1A-B, compared with normal WT mice, the AST and ALT levels were significantly greater in the ALI-WT mice (P < 0.01). Compared with ALI-WT mice, ALI-Grk2± mice exhibited decreased ALT and AST levels (P < 0.05). Moreover, the GSH level in the normal group of Grk2 ± and WT mice was higher than that in the corresponding cisplatin group (P < 0.01). Compared with ALI-WT mice, ALI-Grk2± mice showed increased GSH levels (Fig. 1C, P < 0.05). But, there was no difference in MDA levels between the two groups (Fig. 1D). H&E staining of liver tissues revealed that ALI-Grk2± mice exhibited fewer better pathological manifestations than did ALI-WT mice (Fig. 1E, P < 0.01).
Fig. 1.
Hemizygous Grk2 knockout ameliorated cisplatin-induced liver injury in mice (A: Serum ALT; B: Serum AST; C: GSH levels in the liver homogenates of the mice; D: MDA levels in the liver homogenates of the mice; E: Liver histopathology. The data are expressed as the average ± SD of 5 mice (n = 5). The pathological images are presented with an indication scale (100 μm) and 400 × magnification. **P < 0.01 vs. the normal group (WT mice); @P < 0.05, @@P < 0.01 vs. the normal group (Grk2± mice); #P < 0.05, ##P < 0.01 vs. the ALI group (WT mice))
Hemizygous Grk2 knockout reduced NOX4 levels and inhibited liver cell apoptosis in vivo
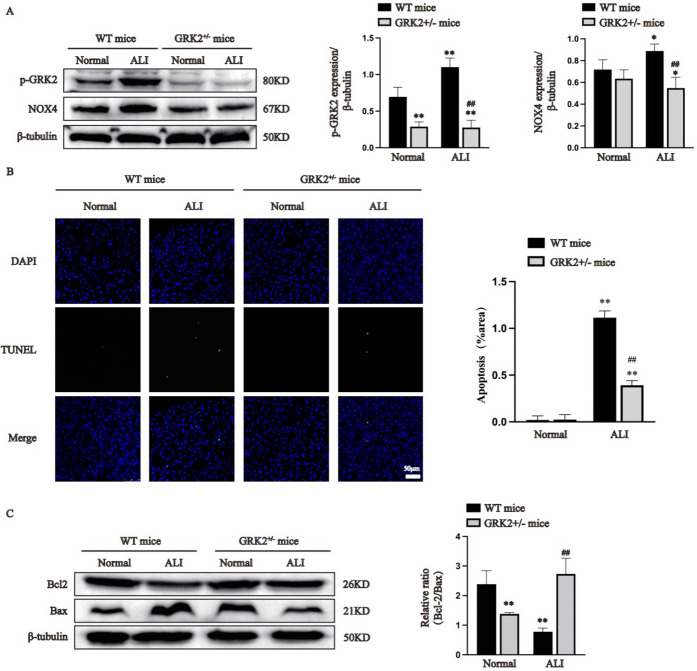
GRK2 and p-GRK2 expression levels in the livers of the Grk2± mice were further verified by Western blot analysis. Compared with WT mice, Grk2± mice exhibited decreased GRK2 and p-GRK2 expression (Supplementary Fig. 1A and Fig. 2A, P < 0.05). There was no significant difference in the expression of NOX4 in WT and Grk2 ± mice. However, cisplatin injection increased NOX4 expression in WT mice but not in Grk2± mice (Fig. 2A, P < 0.05). Our data indicate that GRK2-mediated increased NOX4 expression in the presence of cisplatin. As shown in Fig. 2B, TUNEL staining revealed that the percentage of apoptotic cells in the ALI group (including WT mice and Grk2± mice) was higher than that in the corresponding normal groups (P < 0.01). As expected, the results showed that the rate of apoptotic cells in the ALI group of Grk2± mice was significantly less than that in the ALI group of WT mice (P < 0.01). Moreover, as shown in Fig. 2C, compared with the normal group of Grk2 ± mice, the Bcl-2/Bax ratio was significantly higher in the normal group of WT mice, which suggesting that GRK2 knockdown may promote hepatocyte apoptosis (P < 0.01). However, the Bcl-2/Bax ratio in the ALI group of Grk2± mice was greatly higher than that in the ALI group of WT mice (P < 0.01). Our data suggest that GRK2 knockdown inhibits hepatocyte apoptosis induced by cisplatin.
Fig. 2.
Hemizygous Grk2 knockout reduced NOX4 expression and inhibited liver cell apoptosis in vivo (A: p-GRK2 and NOX4 expression was determined by immunoblotting. B: TUNEL staining; the images are presented with a scale bar (50 μm) and 400 × magnification. C: Bax and Bcl-2 expression in liver tissues was measured by immunoblotting. The data are expressed as the average ± SD of 5 mice (n = 5), *P < 0.05, **P < 0.01 vs. the normal group (WT mice); ##P < 0.01 vs. the ALI group (WT mice); @@P < 0.01 vs. the normal group (Grk2± mice))
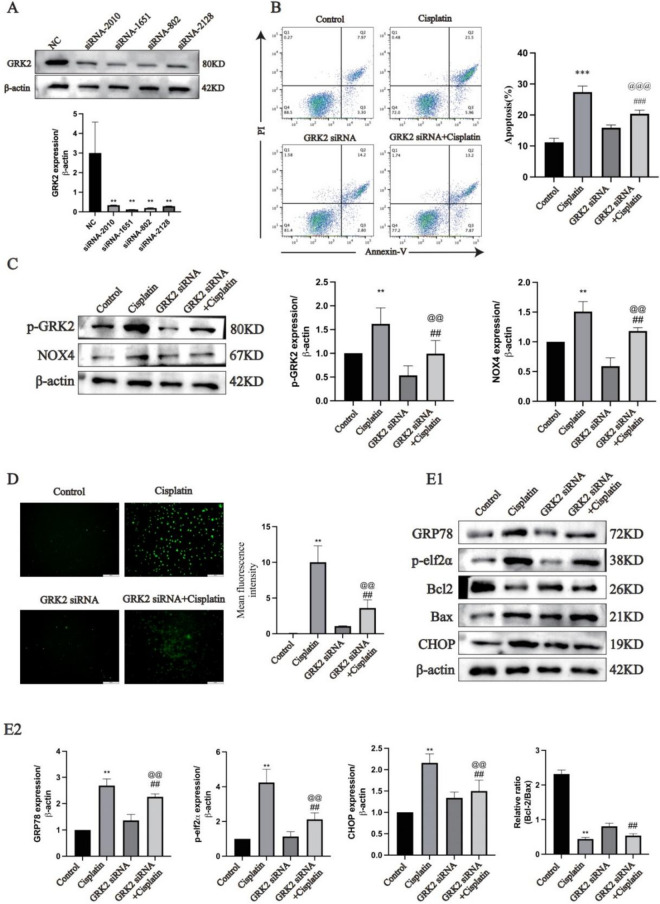
GRK2 knockdown inhibited ER stress and apoptosis of AML12 cells induced by cisplatin stimulation in vitro
Further research was conducted on the mouse liver cell line AML12. GRK2 siRNA-transfected AML12 cells showed decreased GRK2 expression compared with that of control siRNA (NC) (Fig. 3A, P < 0.01). The optimal concentration of cisplatin (20 μM) for stimulating AML12 cells was determined with a CCK-8 assay (Supplementary Fig. 1B) and was used for all subsequent cell tests. Annexin V-FITC/PI double staining revealed that the apoptosis rate in the cisplatin group was greatly higher than that in the control group, and the apoptosis rate in the GRK2 siRNA + cisplatin group was greatly lower than that in the cisplatin group (Fig. 3B, P < 0.001). There was no difference in NOX4 expression between the control group and the GRK2 siRNA group. However, cisplatin stimulation increased NOX4 expression in the cisplatin group but not in the GRK2 siRNA + cisplatin group. These data suggest that silencing GRK2 alters the expression level of NOX4 in response to cisplatin stimulation (Fig. 3C and Supplementary Fig. 1C, P < 0.01). Moreover, ROS release was markedly greater in the cells stimulated by cisplatin than in the normal cells; however, ROS release was greatly lower in the GRK2 siRNA + cisplatin group than the cells treated by cisplatin (Fig. 3D, P < 0.01). Compared with control group, the levels of the ER stress proteins CHOP, p-elF2α and GRP78 in the cisplatin group were markedly higher; however, the levels of these ER stress proteins were greatly lower in the GRK2 siRNA + cisplatin group than in the cisplatin group (Fig. 3E, P < 0.01). The reduced Bcl-2/Bax ratio by cisplatin can be inhibited by GRK2 siRNA (P < 0.01).
Fig. 3.
Knockdown of GRK2 inhibited cisplatin-induced ER stress and apoptosis in AML12 cells (A: GRK2 silencing efficiency in AML12 cells was determined via Western blotting (n = 5). B: AML12 cell apoptosis was measured using flow cytometry (n = 3). C: p-GRK2 and NOX4 expression in AML12 cells was determined using immunoblotting and semiquantitative analysis based of the internal reference protein β-actin (n = 5). D: ROS levels were detected (n = 5). E1-E2: ER stress protein, Bax and Bcl-2 expression in AML12 cells was determined with immunoblotting (n = 5). The data are expressed as the average ± SD. **P < 0.01, ***P < 0.001 vs. the control group;@@P < 0.01, @@@P < 0.001 vs. the GRK2 siRNA group; ##P < 0.01, ###P < 0.001 vs. the cisplatin group.)
GRK2 mediated cisplatin-induced ROS release and apoptosis by modulating NOX4
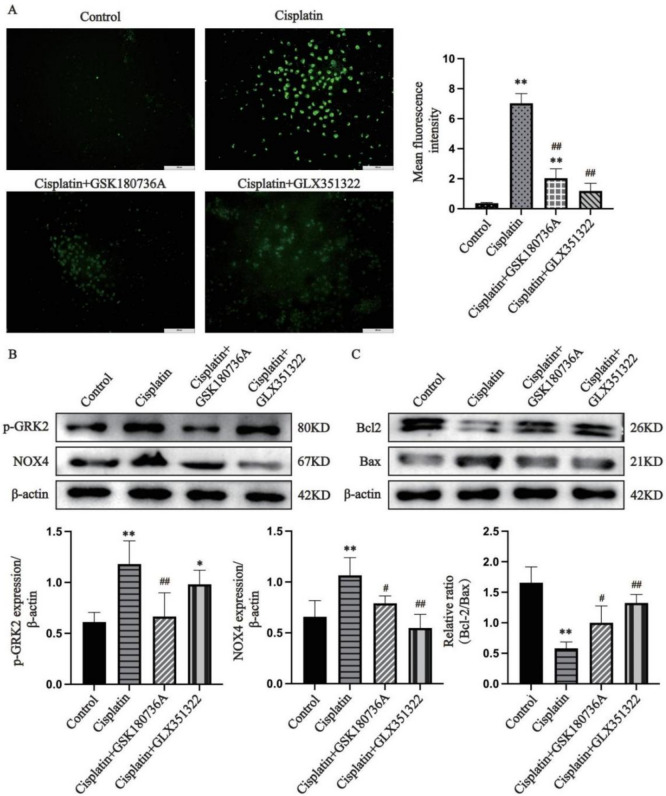
Further studies were performed to determine the role of GRK2 in ALI. As shown in Fig. 4A, cisplatin stimulation increased ROS release in AML12 cells, and this effect was effectively inhibited when the cells received GSK180736A or GLX351322 (P < 0.01). GRK2, p-GRK2, and NOX4 expression in the cisplatin group was markedly greater than that in the control group (Fig. 4B and Supplementary Fig. 1D, P < 0.01). Compared to the cisplatin group, GRK2, p-GRK2 and NOX4 levels were greatly lower in the GSK180736A group, and NOX4 expression was significantly lower in GLX351322-treated cells (Fig. 4B and Supplementary Fig. 1D, P < 0.01). Moreover, the Bcl-2/Bax ratio was greatly elevated and apoptosis was markedly ameliorated in GSK180736A and GLX351322-treated cells compared to the cisplatin group (Fig. 4C, P < 0.05). Our data suggest that GRK2 inhibition effectively attenuated NOX4 expression and ROS release, thereby blocking cisplatin-induced apoptosis in AML12 cells.
Fig. 4.
GRK2 mediates cisplatin-induced ROS release and apoptosis in AML12 cells by modulating NOX4 (A: Detection of ROS; B: Determination of p-GRK2 and NOX4 expression; C: Determination of Bax and Bcl-2 expression. The data are expressed as the average ± SD of 5 tests (n = 5), *P < 0.05, **P < 0.01 vs. the control group; #P < 0.05, ##P < 0.01 vs. the cisplatin group.)
Pharmacological inhibition of GRK2 exerted good protective effects on mice with cisplatin-induced ALI
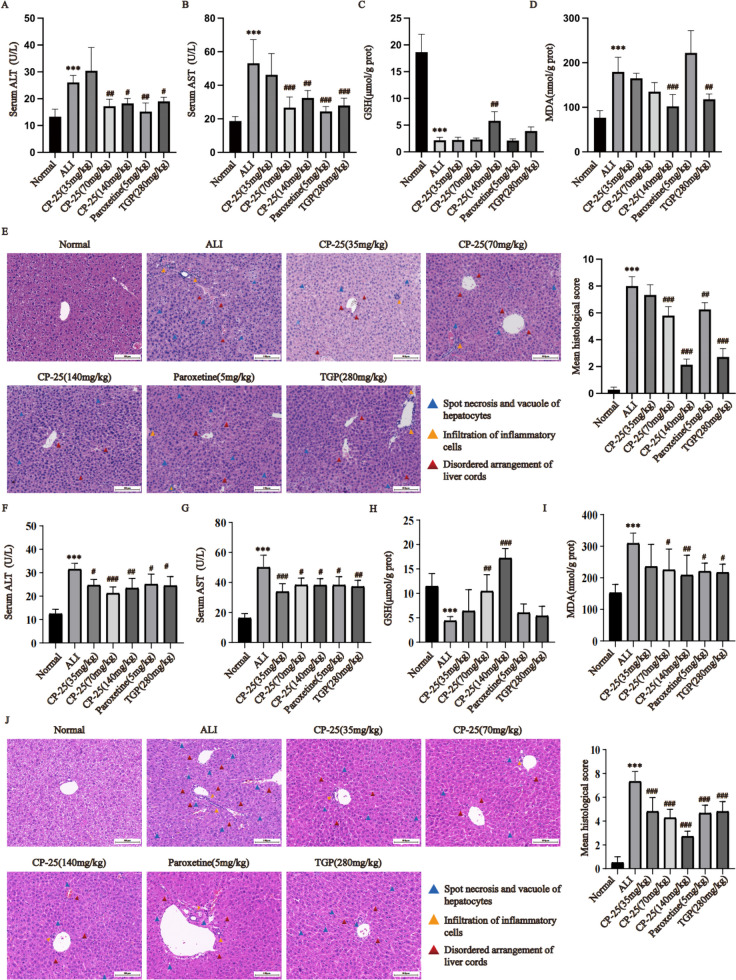
To assess the protective effect of pharmacological inhibition of GRK2, we generated ALI models in both male and female WT mice subjected to a single injection of cisplatin. The results showed that the serum AST and ALT levels of mice in the ALI group were greatly higher than those in the normal group (P < 0.001). Compared to the ALI group, the serum AST and ALT levels of mice were greatly lower in the paroxetine (5 mg/kg) group, TGP (280 mg/kg) group and CP-25 (70 and 140 mg/kg) group, (Fig. 5A-B and Fig. 5F-G, P < 0.05). Moreover, the ALT and AST serum levels in female mice were markedly lower in the CP-25 (35 mg/kg) group than in the ALI group (Fig. 5F-G, P < 0.05). GSH levels were decreased and MDA levels were increased in the ALI group compared to the normal group (Fig. 5C-D and Fig. 5H-I, P < 0.01). Compared to the ALI group, male mice treated by CP-25 (140 mg/kg) exhibited a greatly higher in MDA and a greatly lower in GSH (P < 0.01). In addition, the MDA levels in the liver of male mice treated with TGP were significantly lower than those in the liver of the ALI group (Fig. 5D, P < 0.01). GSH was greatly higher and MDA was greatly lower in CP-25 (70,140 mg/kg) treated female mice compared to the ALI group (Fig. 5H-I, P < 0.05). Moreover, MDA levels in liver tissues of paroxetine or TGP treated female mice were greatly lower than those in the ALI group (Fig. 5I, P < 0.05). Pathological analysis of normal liver tissue revealed that the liver was morphologically intact and that the hepatic cords were arranged radially. In the ALI group, there was significant necrosis and vacuolization of hepatocytes, obvious infiltration of inflammatory cells, and disordered arrangement of liver cords. The pathological manifestations in the ALI group were worse than that of male and female mice that received CP-25 (70 or 140 mg/kg), paroxetine, or TGP, and the pathological scores in the treated mice were significantly decreased (Fig. 5E and 5J, P < 0.01). In addition, the pathological manifestations of female mice treated with CP (35 mg/kg) were significantly decreased (Fig. 5J, P < 0.01). These results revealed that drugs that pharmacologically inhibit GRK2, such as CP-25, exert significant protective effects against cisplatin-induced ALI in mice.
Fig. 5.
Pharmacological inhibition of GRK2 effectively improved liver function and pathological changes in ALI model mice (A : Serum ALT in male mice; B : Serum AST in male mice; C: GSH levels in the liver homogenates of male mice; D: MDA levels in the liver homogenates of male mice; E: Liver histopathology in male mice; F: Serum ALT in female mice; G: Serum AST in female mouse livers; H: GSH levels in the liver homogenates of female mice; I: MDA levels in the liver homogenates of female mice; J: Liver histopathology in female mice. The data are expressed as the average ± SD of 5 mice ( n = 5), *** P < 0.001 vs. the normal group; # P < 0.05; ## P < 0.01; ### P < 0.001 vs. the ALI group. Blue triangle: Spot necrosis and vacuole of hepatocytes; Yellow triangle: Infiltration of inflammatory cells; Red triangle: Disordered arrangement of liver cords.)
Pharmacological inhibition of GRK2 prevents hepatocyte apoptosis in mice with cisplatin-induced ALI
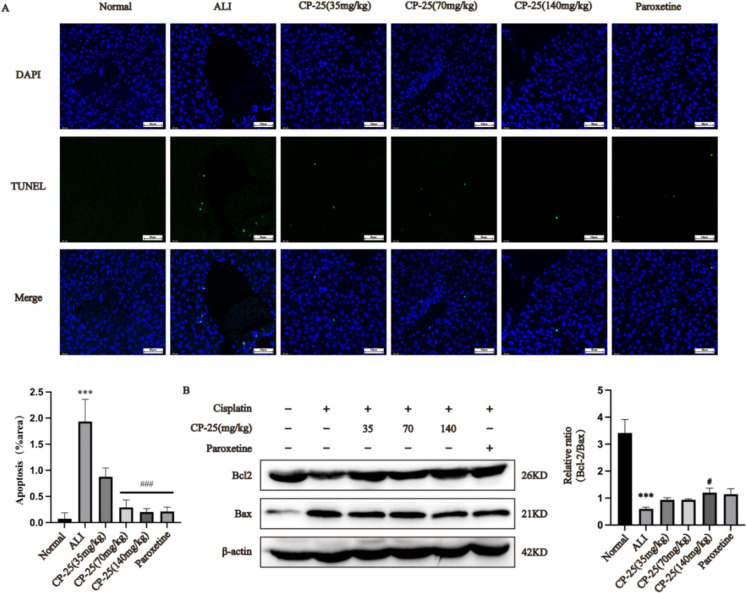
TUNEL staining of liver tissue sections from male mice was conducted. As shown in Fig. 6A, hepatocyte apoptosis was significantly elevated in cisplatin-induced ALI model mice (P < 0.01). Compared to the ALI group, the liver tissue of the mice that received CP-25 (70 or 140 mg/kg) or paroxetine exhibited a decreased rate of apoptosis (P < 0.001). Moreover, in Fig. 6B, compared to the normal group, the Bcl-2/Bax ratio was greatly lower in the ALI group (P < 0.001). However, compared to the ALI group, the Bcl-2/Bax ratio in the group of mice treated with CP-25 (140 mg/kg) was significantly greater (P < 0.05).
Fig. 6.
Pharmacological inhibition of GRK2 reduces hepatocyte apoptosis in ALI mice (A: TUNEL staining; the images are presented with a scale bar (50 μm) and 400 × magnification. (Merge: a merge diagram of TUNEL image and DAPI image); B: The expression of Bax and Bcl-2 in the liver was measured by Western blotting. The TUNEL staining data are expressed as the average ± SD of 5 mice ( n = 5), and the Western blot analysis data are expressed as the average ± SD ( n = 3); *** P < 0.001 vs. the normal group; # P < 0.05, ### P < 0.001 vs. the ALI group.)
Pharmacological inhibition of GRK2 decreases NOX4 expression and the expression of ER stress proteins in mice with cisplatin-induced ALI
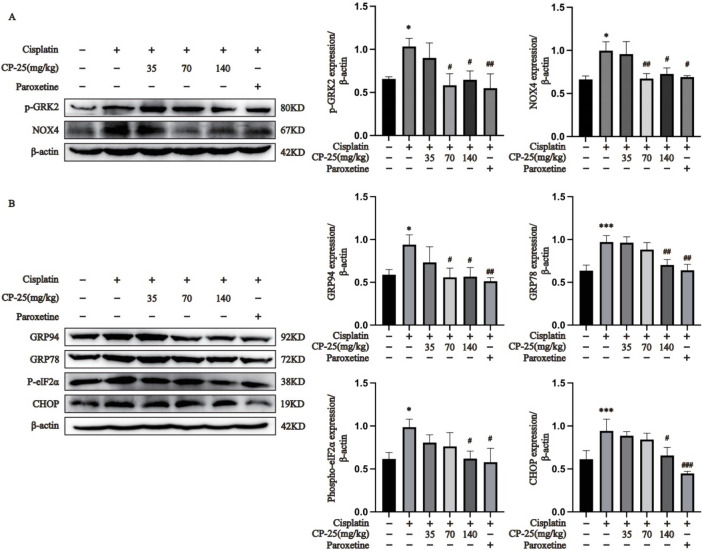
Semiquantitative analysis of liver tissue proteins revealed that the levels of GRK2, p-GRK2 and NOX4 in male and female ALI model mice were significantly greater than in corresponding normal mice (Fig. 7A, Supplementary Fig. 1E and Fig. 1H, P < 0.05). However, the expression levels of GRK2, p-GRK2 and NOX4 in CP-25 (140 mg/kg) or paroxetine-treated mice were greatly lower than those in the ALI group (Fig. 7A, Supplementary Fig. 1E and Fig. 1H; P < 0.05). The levels of ER stress-related proteins in male mice were further investigated through subsequent experiments. As shown in Fig. 7B, compared to the the normal group, GRP94, GRP78, p-elF2α and CHOP levels in the liver tissues of the ALI group were markedly higher (P < 0.05). Additionally, compared to the ALI group, the GRP94, GRP78, p-elF2α and CHOP levels in CP-25 (140 mg/kg) or paroxetine-treated mice groups were significantly lower (P < 0.05). Moreover, CP-25 (35 mg/kg) had no significant effect on GRP94, GRP78, p-elF2α or CHOP expression in ALI mice, and CP-25 (70 mg/kg) effectively downregulated the expression level of GRP94.
Fig. 7.
Pharmacological inhibition of GRK2 inhibited ER stress via the modulation of NOX4 in the ALI mice (A: Determination of hepatic protein p-GRK2 and NOX4 expression via Western blotting; B: Determination of the expression of ER stress proteins in liver tissue. The data are expressed as the average ± SD (n = 3), *P < 0.05, ***P < 0.001 vs. the normal group; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. the ALI group.)
CP-25 inhibited cisplatin-mediated ER stress and apoptosis in vitro
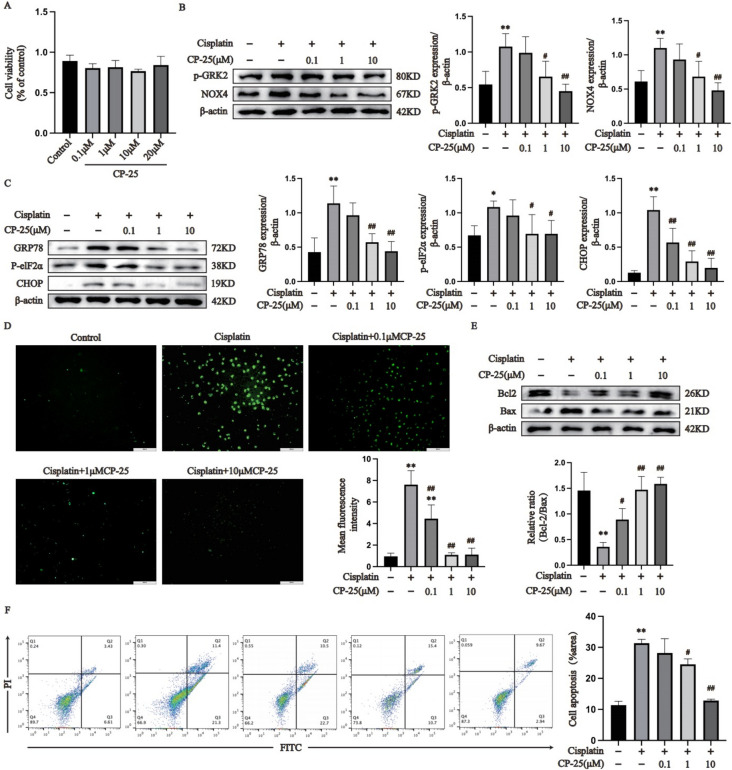
The protective function of CP-25 on AML12 cells stimulated with cisplatin was examined. CCK-8 assay to detect the effect of different concentrations of CP-25 on AML12 cell viability showed that different concentrations of CP-25 had no effect on AML12 cell viability (Fig. 8A). We subsequently stimulated AML12 cells with 20 μM cisplatin and administered three concentrations of CP-25 (0.1 μM, 1 μM and 10 μM), the effects of which were subsequently detected via a CCK-8 assay. The results suggested that all three concentrations greatly improved cell viability under cisplatin stimulation (Supplementary Fig. 1F). Therefore, CP-25 (0.1 μM, 1 μM and 10 μM) was selected for subsequent tests. As shown in Fig. 8B and Supplementary Fig. 1G, the expression of GRK2, p-GRK2 and NOX4 were markedly upregulated in the cisplatin group compared with the control group (P < 0.01), whereas, the expression of GRK2, p-GRK2 and NOX4 were greatly downregulated in the CP-25 group (1 μM and 10 μM) compared with the cisplatin group (P < 0.05). In addition, the expression levels of the ER stress proteins GRP78, p-elF2α and CHOP in the cisplatin group were markedly greater than those in the normal group (P < 0.05). However, compared to the cisplatin group, the expression levels of GRP78, p-elF2α, CHOP, and CP-25 (0.1 μM) in the CP-25 group were lower (Fig. 8C, P < 0.05). In addition, compared to normal group, ROS release was markedly more in the cisplatin group, whereas ROS release was markedly lower in the cells treated with CP-25 (0.1 μM, 1 μM, or 10 μM) than in the cisplatin group (Fig. 8D, P < 0.01). The Bcl-2/Bax ratio in the cisplatin group was greatly lower than that in the normal group, whereas the Bcl-2/Bax ratio in the CP-25 (0.1 μM, 1 μM and 10 μM)-treated groups was markedly greater than that in the cisplatin group (Fig. 8E, P < 0.05). Annexin V-FITC/PI double staining showed that the apoptosis level of cisplatin-treated cells was markedly higher than that of normal cells, whereas CP-25 (1 μM and 10 μM) greatly reduced cisplatin-induced apoptosis (Fig. 8F, P < 0.05).
Fig. 8.
CP-25 effectively ameliorated cisplatin-induced apoptosis in AML12 cells by regulating ER stress (A: Selection of the CP-25 concentration; B: Determination of p-GRK2 and NOX4 expression; C: Determination of ER stress protein expression in AML12 cells by immunoblotting; D: Detection of ROS; E: The expression of Bax and Bcl-2 in AML12 cells was measured by immunoblotting; F: AML12 cell apoptosis was measured via flow cytometry. The data are expressed as the average ± SD of 5 tests (n = 5), *P < 0.05, **P < 0.01 vs. the control group; #P < 0.05, ##P < 0.01 vs. the cisplatin group.)
Discussion
Cisplatin induces multiorgan impairment by inducing apoptotic necrosis. GRK2 reportedly mediates several types of tissue injury. For example, inhibiting the upregulation of GRK2 induced by ischemia‒reperfusion injury can antagonize cardiomyocyte apoptosis (Li et al. 2022). Moreover, it has been shown that GRK2 expression levels were significantly elevated in response to cisplatin treatment (Ammone et al. 2021). Therefore, GRK2 may play an key role in cisplatin-induced ALI. To demonstrate the role of GRK2 in cisplatin-induced ALI, Grk2± and WT mice were generated and injected with cisplatin. Jaber et al. (1996) demonstrated that homozygosity for Grk2 results in embryonic death, as Grk2± embryonic mice exhibit pronounced hypoplasia of the ventricular myocardium. This study elucidated a significant reduction in the cardiac ejection fraction and severe heart failure in Grk2± embryonic mice. However, the use of global adult hemizygous (Grk2±) mice can circumvent the embryonic lethality associated with homozygosity. According to the Mouse Genome Informatics database, mice with a genetic modification of Grk2 are also designated Adrbk1 mice. Furthermore, Grk2± are denoted as Adrbkl±. p-GRK2 expression was greatly elevated in the hepatic tissue of ALI mice, and GRK2 knockdown significantly reduced the levels of serum ALT and AST and improved liver pathology by decreasing liver cell apoptosis. In vitro, we confirmed that siRNA-mediated GRK2 knockdown effectively reduced AML12 cell apoptosis.
Oxidative stress constitutes the pathological basis of ALI. The overproduction of ROS is accompanied by the occurrence and development of ALI. Cellular ROS are mainly produced and regulated by the NADPH oxidase family, which comprises seven members. In contrast to other NADPH oxidase family members, NOX4 is constitutively active and produces H2O2. Overactivation of NOX4 has been implicated in ALI(Matuz-Mares et al. 2022). GRK2 has been shown to bind with NOX4 in cardiomyocytes and HeLa cervical cancer cells (Theccanat et al. 2016; Ammone et al. 2021). We previously confirmed that GRK2 and NOX4 interact directly with each other by via immunoprecipitation in NRK-52E cells (not yet published). In the current study, it was demonstrated that downregulation of GRK2 significantly reduced NOX4 expression and ROS production both in vivo and in vitro, suggesting that GRK2 plays an important role in oxidative stress by regulating NOX4 expression.
The massive release of ROS promotes ER stress, which facilitates cell apoptosis and tissue damage (Sun et al. 2021). Cisplatin has been reported to induce apoptosis by promoting ER stress (Huang et al. 2020). ER stress is a cellular process characterized by the accumulation of unfolded and misfolded proteins in the lumen of the ER. ER stress results from disruption of ER homeostasis, and ER stress activates the unfolded protein response (UPR). During this process, several key molecules involved in the canonical UPR signaling pathways are activated and participate in ER stress (Oakes and Papa 2015). Briefly, CHOP inhibits the expression of the Bcl-2 family, promotes the expression of proapoptotic genes, and transfers calcium directly from the ER to the mitochondria, thereby triggering mitochondrial apoptosis during ER stress. GRP78, an ER calcium-binding protein, is the gatekeeper of the mammalian UPR. Under nonstress conditions, GRP78 is associated with transmembrane ER stress sensors. However, under stress conditions, misfolded proteins disengage GRP78 from binding to these proteins, allowing them to activate the UPR. GRP94 is another major ER chaperone. Like GPR78, GRP94 is involved in protein folding, interacts with other components of the ER protein folding machinery, helps target misfolded proteins for ER-associated degradation, and stores ER calcium (Zhu ang Lee 2015; Yuan et al. 2022). In the current study, GRK2 knockdown in vivo and in vitro significantly reduced the expression of NOX4 and ER stress proteins (GRP78, GRP94, p-elF2α, and CHOP), thereby decreasing the expression levels of apoptosis-related proteins (Bax and Bcl-2). We found that the Bcl-2/Bax ratio was greater in the ALI group than in the normal group in the Grk2 ± mouse experiments (Fig. 2C). Whereas in AML12 cells, the Bcl-2/Bax ratio was lower in the GRK2 siRNA + cisplatin group than in the GRK2 siRNA group (Fig. 3E2). This difference may be due to the difference between transgenic live animals and simple cell lines. Additionally, GRK2 knockdown in vitro was confirmed to significantly reduce the release of ROS. Therefore, the knockdown of GRK2 reduced the percentage of apoptotic cells. Our current results are similar to those previously reported. Silencing GRK2 in mice has been reported to protect against nonalcoholic fatty liver disease through increased autophagy and mitochondrial fusion and biogenesis, together with reduced ER stress (Cruces-Sande et al. 2018). Therefore, our data suggest that GRK2 participates in the progression of ALI by modulating ER stress.
Natural plant-derived drugs and their active extracts are potential options for developing treatments for ALI (Sun et al. 2022). CP-25, an esterified derivative of paeoniflorin, has good anti-inflammatory and immunomodulatory activity, and has regulatory effects on a variety of autoimmune diseases (Yang and Wei 2020). In a previous animal model, we demonstrated that CP-25 ameliorated methotrexate-induced renal and hepatic injury. (Wei et al. 2021; Yang et al. 2019). Like CP-25, paroxetine has significant anti-inflammatory effects. Briefly, T-lymphocyte activation and joint infiltration in collagenous arthritis rats are ameliorated by paroxetine (Wang et al. 2017). Moreover, paroxetine has been confirmed to ameliorate tissue injury, such as cerebral ischemia (Naderi et al. 2018). The commonality of their tissue-protective effects may be associated with the inhibition of GRK2 activity. We previously showed that CP-25 directly binds to Ala321 of GRK2, inhibiting GRK2 translocation from the cytoplasm to the cell membrane and GRK2 kinase activity (Han et al. 2021). Moreover, paroxetine and its derivative GSK180736A were able to inhibit GRK2 kinase activity due to their ability to specifically interact with the kinase domain of GRK2 by forming hydrogen bonds at D272 and M274. (Waldschmidt et al. 2016). Therefore, we hypothesize that drugs that pharmacologically inhibit GRK2, such as CP-25, paroxetine and GSK180736A, may improve cisplatin-induced ALI by regulating GRK2. In the current study, we explored and evaluated the effect of GRK2 on a cisplatin-induced ALI model. The CP-25 doses used were determined based on previous reports (Yang et al. 2019; Wei et al. 2021). Our data revealed that paroxetine (5 mg/kg) and CP-25 (70 and 140 mg/kg) effectively downregulated AST and ALT and significantly improved liver pathological manifestations in ALI model mice, suggesting good protection against ALI. Further study revealed that CP-25 effectively downregulated GRK2 and NOX4, resulting in reduced hepatic oxidative stress, decreased ROS production and cellular ER stress and increased apoptosis. In the in vitro study, we observed similar results, which were consistent with the in vivo results. To our knowledge, GRK2 as a potential new therapeutic target for cisplatin-induced ALI was identified for the first time. The present study may provide a new horizon for the future clinical application of pharmacological GRK2 inhibition.
Previous studies have reported that reducing GRK2 levels in myeloid cells can prevent high-fat diet-induced changes in liver metabolism, thereby protecting the liver (Vila-Bedmar et al. 2020). However, that research was limited to myeloid cells rather than focusing on the liver itself, and the mechanism of the downstream effects on the liver has not been fully explained. For the first time, we showed that GRK2 participates in the process of ALI and further revealed that GRK2 regulates NOX4-mediated oxidative stress. On this basis, the small molecule drug CP-25 was used to intervene in ALI model mice, providing a new therapeutic idea for the clinical prevention of ALI.
This study has certain limitations. Our study utilized hemizygous whole-body Grk2 knockout mice rather than a liver-specific gene knockout model and used only siRNA in vitro and not gene knockout. Compared with conventional global knockout animals, conditional knockout (CKO) animals provide a more precise, accurate, and reliable method for targeting gene modifications in both temporal and spatial dimensions. Therefore, the CKO model effectively addresses some of the inherent limitations of traditional knockout methods methods and is indispensable for investigating target genes involved in embryonic mortality as well as elucidating the physiological and pathological roles of genes in specific tissues or cells (Wang and Abate-Shen 2014; Hu et al. 2012). To avoid the embryonic lethality associated with complete Grk2 knockout and facilitate investigations into the role and mechanism of Grk2 in liver development, growth, disease occurrence, and therapy, it is imperative to employ liver-specific conditional knockout mice as an alternative approach. Future research will systematically conduct relevant work. Although siRNA-mediated cell transfection has been used for cell function inhibition in vitro, several limitations remain. Our siRNA-transfected cells did not completely silence Grk2 expression, so we used Grk2-knockout cells for further verification in subsequent experiments.
Conclusions
In conclusion, GRK2 is abnormally upregulated and plays a crucial role in cisplatin-induced ALI via the modulation of NOX4 expression, ROS release, and ER stress. Pharmacological inhibition of GRK2 with CP-25 or paroxetine effectively alleviated ALI. GRK2 is a novel therapeutic sit for the prevention and treatment of liver injury.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ALI
Acute liver injury
- ER
Endoplasmic reticulum
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- GSH
L-glutathione
- MDA
Malonaldehyde
- GRK2
G protein-coupled receptor kinase 2
- NOX4
NADPH oxidase 4
- ROS
Reactive oxygen species
- CP-25
Paeoniflorin-6'-O-benzene sulfonate
- CKO
Conditional knockout
- Grk2± mice
Hemizygous whole-body Grk2 knockout mice
- WT
Wild type
- SPF
Specific pathogen free
Authors’ contributions
All authors contributed to the study conception and design. QLW, MYL & FD: Formal analysis, Validation, Curation of animal experimental data, Writing-original draft, Writing-review & editing. They have contributed equally to this work and share first authorship. KJX, LN, QQS, JRC & ZKX: Software, Writing—review & editing. JJK, BFX, FX: Project administration. WW: Validation, Supervision, Writing-review & editing. CW: Funding acquisition, Project administration.
Funding
This work was financially supported by the University Synergy Innovation Program of Anhui Province (grant number GXXT-2021–064), Key Research and Development Plan of Anhui Province (grant number 2023s07020003), Key Project of Provincial Natural Science Foundation of universities in Anhui (grant number 2023AH040075), Anhui Province National Science Foundation in University (grant number no. KJ2021A0241), National Natural Science Foundation of China (grant number 82204402) and the Basic and Clinical Cooperation Research Promotion Plan of the Third Affiliated Hospital of Anhui Medical University (grant number 2022sfy016).
Data availability
Data and materials used and/or analyzed during the current study are available from the corresponding authors on reasonable request. All data and legends are original.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
The present study was approved by the Subcommittee of the Institute of Clinical Pharmacology, Ethical Committee on Animal Research of Anhui Medical University (No. PZ-2021–015; Date: May 8th, 2021) and conducted according to the U.K. Animals (Scientific Procedures) Act, 1986 and our institutional guidelines for the care and use of animals.
Consent for publication
No applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Qianlei Wang, Mengyang Li and Fei Duan have contributed equally to this work and share first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wei Wei, Email: wwei@ahmu.edu.cn.
Chun Wang, Email: wangchun@ahmu.edu.cn.
References
- Alrashed AA, El-Kordy EA. Possible Protective Role of Panax Ginseng on Cisplatin-Induced Hepatotoxicity in Adult Male Albino Rats (Biochemical and Histological Study). Journal of Microscopy and Ultrastructure. 2019;7(2):84–90. 10.4103/JMAU.JMAU419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammon JC, Valls D, Eldemerdash M, Patel JR, Tran PD, Feng L, Gi M, Gonzalez TT, Phan C, Zendejas AE, So CH. G protein-coupled receptor kinase 2 modifies the cellular reaction to cisplatin through interactions with NADPH oxidase 4. Mol Cell Biochem. 2021;476(3):1505–16. 10.1007/s11010-020-03969-3. [DOI] [PubMed] [Google Scholar]
- Cannavo A, Liccardo D, Koch WJ. Targeting cardiac β-adrenergic signaling via GRK2 inhibition for heart failure therapy. Front Physiol. 2013;4:264. 10.3389/fphys.2013.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Zhu J, Wang YH, Hang CH. ROS-Mediated Mitochondrial Dysfunction and ER Stress Contribute to Compression-Induced Neuronal Injury. Neuroscience. 2019;416:268–80. 10.1016/j.neuroscience.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Cruces-Sande M, Vila-Bedmar R, Arcones AC, González-Rodríguez Á, Rada P, Gutiérrez-de-Juan V, Vargas-Castrillón J, Iruzubieta P, Sánchez-González C, Formentini L, Crespo J, García-Monzón C, Martínez-Chantar ML, Valverde ÁM, Mayor F Jr, Murga C. Involvement of G protein-coupled receptor kinase 2 (GRK2) in the development of non-alcoholic steatosis and steatohepatitis in mice and humans. Biochim Biophys Acta, Mol Basis Dis. 2018;1864(12):3655–67. 10.1016/j.bbadis.2018.09.027. [DOI] [PubMed] [Google Scholar]
- Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. 2019;88:102925. 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- Gong S, Feng Y, Zeng Y, Zhang H, Pan M, He F, Wu R, Chen J, Lu J, Zhang S, Yuan S, Chen X. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J Transl Med. 2021;19(1):147. 10.1186/s12967-021-02814-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Li Y, Zhang Y, Wang Y, Cui D, Luo T, Zhang Y, Liu Q, Li H, Wang C, Xu D, Ma Y, Wei W. Targeted inhibition of GRK2 kinase domain by CP-25 to reverse fibroblast-like synoviocytes dysfunction and improve collagen-induced arthritis in rats. Acta Pharmaceutica Sinica B. 2021;11(7):1835–52. 10.1016/j.apsb.2021.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MW, Wang ZB, Schatten H, Sun QY. New understandings on folliculogenesis/oogenesis regulation in mouse as revealed by conditional knockout. J Genet Genomics. 2012;39(2):61–8. 10.1016/j.jgg.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Huang Z, Guo F, Xia Z, Liang Y, Lei S, Tan Z, Ma L, Fu P. Activation of GPR120 by TUG891 ameliorated cisplatin-induced acute kidney injury via repressing ER stress and apoptosis. Biomedicine & pharmacotherapy Biomedecine & pharmacotherapie. 2020;126:110056. 10.1016/j.biopha.2020.110056. [DOI] [PubMed] [Google Scholar]
- Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93(23):12974–9. 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yang B, Zhang X, Shen X, Ma Y, Jing L. Lycium barbarum polysaccharide antagonizes cardiomyocyte apoptosis by inhibiting the upregulation of GRK2 induced by I/R injury, and salvage mitochondrial fission/fusion imbalance and AKT/eNOS signaling. Cell Signal. 2022;92:110252. 10.1016/j.cellsig.2022.110252. [DOI] [PubMed] [Google Scholar]
- Matuz-Mares D, Vázquez-Meza H, Vilchis-Landeros MM. NOX as a Therapeutic Target in Liver Disease. Antioxidants (Basel, Switzerland). 2022;11(10):2038. 10.3390/antiox11102038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderi Y, Parvardeh S, Moini Zanjani T, Sabetkasaei M. Neuroprotective Effect of Paroxetine on Memory Deficit Induced by Cerebral Ischemia after Transient Bilateral Occlusion of Common Carotid Arteries in Rat. Iranian J Pharm Res: IJPR. 2018;17(1):215–24. [PMC free article] [PubMed] [Google Scholar]
- Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–94. 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oun, R., , Moussa, Y. E. & Wheate, N. J., The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton transactions (Cambridge, England: 2003),(2018); 47(19), 6645–6653. 10.1039/c8dt00838h [DOI] [PubMed]
- Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, Akhter SA, Archer SL. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation. 2012;126(24):2859–69. 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piera-Velazquez S, Jimenez SA. Oxidative Stress Induced by Reactive Oxygen Species (ROS) and NADPH Oxidase 4 (NOX4) in the Pathogenesis of the Fibrotic Process in Systemic Sclerosis: A Promising Therapeutic Target. J Clin Med. 2021;10(20):4791. 10.3390/jcm10204791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem Res Toxicol. 2019;32(8):1469–86. 10.1021/acs.chemrestox.9b00204. [DOI] [PubMed] [Google Scholar]
- Su VY, Chen WC, Yu WK, Wu HH, Chen H, Yang KY. Nintedanib Regulates GRK2 and CXCR2 to Reduce Neutrophil Recruitment in Endotoxin-Induced Lung Injury. Int J Mol Sci. 2021;22(18):9898. 10.3390/ijms22189898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. 2012;18(3):249–57. 10.3350/cmh.2012.18.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Jin J, Wang L, Wang J, Zhou H, Zhang Q, Xu X. Porcine epidemic diarrhea virus infections induce autophagy in Vero cells via ROS-dependent endoplasmic reticulum stress through PERK and IRE1 pathways. Vet Microbiol. 2021;253:108959. 10.1016/j.vetmic.2020.108959. [DOI] [PubMed] [Google Scholar]
- Sun YK, Zhang YF, Xie L, Rong F, Zhu XY, Xie J, Zhou H, Xu T. Progress in the treatment of drug-induced liver injury with natural products. Pharmacol Res. 2022;183:106361. 10.1016/j.phrs.2022.106361. [DOI] [PubMed] [Google Scholar]
- Theccanat T, Philip JL, Razzaque AM, Ludmer N, Li J, Xu X, Akhter SA. Regulation of cellular oxidative stress and apoptosis by G protein-coupled receptor kinase-2; The role of NADPH oxidase 4. Cell Signal. 2016;28(3):190–203. 10.1016/j.cellsig.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19(11):1880–91. 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Bedmar R, Cruces-Sande M, Arcones AC, Willemen HLDM, Prieto P, Moreno-Indias I, Díaz-Rodríguez D, Francisco S, Jaén RI, Gutiérrez-Repiso C, Heijnen CJ, Boscá L, Fresno M, Kavelaars A, Mayor F Jr, Murga C. GRK2 levels in myeloid cells modulate adipose-liver crosstalk in high fat diet-induced obesity. Cellular and Molecular Life Sciences : CMLS. 2020;77(23):4957–76. 10.1007/s00018-019-03442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldschmidt HV, Homan KT, Cruz-Rodríguez O, Cato MC, Waninger-Saroni J, Larimore KM, Cannavo A, Song J, Cheung JY, Kirchhoff PD, Koch WJ, Tesmer JJ, Larsen SD. Structure-Based Design, Synthesis, and Biological Evaluation of Highly Selective and Potent G Protein-Coupled Receptor Kinase 2 Inhibitors. J Med Chem. 2016;59(8):3793–807. 10.1021/acs.jmedchem.5b02000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Abate-Shen C. Analyses of tumor-suppressor genes in germline mouse models of cancer. Cold Spring Harb Protoc. 2014;2014(8):807–12. 10.1101/pdb.top069773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang L, Wu L, Zhang M, Hu S, Wang R, Han Y, Wu Y, Zhang L, Wang X, Sun W, Wei W. Paroxetine alleviates T lymphocyte activation and infiltration to joints of collagen-induced arthritis. Sci Rep. 2017;7:45364. 10.1038/srep45364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dong XL, Qin XM, Li ZY. Investigating the inter-individual variability of Astragali Radix against cisplatin-induced liver injury via 16S rRNA gene sequencing and LC/MS-based metabolomics. Phytomedicine : International Journal of Phytotherapy and Phytopharmacology. 2022;101:154107. 10.1016/j.phymed.2022.154107. [DOI] [PubMed] [Google Scholar]
- Wei X, Wu Y, Tang H, Wang B, Wang Y, Sun W, Asenso J, Xiao F, Wang C. CP-25 ameliorates methotrexate induced nephrotoxicity via improving renal apoptosis and methotrexate excretion. J Pharmacol Sci. 2021;146(1):21–8. 10.1016/j.jphs.2021.02.007. [DOI] [PubMed] [Google Scholar]
- Yang XZ, Wei W. CP-25, a compound derived from paeoniflorin: research advance on its pharmacological actions and mechanisms in the treatment of inflammation and immune diseases. Acta Pharmacol Sin. 2020;41(11):1387–94. 10.1038/s41401-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zhao Y, Jia X, Wang C, Wu Y, Zhang L, Chang Y, Wei W. CP-25 combined with MTX/ LEF ameliorates the progression of adjuvant-induced arthritis by the inhibition on GRK2 translocation. Biomed Pharmacother. 2019;110:834–43. 10.1016/j.biopha.2018.12.040. [DOI] [PubMed] [Google Scholar]
- Yuan M, Gong M, He J, Xie B, Zhang Z, Meng L, Tse G, Zhao Y, Bao Q, Zhang Y, Yuan M, Liu X, Luo C, Wang F, Li G, Liu T. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 2022;52:102289. 10.1016/j.redox.2022.102289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230(7):1413–20. 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials used and/or analyzed during the current study are available from the corresponding authors on reasonable request. All data and legends are original.
Not applicable.