Abstract
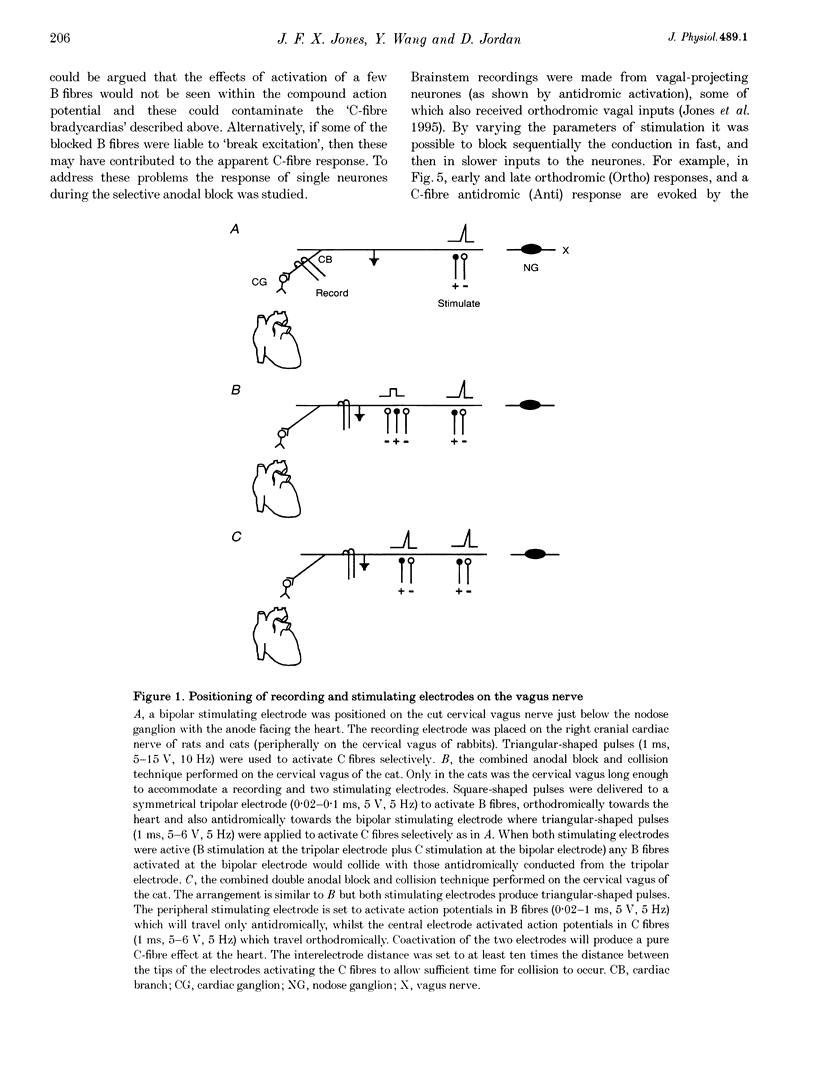
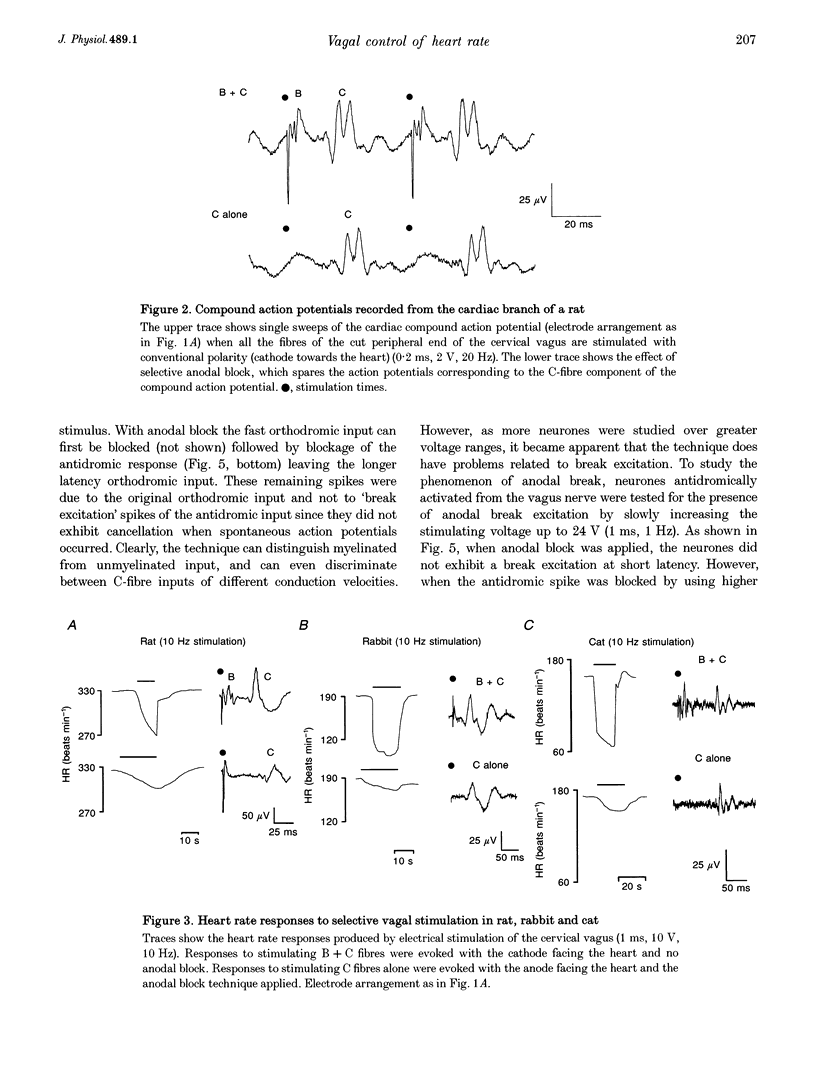
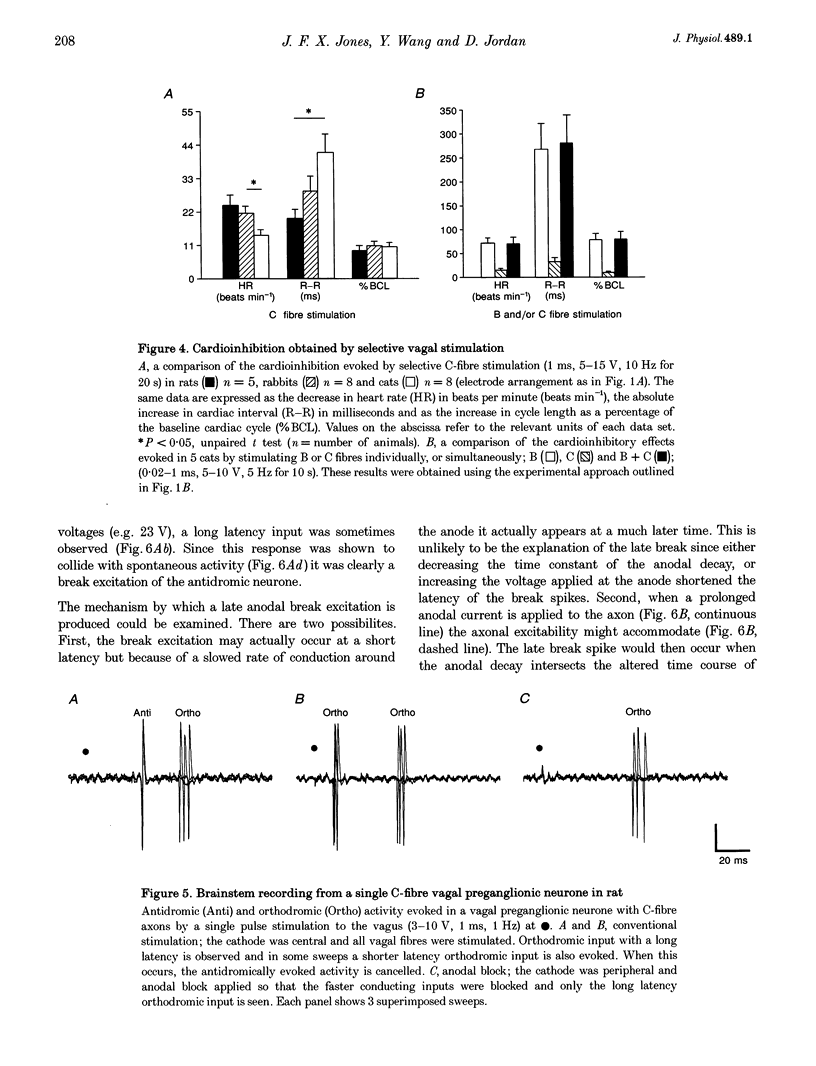
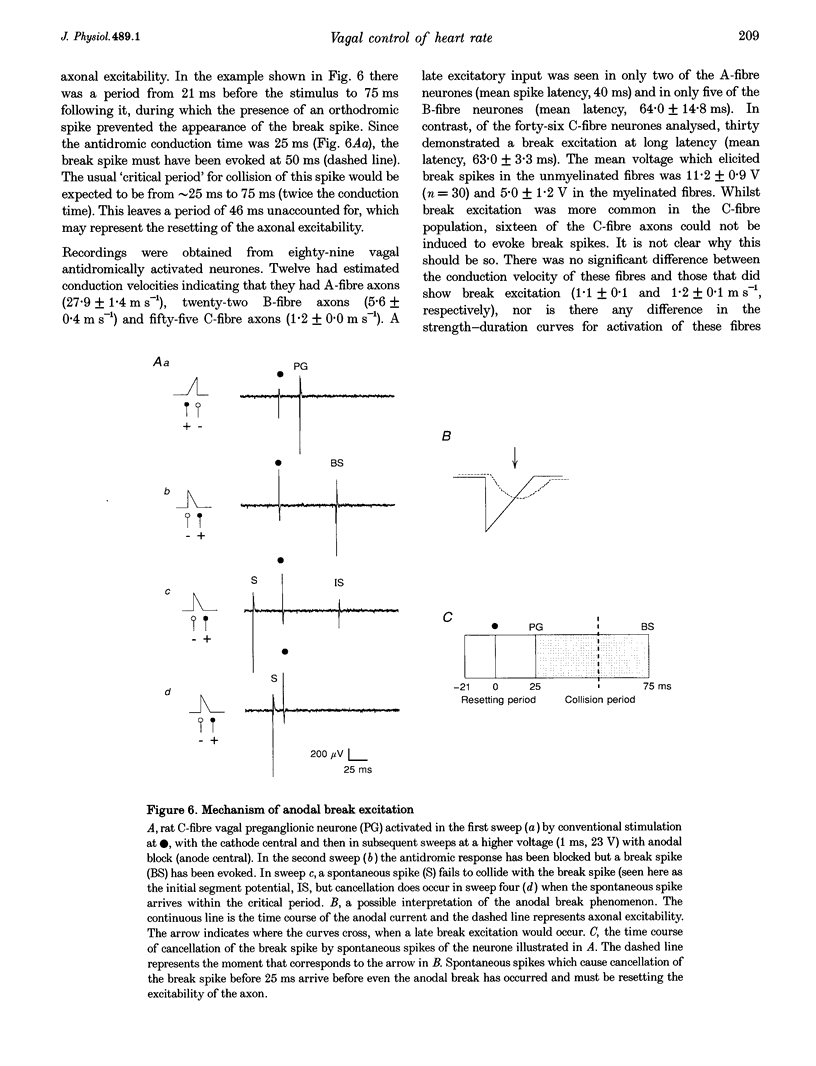
1. The contribution of cardiac vagal C fibres to vagal chronotropic control in anaesthetized cats, rats and rabbits was analysed using electrical stimulation of the vagus nerve with a selective anodal block technique. 2. After bilateral vagotomy and pretreatment with atenolol, 10 Hz continuous selective stimulation of unmyelinated fibres in the cut peripheral end of the cervical vagus evoked a bradycardia in anaesthetized rats, cats and rabbits. With this stimulation protocol the three species exhibited a similar lengthening of the heart period (R-R interval) when expressed as a percentage of their basal cardiac interval. 3. The mechanism of action of the selective blocking technique was analysed by recording eighty-nine single A- (n = 12), B- (n = 22) and C-fibre (n = 55) vagal-projecting neurones in the medulla of the rat. This demonstrated that the technique can selectively block conduction in myelinated fibres and that 'break excitation' is seen mainly in unmyelinated fibres. Although thirty C fibres showed break excitation sixteen did not and this difference could not be correlated with their axonal conduction velocity, chronaxie or initial segment frequency following. 4. Using the anodal block technique the vagal effects on heart rate were reanalysed in the cat by incorporating a collision technique. B fibres were activated orthodromically to evoke cardioinhibition and simultaneously antidromically to collide with errant B-fibre spikes activated at the electrode producing anodal block. With this protocol it was noted that the B- and C-fibre bradycardias were not additive. Using a double anodal block and collision technique, it was demonstrated that this phenomenon was likely to be due to occlusion of the effects of B and C fibres. 5. In conclusion, in addition to the well-defined effects of vagal B fibres on heart rate, selective stimulation of vagal C fibres also had a cardioinhibitory effect in all three species studied. However, since the effects of cardiac C fibres on heart rate was small, these neurones alone cannot account for the cardioinhibition of the pulmonary chemoreflex. It is likely that activation of both B- and C-fibre cardiac vagal preganglionic neurones accounts for this reflex cardioinhibition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accornero N., Bini G., Lenzi G. L., Manfredi M. Selective Activation of peripheral nerve fibre groups of different diameter by triangular shaped stimulus pulses. J Physiol. 1977 Dec;273(3):539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T. G., Burnstock G. M1 and M2 muscarinic receptors mediate excitation and inhibition of guinea-pig intracardiac neurones in culture. J Physiol. 1990 Mar;422:463–480. doi: 10.1113/jphysiol.1990.sp017995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES G. S., MOTT J. C., WIDDICOMBE J. G. Respiratory and cardiovascular reflexes from the heart and lungs. J Physiol. 1951 Nov 28;115(3):258–291. doi: 10.1113/jphysiol.1951.sp004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M. D. Some reflex cardioinhibitory responses in the cat and their modulation by central inspiratory neuronal activity. J Physiol. 1991 Aug;439:559–577. doi: 10.1113/jphysiol.1991.sp018682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford T. W., Bennett J. A., Kidd C., McWilliam P. N. Neurones in the dorsal motor vagal nucleus of the cat with non-myelinated axons projecting to the heart and lungs. Exp Physiol. 1990 Jul;75(4):459–473. doi: 10.1113/expphysiol.1990.sp003423. [DOI] [PubMed] [Google Scholar]
- Ford T. W., McWilliam P. N. The effects of electrical stimulation of myelinated and non-myelinated vagal fibres on heart rate in the rabbit. J Physiol. 1986 Nov;380:341–347. doi: 10.1113/jphysiol.1986.sp016289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis G. S., Wurster R. D. Cardiac responses during stimulation of the dorsal motor nucleus and nucleus ambiguus in the cat. Circ Res. 1980 May;46(5):606–611. doi: 10.1161/01.res.46.5.606. [DOI] [PubMed] [Google Scholar]
- Heavner J. E., de Jong R. H. Lidocaine blocking concentrations for B- and C-nerve fibers. Anesthesiology. 1974 Mar;40(3):228–233. doi: 10.1097/00000542-197403000-00004. [DOI] [PubMed] [Google Scholar]
- Jones J. F., Wang Y., Jordan D. Activity of cardiac vagal preganglionic neurones during the pulmonary chemoreflex in the anaesthetized cat. Adv Exp Med Biol. 1994;360:301–303. doi: 10.1007/978-1-4615-2572-1_52. [DOI] [PubMed] [Google Scholar]
- Katona P. G., Poitras J. W., Barnett G. O., Terry B. S. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. Am J Physiol. 1970 Apr;218(4):1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- MIDDLETON S., MIDDLETON H. H., GRUNDFEST H. Spike potentials and cardiac effects of mammalian vagus nerve. Am J Physiol. 1950 Sep;162(3):545–552. doi: 10.1152/ajplegacy.1950.162.3.545. [DOI] [PubMed] [Google Scholar]
- McAllen R. M., Spyer K. M. Two types of vagal preganglionic motoneurones projecting to the heart and lungs. J Physiol. 1978 Sep;282:353–364. doi: 10.1113/jphysiol.1978.sp012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam P. N., Woolley D. C. The effect of supranodose vagotomy on the hexamethonium-resistant bradycardia in the anaesthetized rabbit. J Auton Nerv Syst. 1990 Mar;29(3):227–230. doi: 10.1016/0165-1838(90)90148-c. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SEARS T. A. Some factors concerned in differential nerve block by local anaesthetics. J Physiol. 1961 Aug;157:565–580. doi: 10.1113/jphysiol.1961.sp006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka S. Electrophysiologic identification of preganglionic neurons in rat dorsal motor nucleus and analysis of vagus afferent projections. Exp Neurol. 1986 Feb;91(2):366–381. doi: 10.1016/0014-4886(86)90076-2. [DOI] [PubMed] [Google Scholar]
- Nosaka S., Yasunaga K., Kawano M. Vagus cardioinhibitory fibers in rats. Pflugers Arch. 1979 Apr 30;379(3):281–285. doi: 10.1007/BF00581432. [DOI] [PubMed] [Google Scholar]
- Paintal A. S. Block of conduction in mammalian myelinated nerve fibres by low temperatures. J Physiol. 1965 Sep;180(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Parker P., Celler B. G., Potter E. K., McCloskey D. I. Vagal stimulation and cardiac slowing. J Auton Nerv Syst. 1984 Sep;11(2):226–231. doi: 10.1016/0165-1838(84)90080-8. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Fieber L. A., Adams D. J. Neurotransmission in neonatal rat cardiac ganglion in situ. Am J Physiol. 1990 Oct;259(4 Pt 2):H997–1005. doi: 10.1152/ajpheart.1990.259.4.H997. [DOI] [PubMed] [Google Scholar]
- Whitwam J. G., Kidd C. The use of direct current to cause selective block of large fibres in peripheral nerves. Br J Anaesth. 1975 Nov;47(11):1123–1133. doi: 10.1093/bja/47.11.1123-b. [DOI] [PubMed] [Google Scholar]
- Woolley D. C., McWilliam P. N., Ford T. W., Clarke R. W. The effect of selective electrical stimulation of non-myelinated vagal fibres on heart rate in the rabbit. J Auton Nerv Syst. 1987 Dec;21(2-3):215–221. doi: 10.1016/0165-1838(87)90024-5. [DOI] [PubMed] [Google Scholar]
- Xi-Moy S. X., Randall W. C., Wurster R. D. Nicotinic and muscarinic synaptic transmission in canine intracardiac ganglion cells innervating the sinoatrial node. J Auton Nerv Syst. 1993 Mar;42(3):201–213. doi: 10.1016/0165-1838(93)90365-2. [DOI] [PubMed] [Google Scholar]


