Abstract
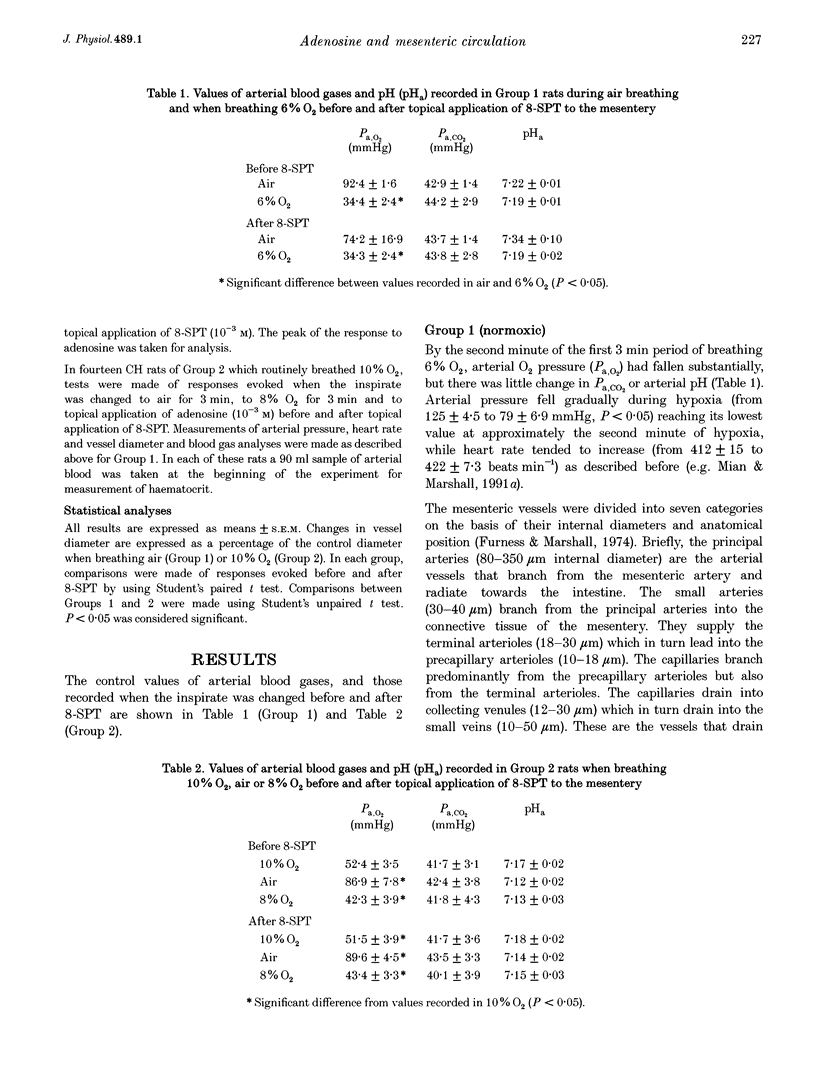
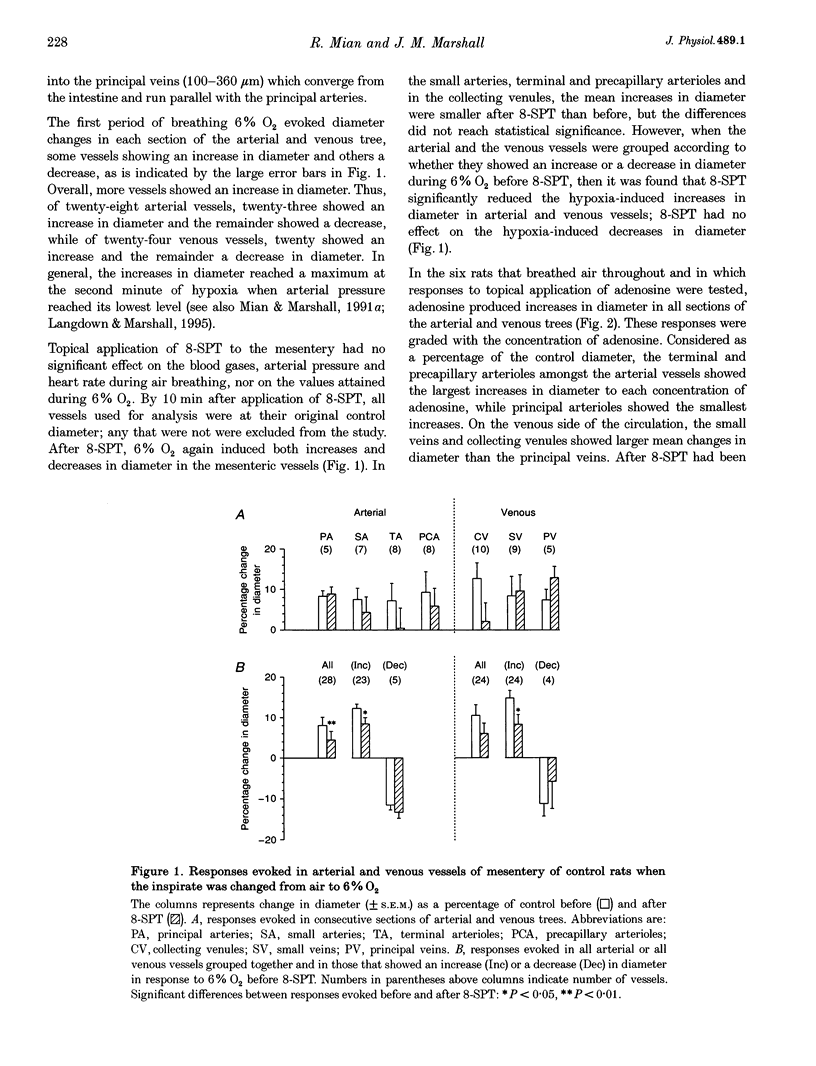
1. We have compared the roles of adenosine in mediating dilator responses to acute hypoxia in mesenteric microcirculation of control, normoxic (N) rats and in chronically hypoxic (CH) rats kept in an hypoxic chamber at 10% O2 for 3-4 weeks. 2. In fifteen N rats, acute hypoxia (breathing 6% O2 for 3 min) induced mean increases in the diameter of arterial vessels of mesentery (whose internal diameter was 10-350 microns) of 8.0 +/- 1.9% (mean +/- S.E.M.) and of venous vessels (whose internal diameter was 12-360 microns) of 10.4 +/- 2.6%. These diameter changes were reduced by approximately 30% when the adenosine receptor antagonist 8-sulpho-phenyltheophylline (8-SPT, 10(-3) M) was applied topically to the mesentery. 3. In a further six N rats, topical application of graded concentrations of adenosine (10(-7)-10(-3) M) to the mesentery evoked graded increases in the diameter of all arterial and venous vessels, maximum increases with 10(-3) M being 12.5 +/- 3.3 and 8.4 +/- 4.3%, respectively; these responses were abolished by 8-SPT. 4. By contrast, in fourteen CH rats, the smaller change in inspirate from 10 to 8% O2 induced increases in diameter of arterial and venous vessels which had control diameters that were comparable to those of N rats, of 14.1 +/- 2.4 and 12.9 +/- 2.7%, respectively, and which were virtually equivalent to the responses induced by topical application of 10(-3) M adenosine (13.3 +/- 1.3 and 16.3 +/- 2.0% in arterial and venous vessels, respectively). The changes induced by acute hypoxia were abolished by 8-SPT, as were those induced by adenosine. 5. These results suggest that in the intestinal mesentery, where the blood vessels have negligible tissue parenchyma around them, locally released or synthesized adenosine makes a substantial contribution to the dilatation that is evoked in arteriolar vessels by acute hypoxia and to the active dilatation, or passive distension of the venous vessels. The results also suggest that this contribution is accentuated in chronic hypoxia either by greater release of adenosine or greater vascular sensitivity to it.
Full text
PDF

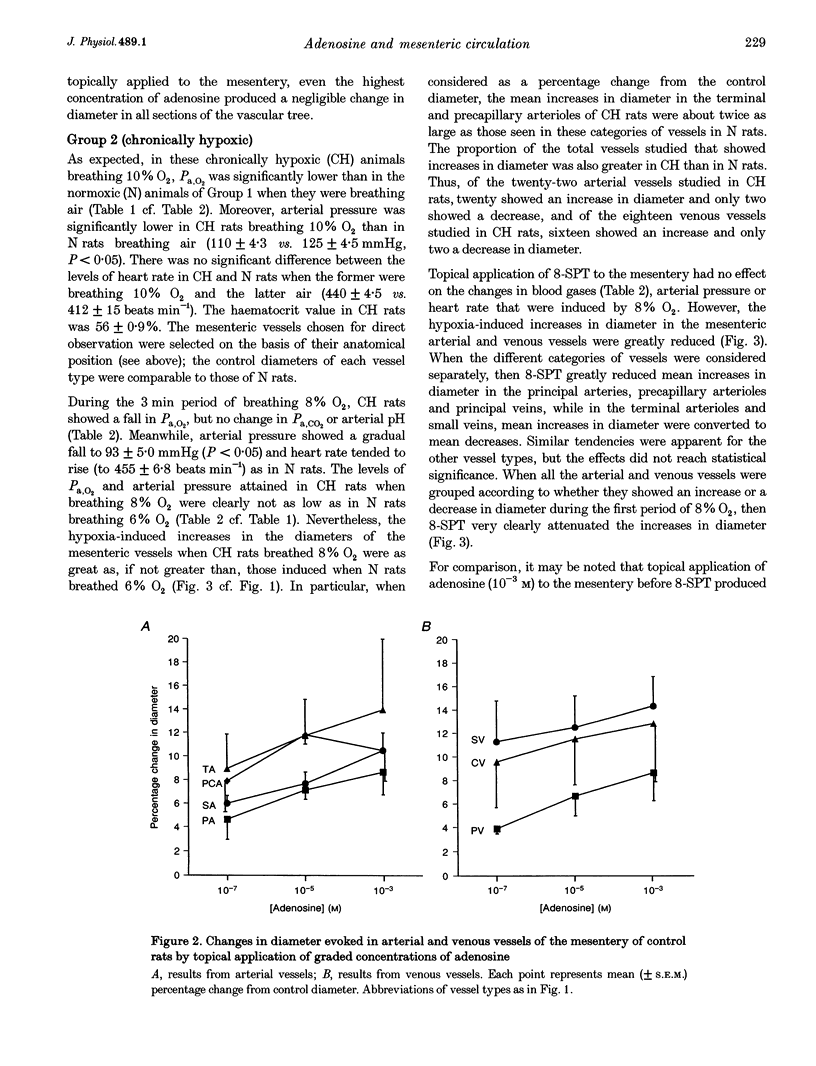
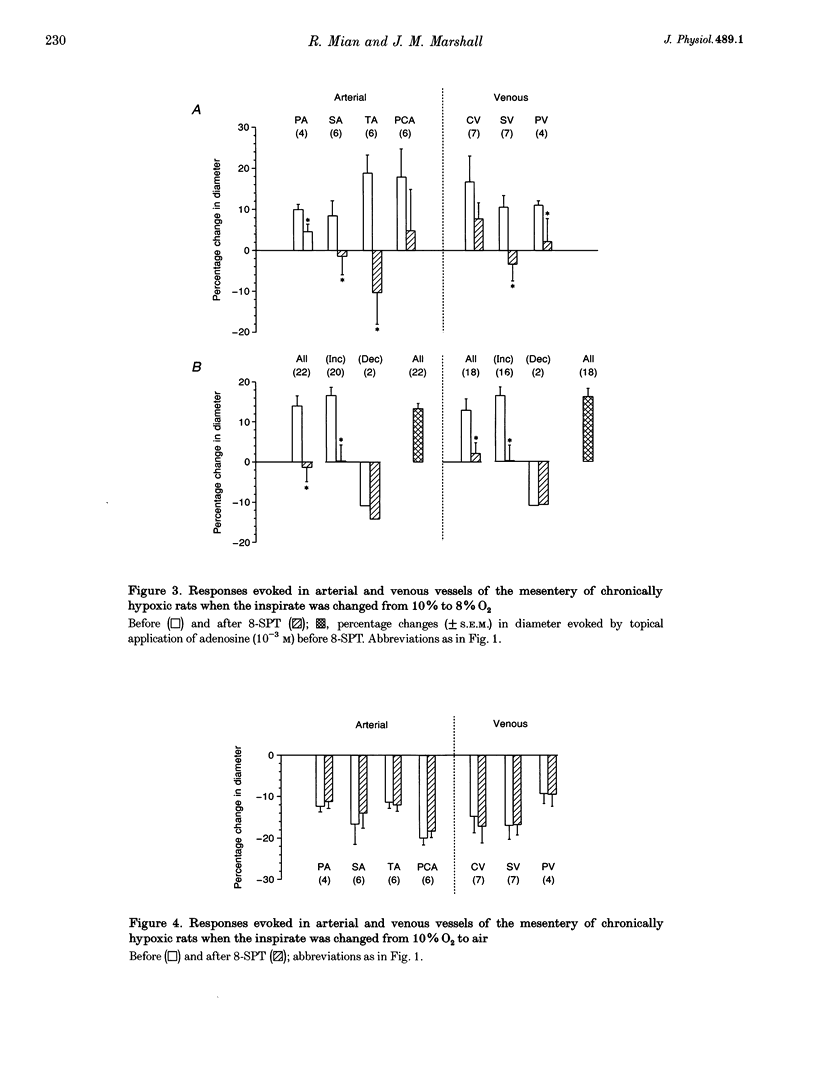




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. H., Sutton E. T. Antagonism of acetylcholine and adenosine rat cremaster arteriolar vasodilation by combination of NO antagonists. Int J Microcirc Clin Exp. 1993 Jun;12(3):275–286. [PubMed] [Google Scholar]
- Burnstock G. Sympathetic purinergic transmission in small blood vessels. Trends Pharmacol Sci. 1988 Apr;9(4):116–117. doi: 10.1016/0165-6147(88)90185-x. [DOI] [PubMed] [Google Scholar]
- Deussen A., Möser G., Schrader J. Contribution of coronary endothelial cells to cardiac adenosine production. Pflugers Arch. 1986 Jun;406(6):608–614. doi: 10.1007/BF00584028. [DOI] [PubMed] [Google Scholar]
- Fuglsang A., Therkildsen P., Crone C. Presynaptic modulation of sympathetic nerve transmission--an element in vasomotor control. Int J Microcirc Clin Exp. 1989 Feb;8(1):71–84. [PubMed] [Google Scholar]
- Furness J. B., Marshall J. M. Correlation of the directly observed responses of mesenteric vessles of the rat to nerve stimulation and noradrenaline with the distribution of adrenergic nerves. J Physiol. 1974 May;239(1):75–88. doi: 10.1113/jphysiol.1974.sp010556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad D. D., Abboud F. M. Dickinson W. Richards Lecture: Circulatory adjustments to hypoxia. Circulation. 1980 Mar;61(3):463–470. doi: 10.1161/01.cir.61.3.463. [DOI] [PubMed] [Google Scholar]
- Hopwood A. M., Lincoln J., Kirkpatrick K. A., Burnstock G. Adenosine 5'-triphosphate, adenosine and endothelium-derived relaxing factor in hypoxic vasodilatation of the heart. Eur J Pharmacol. 1989 Jun 20;165(2-3):323–326. doi: 10.1016/0014-2999(89)90730-9. [DOI] [PubMed] [Google Scholar]
- Hébert M. T., Marshall J. M. Direct observations of effects of baroreceptor stimulation on mesenteric circulation of the rat. J Physiol. 1988 Jun;400:29–44. doi: 10.1113/jphysiol.1988.sp017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. J., Johnson P. C. Elevated ambient oxygen does not affect autoregulation in cat mesentery. Am J Physiol. 1988 Jul;255(1 Pt 2):H131–H137. doi: 10.1152/ajpheart.1988.255.1.H131. [DOI] [PubMed] [Google Scholar]
- Langdown A. J., Marshall J. M. Analysis of responses observed in mesenteric microcirculation of the rat during systemic hypoxia. J Physiol. 1995 Feb 1;482(Pt 3):669–677. doi: 10.1113/jphysiol.1995.sp020549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Metcalfe J. D. Analysis of the cardiovascular changes induced in the rat by graded levels of systemic hypoxia. J Physiol. 1988 Dec;407:385–403. doi: 10.1113/jphysiol.1988.sp017422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall J. M., Thomas T., Turner L. A link between adenosine, ATP-sensitive K+ channels, potassium and muscle vasodilatation in the rat in systemic hypoxia. J Physiol. 1993 Dec;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. Responses observed in individual arterioles and venules of rat skeletal muscle during systemic hypoxia. J Physiol. 1991 May;436:485–497. doi: 10.1113/jphysiol.1991.sp018562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian R., Marshall J. M. The role of adenosine in dilator responses induced in arterioles and venules of rat skeletal muscle by systemic hypoxia. J Physiol. 1991 Nov;443:499–511. doi: 10.1113/jphysiol.1991.sp018847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon M., Marshall J. M. The role of adenosine in the respiratory and cardiovascular response to systemic hypoxia in the rat. J Physiol. 1991;440:529–545. doi: 10.1113/jphysiol.1991.sp018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Milner P., Kirkpatrick K. A., Burnstock G. Flow-induced release of adenosine 5'-triphosphate from endothelial cells of the rat mesenteric arterial bed. Experientia. 1992 Jan 15;48(1):31–34. doi: 10.1007/BF01923600. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Thomas T., Elnazir B. K., Marshall J. M. Differentiation of the peripherally mediated from the centrally mediated influences of adenosine in the rat during systemic hypoxia. Exp Physiol. 1994 Sep;79(5):809–822. doi: 10.1113/expphysiol.1994.sp003809. [DOI] [PubMed] [Google Scholar]
- Thomas T., Marshall J. M. A study on rats of the effects of chronic hypoxia from birth on respiratory and cardiovascular responses evoked by acute hypoxia. J Physiol. 1995 Sep 1;487(Pt 2):513–525. doi: 10.1113/jphysiol.1995.sp020896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Marshall J. M. Interdependence of respiratory and cardiovascular changes induced by systemic hypoxia in the rat: the roles of adenosine. J Physiol. 1994 Nov 1;480(Pt 3):627–636. doi: 10.1113/jphysiol.1994.sp020389. [DOI] [PMC free article] [PubMed] [Google Scholar]


