Abstract
Deficient sleep has been linked to a broad range of physical, cognitive and mental health impacts, in particular during adolescence. It is thus essential to understand its underlying mechanisms, including family factors. The goal of our study was to assess through combined subjective and objective assessments, how family members’ daily variations in sleep are interconnected, between parents and siblings of adolescents. A total of 111 participants within 30 families were included, of whom 30 adolescents (14.5 years +/− 2.1 years, 46.7% boys), 30 siblings (12.8 years + /− 3.8, 40% boys), 30 mothers (43.5 years +/− 5.5) and 21 fathers (47.5 years +/− 7.6). Sleep was objectively assessed using parallel actigraphy recordings during a single week for all family members. Adolescents’ daily variation in sleep duration and timing displayed many concordances with their siblings’ and their mothers’ sleep, but not with their fathers’ sleep. Adolescents whose parents did not set limits on sleep time or screen use went to bed over an hour later (p < 0.001) and slept over 30 min less on school nights. Our study demonstrates the important role of family sleep on adolescent sleep, through rule-setting and congruence between each family members sleep, in particular regarding sleep duration and timing.
Subject terms: Human behaviour, Paediatric research, Paediatric research
Introduction
During adolescence, many biological changes occur, including a reorganization of sleep1 and circadian rhythms, with a progressive delay in chronotype, i.e. the desired timing of sleep2. The underlying biological mechanisms of this vesperal preference are a combination of changes in the homeostatic process of sleep, with a slower building of the sleep pressure3, and in the circadian process, including a delayed secretion of melatonin4. Adolescence is also marked by changes in social and familial organization with increasing autonomy and less adherence to set rules around sleep, which can also contribute to a delayed sleep onset5. Furthermore, adolescents are often faced with academic pressure and social pressure to take part in late networking. The technological advances of the past decades have provided unlimited access to entertainment and have come hand in hand with an explosion in the use of electronic devices during the night, further delaying sleep onset and disrupting sleep6. In addition, the use of screens results in inappropriate exposure to light in late hours, especially blue light, preventing melatonin secretion and resulting in significant phase delay7.
The convergence of factors leading to a reduced sleep duration in adolescence has been conceptualize in a framework described by Carskadon et al. as “the perfect storm”5. Data from national surveys8 and longitudinal studies9 congruently observe a decrease in time spent sleeping throughout adolescence. However, present knowledge supports that this reduction is not driven by a reduced need for sleep but rather by a combination of a delayed sleep onset, due to biological reasons as well as social ones, and a maintained or even advanced wake time due to school schedules5. The sleep debt accumulated during school nights is then compensated on weekends, with an even further delayed wake time10. Sleep debt in adolescence is considered today a significant public health issue, as it has been linked to a broad range of physical, cognitive and mental health impacts, in particular during adolescence, leading to a higher risk of excessive daytime sleepiness, inattention, low school performance, as well as mood and behavioral disorders, substance abuse and even suicidal ideation and suicide attempts in this age group11,12.
In addition to biological and social factors, the family context is likely to play a significant role in adolescent sleep. A negative family environment has been associated with shorter sleep duration and longer sleep onset latency13, whereas a positive parenting style14 and rule setting regarding sleep15 correlated with increased sleep quality and longer sleep duration. Beyond rule setting, parents’ sleep may also influence adolescents’ sleep through an imitation process. There is increasing evidence of an association between parents’ and children’s sleep–wake rhythms, although most studies have focused on younger children16,17. Regarding adolescents, studies based on subjective sleep logs18,19, actigraphy20 and EEG21 have all observed associations between family members’ sleep. A greater concordance was found between adolescents and mothers than with fathers18–21. Additionally, the interrelations between family member’s sleep were greater in larger families19, but the potential interacting effect of child’s gender, age and depressive symptoms remain unclear, although these factors affect adolescent’s sleep in general22,23. Also, little is known about how the presence of a sibling might affect adolescent’s sleep24.
Although the current literature is sparse, especially in adolescents, these studies underline within-family correlations regarding sleep, measured both subjectively and objectively. We hypothesize that day-to-day variations in sleep are correlated between all family members. The aim of the present study was to investigate how day-to-day variation in sleep correlates between adolescents, mothers, fathers and siblings and how age, gender, type of night (preceding a weekend or a work day), bedroom arrangement and depressive symptoms influenced these associations.
Methods
Participants
Recruitment took place between October 2020 and October 2021 in two regions of France (Normandy and Haute Savoie). No protocols were conducted during the lockdowns implemented due to the COVID-19 pandemic. Advertisements for the study was carried out on social media and within different local non-governmental organizations, especially the Schools of Parents and Educators (“École des Parents et des Éducateurs” (EPE)) which specializes in supporting parenthood. The inclusion criteria for the families were to be comprised of at least one adolescent (between 10 and 20 years old), one sibling (aged 8 or older) and one parent. One adolescent, one sibling and one or two parents were recruited per family unit. We did not include younger siblings to limit the age gap, and only one sibling was included due to a limited number of actigraphs. Although exploring the association with the father’s sleep was one of the aims of the study, the absence of a father was not defined as an exclusion criterion, to avoid selection bias. Families where the adolescent or the sibling lived more than half of the time in a separate house (e.g. step family, boarding school) where not included, and there had to be at least two siblings living within the family. Exclusion criteria were any difficulties that could hinder participation or compliance with the study. All participants were asked to participate to the full study protocol. The study was approved by the ‘Comité éthique pour la recherche’ (CER), the ethics committee of the University of Strasbourg (Unistra/CER/2020-20).
Study protocol
We conducted a cross-sectional observational study, including qualitative and quantitative measures. The first step of the protocol was a semi-directive interview led by trained psychologists (LE and EE), which lasted on average an hour. The demographic and socio-economic characteristics of the family were extracted from these interviews. Following the interview, validated questionnaires (namely, the Epworth sleepiness scale (ESS), the Pittsburg Sleep Quality Index (PSQI), the Center for Epidemiologic Studies—Depression Scale (CES-D), detailed in the following section) were completed by the participants, then actigraphy devices and paper-based sleep diary were given to the participants. They were instructed to wear the actigraph until the next visit, which occurred 7 to 11 days later, and to complete the diaries daily in parallel.
Measures
The demographic and socio-economic characteristics of the family were extracted from the semi-directive qualitative interviews and were used to define the parents’ living arrangement (both biological parents living together, step family, single-parent home), education level (less than high-school, high-school and above high-school diploma), employment status (official French job class: independent job, senior level salaried job, mid-level salaried job, salaried job: skilled, salaried job: unskilled, unemployed), the number of children within the family. Sleep related family functioning outcomes were also considered. Teenagers were asked if they shared their bedroom (sleeping alone in their bedroom or sharing the bedroom with a sibling or a parent). Parents were asked if they implemented rules regarding sleep schedule (presence of rules vs absence) and screen use. The adolescent’s gender was coded with girl as the reference (girl = 0, boy = 1).
Validated questionnaires were used to assess subjective sleep and self-rated depressive symptoms. Sleepiness was assessed using the Epworth sleepiness scale (ESS) for adults25, and the child and adolescent adapted version for younger participants26; with a higher score indicating higher sleepiness. Sleep quality was assessed with the Pittsburg Sleep Quality Index (PSQI)27 for adolescents and adults and the Children’s Sleep Habits Questionnaire28 for siblings under the age of 11 years, higher scores indicating worse sleep quality. Depressive symptoms were assessed with the Center for Epidemiologic Studies—Depression Scale (CES-D) for adults29 and for adolescents30, with higher scores indicating greater depressive symptoms.
Actigraphy is a noninvasive and objective method for estimating rest-activity rhythms in the home environment. Each participant was asked to wear an actigraph (MotionWatch 8®, CamNtech Ltd., Cambridge, UK) over 7 days, worn on the non-dominant wrist. The participants indicated the time they went to bed by pushing an event marker button on the device. The actigraph contains a piezo-electric accelerometer that measures movement intensity in all directions 32 times per second. Activity counts were sampled into epochs of 1 min. The activity/rest threshold was set to 40 counts (the default setting). The data were analyzed with the MotionWare Sleep Analysis software (CamNtech MotionWare 1.1.20) with medium threshold to determine total sleep time (TST), sleep efficiency (time spent sleeping divided by the time spent in bed) and the timing of sleep onset and sleep offset. When compared to polysomnography, actigraphy shows good psychometric properties, with reported sensitivity and accuracy above 0.87 in adolescence31. In parallel to the actigraphy recording, participants filled in a paper-based sleep diary to indicate at what time they believe they went to bed, fell asleep, woke and got out of bed. Work nights were defined as nights preceding a day of work or a day of school; conversely a free night was defined as a night preceding a weekend day. This variable, named “type of night”, was dummy coded with “work night” as the reference (coded as work night = 0, free night = 1).
Analysis plan
All analyses were conducted with the R software, version 4.3.032.
We first conducted descriptive analyses, to illustrate the family members’ sleep, according to gender and the type of night. Differences between genders were conducted using the Mann–Whitney U test, and differences by type of night with paired Wilcoxon tests. Pearson correlations between the average sleep of all family members were also calculated.
Then we analyzed the daily concordance between the adolescents’ sleep and the sleep of the other family members, using linear mixed models (LME4 package33). Mixed models were necessary to take into account the repeated nature of the data, as sleep measures were considered separately by night in order to study daily variations. Specifically, each line of the data corresponded to one night of sleep and included the adolescent’s, the sibling’s, the mother’s and the father’s sleep on that same night. Models were constructed so that level 1 predictors were those that assessed daily variation (daily sleep measures of all family members and type of night) and level 2 predictors were those that were stable within the subject. Following a selection model procedure (based on Akaike and BIC) only gender and age were retained as level 2 predictors.
Each sleep parameter (TST, sleep efficiency and sleep onset and wake time) was studied separately in correspondence with the family members’ same sleep parameter. For example, when studying daily variation of the adolescent TST, the other family members’ daily variations in TST were included as predictors. The adolescent’s sleep was used as the dependent variable (outcome), the adolescent’s ID was the cluster variable, each of the family members’ daily sleep measures and the type of night were used as level 1 predictors; age and gender were used as level 2 predictors. The family members’ daily sleep predictors were transformed to be person-centered, meaning that they represented how a single night’s sleep differed from the average night sleep of that person. As such, the estimates of family members sleep indicate how within-person daily fluctuation in a given family member predicts the adolescent’s sleep that same night.
Additionally, we investigated separately the interaction effect of age, gender, bedroom arrangement, type of night and the mother’s and the adolescent’s depressive symptoms. This step allowed to understand if the daily concordance between the adolescent’s sleep and other family members sleep varied according to those factors. (i.e. whether the association between the sleep of family member A and the sleep of family member B was modified by the interaction factor).
To complete previous analyses focusing rather on a potential imitation process between adolescents and parents, we carried out Student’s t-tests to investigate if sleep outcomes differed between adolescents who had rules set regarding sleep schedule and those who did not, and similarly for rules regarding screen use.
To ease interpretation, time was reported in hh:mm format (the first two digits represent hours and last two are minutes); however, in the statistical analyses, durations (e.g. TST) were expressed in minutes and timing (e.g. wake-up time) in decimal hours.
Results
Descriptive statistics
Family and individual characteristics are detailed in Tables 1 and 2 respectively. A total of 111 participants within 30 families were included in the quantitative part of the study, including 30 adolescents (mean age 14.5 years; SD = 2.1 years, 46.7% boys), 30 siblings (mean age 12.8 years, SD = 3.8, 40% boys), 30 mothers (mean age 43.5 years, SD = 5.5) and 21 fathers (mean age 47.5, SD 7.6). In two-thirds of the families, there were exactly two children (which was the minimum required to be included in the study), and in three-quarters of the families both of the biological parents were living together with the adolescent. Slightly less than half (45%) of the adolescents had a bedroom of their own. For 44% of the adolescents, the family set rules regarding their sleep and 55.5% regarding screen use.
Table 1.
Family characteristics.
| Family characteristics | |
|---|---|
| Household arrangement | |
| Both biological parents | 83.0% (N = 39) |
| Step family | 6.4% (N = 3) |
| Single parent | 10.6% (N = 5) |
| Number of children in the household | 2.4 (SD = 0.6) |
| Education level | |
| Less than high-school | 30.0% (N = 9) |
| High-school | 13.3% (N = 4) |
| More than high-school | 56.7% (N = 17) |
| Employment status | |
| Independent job | 9.0% (N = 4) |
| Senior level salaried job | 20.5% (N = 9) |
| Mid-level salaried job | 27.3% (N = 12) |
| Salaried job: skilled | 22.7% (N = 10) |
| Salaried job: unskilled | 6.8% (N = 3) |
| Unemployed | 13.6% (N = 5) |
Table 2.
Individual characteristics.
| Adolescents | Parents | |||
|---|---|---|---|---|
| Girls | Boys | Mother | Father | |
| Age (years) | 14.0 (SD = 2.1) | 15.0 (SD = 2.1) | 43.5 (SD = 5.5) | 47.5 (SD = 7.6) |
| Sleepiness (ESS) | 9.3 (SD = 5.3) | 8.2 (SD = 5.0) | 8.7 (SD = 5.4) | 9.9 (SD = 5.4) |
| Sleep quality (PSQI) | 5.5 (SD = 3.0) | 3.7 (SD = 2.3) | 6.5 (SD = 3.9) | 4.7 (SD = 2.8) |
| Depressive symptoms (CES-D) | 15.4 (SD = 11.1) | 9.6 (SD = 6.7) | 14.0 (SD = 10.9) | 8.0 (SD = 4.3) |
ESS Epworth sleepiness scale (range 0 to 24, cutoff > 11), PSQI Pittsburg Sleep Quality Index (range 0 to 21, cutoff > 5), CES-D Center for Epidemiologic Studies—Depression Scale (range 0 to 60, cutoff > 15).
Actigraphy derived sleep parameters are described in Table 3, according to the type of night (night preceding work or school or a night preceding a free day). Participants all wore the device for at least 3 days, with an average of 6.9 days. On school nights, adolescents went to bed at 23:12 (SD = 61 min), slept 6:56 h (SD = 53 min) and woke-up at 7:26 (SD = 43min). On average on free nights, adolescents went to bed 34 min later (p = 0.003), slept 57 min more (p < 0.001) and woke-up 103 min later (p < 0.001). Boys’ and girls’ sleep did not differ on school nights however TST on free nights was 49 min longer for girls (p = 0.003).
Table 3.
Actigraphy derived sleep by sex and type of night.
| Adolescents | Parents | |||||||
|---|---|---|---|---|---|---|---|---|
| Girls | Boys | Mother | Father | |||||
| Work night | Free night | Work night | Free night | Work night | Free night | Work night | Free night | |
| Sleep onset (clock time) | 23:16 (0:58) | 23:48 (1:14) | 23:07 (1:08) | 23:43 (1:08) | 23:22 (0:52) | 23:44 (1:04) | 23:52 (1:15) | 24:06 (1:10) |
| Sleep offset (clock time) | 07:32 (0:41) | 09:28 (1:17) | 07:17 (0:46) | 08:42 (1:02) | 07:13 (0:51) | 08:31 (0:57) | 06:53 (0:55) | 07:44 (1:18) |
| TST (hh:mm) | 07:04 (0:53) | 08:14 (0:34) | 06:45 (0:52) | 07:24 (0:43) | 06:49 (0:49) | 07:40 (1:16) | 06:09 (0:33) | 06:36 (0:43) |
| Sleep efficiency (%) | 81.8 (6.8) | 82.3 (6.7) | 80.3 (7.1) | 79.8 (6.7) | 85.5 (5.4) | 85.1 (7.8) | 86.3 (6) | 85.2 (6.7) |
TST Total Sleep Time. Work nights are nights preceding a day of work or a day of school, conversely a free night is a night preceding a free day.
hh:mm: duration expressed as clock time, with 2 first digits representing the hours and last 2 digits representing the minutes.
Average concordance in family member’s sleep
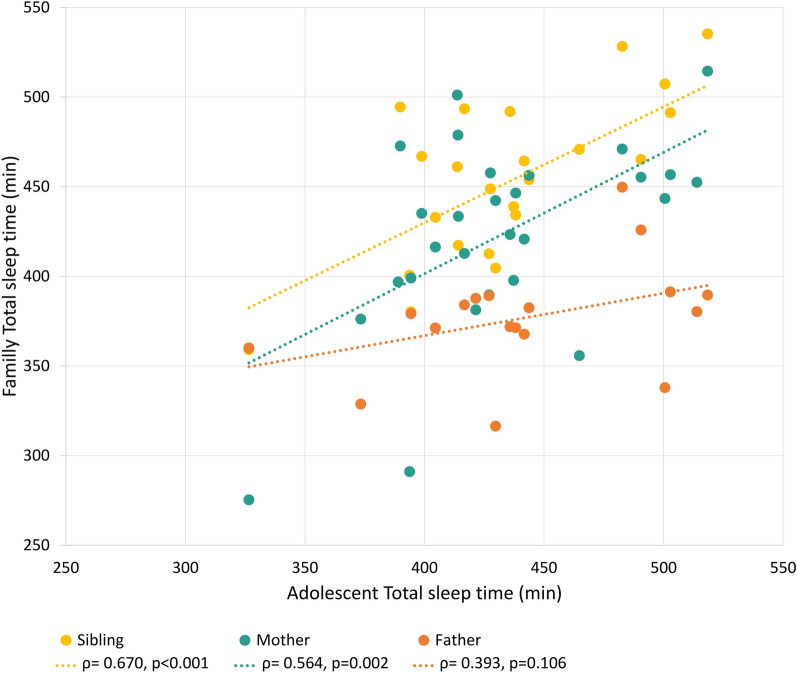
The adolescent’s TST was correlated with that of their mother and sibling (r = 0.564, p = 0.002 and r = 0.670, p < 0.001 respectively). Sleep onset was significantly correlated between siblings (r = 0.616, p = 0.001), and sleep offset between mothers and adolescents (r = 0.454, p = 0.015). However, no correlations were found with the father (Fig. 1).
Fig. 1.
Correlation between family members average total sleep time, measured by actigraphy. In yellow, correlation with the sibling, in green with the mother and in red with the father. TST Total sleep time. Pearson correlation.
Day-to-day concordance between family members’ sleep
The results of the mixed models are detailed in Table 4. As a reminder, models were constructed so that level 1 estimates represent how the daily variation of a given family member from their own mean sleep concords with the adolescent’s sleep that same night. Whereas level 2 estimates represent how parameters that are stable throughout the follow-up (adolescent’s gender and age) are associated with the adolescent’s sleep. Estimates and p-values of statistical interactions are detailed in Supplementary Table 1.
Table 4.
Parameters estimate of the linear mixed models on the association between family members’ sleep.
| TST (minutes) | Sleep onset (hrs) | Sleep offset (hrs) | Sleep efficiency (%) | |||||
|---|---|---|---|---|---|---|---|---|
| b (SE) | p | b (SE) | p | b (SE) | p | b (SE) | p | |
| Level 1 | ||||||||
| Sibling’s daily sleep | 0.18 (0.08) | 0.032 | 0.48 (0.07) | p < 0.001 | 0.25 (0.08) | 0.002 | 0.04 (0.08) | 0.647 |
| Mother’s daily sleep | 0.19 (0.11) | 0.080 | 0.20 (0.09) | 0.026 | 0.21 (0.12) | 0.088 | 0.04 (0.09) | 0.645 |
| Father’s daily sleep | 0.10 (0.09) | 0.281 | − 0.04 (0.05) | 0.438 | 0.01 (0.07) | 0.898 | 0.13 (0.09) | 0.150 |
| Type of night (Free night) | 18.81 (9.40) | 0.047 | 0.30 (0.10) | 0.004 | 0.84 (0.22) | p < 0.001 | 0.32 (0.64) | 0.621 |
| Level 2 | ||||||||
| Adolescent’s Age | − 5.95 (2.34) | 0.017 | 0.27 (0.05) | p < 0.001 | 0.12 (0.03) | 0.002 | 0.62 (0.24) | 0.015 |
| Adolescent’s gender (Boy) | − 47.86 (15.88) | 0.006 | 0.66 (0.33) | 0.060 | 0.16 (0.23) | 0.490 | − 3.56 (1.63) | 0.038 |
Significant values are in bold.
Hrs decimal hours, b (SE) regression estimate and standard error, TST Total Sleep Time, WASO Wake after sleep onset, SOL Sleep onset latency. Type of night is a two category variable distinguishing Work nights (nights preceding a day of work or a day of school, dummy coded as 1) and free nights (night preceding a free day, reference, dummy coded as 0). Gender was a two category variable with girl group as reference (dummy coded as 0) and boy (dummy coded as 1).
Among older adolescents, TST was shorter (b = − 5.95(2.34), p = 0.017), sleep onset and offset were later (b = 0.27 (0.05), p < 0.001 and b = 0.12 (0.03), p = 0.002 respectively) and sleep efficiency was higher (b = 0.62 (0.24), p = 0.015). Among boys, TST was shorter (b = − 47.86 (15.88), p = 0.006) and sleep efficiency was lower (b = − 3.56 (1.63), p = 0.038) than in girls.
The adolescent’s TST was associated with their sibling’s TST that same night; in terms of minutes, an 11-min increase in the sibling’s TST (compared to their own average sleep) was associated with a one-minute increase in the adolescent’s TST. The association between siblings’ daily variation in TST was stronger on free nights (interaction with type of night, with work night as reference: b = 0.43, SE = 0.18, p = 0.020), and when the adolescent shared their bedroom (interaction with bedroom arrangement, with being alone in the bedroom as reference, b = 0.667, SE = 0.162, p < 0.001). Of note, results were similar when replacing bedroom arrangement with the family size. Although not significant, the association between mother’s daily variation in TST and her child had a tendency to be lower when the mother displayed more depressive symptoms (interaction of CES-D b = − 0.026, SE = 0.014, p = 0.057). TST was longer in all participants on free nights. There was no association between the fathers’ and adolescent’s daily variation in TST.
The adolescent’s sleep onset time was associated with their sibling’s and their mother’s variation in sleep onset that same night. An hour delay in the adolescent’s sleep onset was associated with a delay of 29 min in the sibling’s sleep onset and of 12 min in the mother’s sleep onset. No association was found with the father’s sleep onset. Sleep onset was later on free nights for all participants.
Similarly, the adolescent’s sleep offset time was associated with their sibling’s and their mother’s variation in sleep offset that same night, but not the father’s. An hour delay in the adolescent’s sleep offset was associated with a delay of 15 min in the sibling’s sleep offset and of 12 min in the mother’s sleep offset. The association between sibling’s daily variation in sleep offset was stronger on free nights (interaction with type of night, with work days as reference: b = 0.41, SE = 0.18, p = 0.024), among older adolescents (interaction with age b = 0.07, SE = 0.03, p = 0.01) and when the adolescent shared their bedroom (interaction with bedroom arrangement, with being alone in the bedroom as reference, b = 0.351, SE = 0.135, p = 0.011). The association between the mother’s and the adolescent’s daily variation in sleep offset was weaker in mothers with higher depressive symptoms (interaction with mother’s CESD score b = − 0.027, SE = 0.018, p = 0.024). Sleep offset was later on free nights in all participants. However, the depressive symptoms of the adolescent did not affect the association between their sleep and their mother’s or siblings’ sleep.
There was no significant concordance between family members’ daily variation in sleep efficiency when interaction factors were not considered. However, after adding age as an interaction factor in the association between the mother’s and adolescent’s daily variation in sleep efficiency, the mother’s daily variation in sleep efficiency became significant (b = 1.49, SE = 0.36, p < 0.001). Indeed, the association between the mother’s and the adolescent’s variation in sleep efficiency was stronger in younger adolescents (interaction with age b = − 0.10, SE = 0.02, p < 0.001). Similarly, after adding bedroom arrangement as an interaction factor in the association between the sibling’s and adolescent’s daily variation in sleep efficiency, the sibling’s daily variation in sleep efficiency became significant (b = 0.610, SE = 0.147, p < 0.001). Indeed, the association between the sibling’s and the adolescent’s variation in sleep efficiency was stronger when the bedroom was shared (interaction with bedroom arrangement with being alone in the bedroom as reference b = − 0.84, SE = 0.18, p < 0.001). There was no association between the fathers’ and adolescent’s daily variation in sleep efficiency.
Rule setting around sleep and screen use
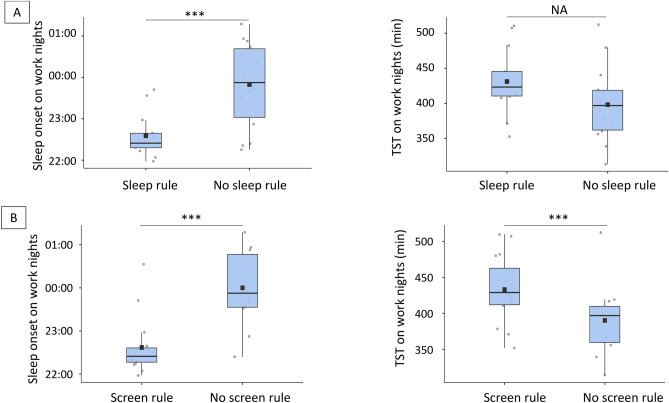
Adolescent whose parents’ declared setting rules regarding sleep had, on schooldays, earlier sleep onset (22:35 (SD = 0:32) vs 23:50 (SD = 1:02), p < 0.001). However, they also had a tendency to wake-up earlier, so although TST was longer, it did not significantly differ between groups: adolescents with rules slept on average 431 min (SD = 46) vs 398 min (SD = 54) for those without (p = 0.102). No differences were observed on free nights.
Adolescent whose parents’ declared setting rules regarding screen use had on schooldays earlier sleep onset (22:37 (SD = 0:41) vs 00:00 (SD = 0:53), p < 0.001), and also longer TST (433 min, SD = 47.0 vs 390 min, SD = 50.6; p = 0.032) than those without. Sleep onset was also earlier for them on free nights (23:35 (SD = 1:00) vs 00:20 (SD = 1:13), p = 0.030) but not TST (473 min, SD = 43) vs 469 min (SD = 49), p = 0.832). Results are represented Fig. 2.
Fig. 2.
Difference in sleep onset and total sleep time according to parent-set rules regarding sleep and screen use. Box plot representing the difference in sleep onset and total sleep time on school/workdays between (A) adolescents who have been set rules regarding their sleep schedule and those who did not, (B) adolescents who have been set rules regarding their screen use and those who did not. TST Total sleep time. Black squares represent the average while black horizontal bars represent the median.
Discussion
Adolescent’s daily variation in sleep duration and timing displayed many concordances with their sibling’s and their mother’s sleep, but not with their father’s sleep. These associations were often stronger on free nights (when the family members had fewer social and school-related constraints regarding their sleep schedules), when adolescents shared their bedroom with their siblings and when the mother displayed fewer depressive symptoms. Sleep efficiency, however, was associated with the adolescent’s personal characteristics (higher efficiency in older adolescents and in girls) but not with family members’ daily variations. To the best of our knowledge, this is the first study investigating within-family correlations between adolescents’ sleep–wake rhythms compared not only to their parents’ but also to their siblings’ sleep.
Due to a lack of literature on the subject, our results could not be compared regarding concordance between siblings’ sleep. However, our findings were in congruence with previous studies regarding parental sleep. To the best of our knowledge, Kouros & El-Sheikh’s20 study is the only previous work investigating daily recordings of actigraphy of an adolescent and both parents separately. They included 163 families with a follow-up ranging from 2 to 7 days. Although children were younger in their study (mean age = 10.45 years, range 9 to 12 years) the results were similar. More specifically, they found a strong association between the adolescent’s and the mother’s daily variation in sleep (TST, sleep efficiency and wake time), but not with the father’s. In their study, Fuligni et al.19 included 421 adolescents (mean age 15 years) and one of their primary caregivers (83% mothers, 14% fathers and 4% other family members). Sleep was assessed subjectively daily by questionnaires, separately by the adolescent and the caregiver. The authors found a daily concordance between TST, bedtime and wake time between the adolescent and the caregiver. Regarding interactional effects, age and gender seldom played a role in the association between family members’ daily sleep variation. However, similarly to our results, associations were stronger in families of larger size, and when the caregiver showed higher interpersonal support.
Although studies investigating daily variations in sleep are sparse, it should be noted that a larger body of literature has investigated how sleep on average correlates between family members. Kalak et al.21 recorded within 47 families one night of sleep using home-based EEG, including per family one or several adolescents (mean age = 16.3, SD = 2.0 years) and both parents. They found a correlation between the adolescent’s TST and both parents’; however, SOL, SE and sleep structure (namely stage 2 duration and percentage) were associated between the adolescents and the mothers only. Other studies based on subjective measures of sleep have also described great concordance between adolescents and parents sleep18,34.
Most of the literature describes a stronger association between adolescents’ sleep and that of their mothers than that of their fathers18,20,21,34. Authors hypothesize that there could be a stronger implication of mothers in their adolescent’s sleep schedule, alongside a stronger implication in parenting in general. Kouros et al.20 suggest that the stronger implication of mothers in adolescents’ sleep could be the continuity of a pattern established since infancy, where mothers are still most commonly those in charge of nighttime feeding and are more likely to respond to the child’s cries at night.
It is also possible that the association between mothers’ and adolescents’ sleep is of an indirect nature. Bai et al.35, in their study which included daily actigraphy recordings of 517 adolescents (mean age = 15.4 years, range = 15–18), found that on days when adolescents reported getting along with their parents, they slept 26 min longer. Similarly, Sasser et al.36, found that adolescents slept longer on days when they spent more time with siblings. Interestingly, it was rather the general connection between adolescent and parents, rather than its daily variation, that was associated with adolescents’ sleep duration. Brand et al.18, in their study focusing on average sleep, found that the association between mothers’ and adolescents’ general sleep quality was mediated by parenting style. Indeed, the results of the structural equation modeling analyses (SEM) indicated that mothers’ poor sleep was associated with poor parenting style, which was in turn associated with adolescents’ poor sleep.
Our results suggest that adolescents on average are influenced by their parent’s own behavior around sleep and screen use. Additionally, setting rules around sleep explicitly remains important, even though adolescents seem to respect these rules less. Interestingly, our results demonstrate earlier sleep-onset times and longer TST on school days for adolescents where rules are set in the family. This result is in line with Bauducco et al.’s longitudinal study37, which included 2509 Adolescents (mean age 12.6(0.5) years), who self-reported on two consecutive years their sleep patterns and the rules set by their parents regarding bedtime. They found that adolescents who had parent-set bedtimes in the second year of follow-up, had earlier sleep onset and longer TST (about 20 min), regardless of the rules set in the first year.
The present study adds to the current literature’s insight regarding daily interactions of sleep within the family; however, several limitations should be noted. First, the number of recruited families was relatively small. This limited some of the analyses, thus we focused on daily variations only whereas Kouros et al.20 integrated in the same model daily variation and average sleep. Also, the lack of associations found with fathers could be due to insufficient statistical power, since fewer fathers wore the actigraph than mothers. Secondly, the sample was not representative of the national population. We did not include siblings under the age of 8, and only one sibling was considered in the analysis. Another limitation is the investigation of socio-economic factors as well as sleep problems and medical conditions. The familial socio-economic environment was assessed by semi-directive interviews and was thus qualitative in nature and not directly implementable in this quantitative study (e.g. the income-to-need ratio was not expressed with the same verbatim across families, rendering comparison difficult). Due to our recruitment strategy, which favored families where the father could be contacted, the great majority of families were married couples, so marital status could not be taken into account. Also, ethnicity and race were not investigated in accordance with French legislation. A separate study using qualitative approaches will be conducted to address this limitation. However, since the focus of our study was on daily variations of sleep (if the adolescent slept more on a given day compared to his/her own average) rather than sleep in general, this confounding factor is limited. Mediation analyses were not tested in this study and present an opportunity for future research.
In conclusion, the present study corroborates previous research regarding the interplay between adolescents’ and parents’ sleep. We additionally demonstrated the influence of the sibling, highlighting the different roles of family members’ sleep on adolescents’ sleep, especially regarding timing and duration. Taken together, the literature stresses the fact that a holistic approach to adolescents’ families is crucial. Considering the whole family when implementing sleep hygiene measures for an adolescent could increase adherence and the global benefits of interventions. Furthermore, future research and clinical care should consider both role-modeling and limit-setting by parents as both risk factors and preventive factors for adolescent sleep deficit.
Supplementary Information
Acknowledgements
We are deeply thankful for the involvement of our deceased colleague, Professor Régine Scelles, without whom this research would not have been possible and to who we dedicate this article. We would like to thank the National Federation of the School of Parents and Educators (Fédération Nationale des Ecoles de Parents et des Educateurs, Paris, France) for their logistic support (in particular Béatrice Bayo and Elodie Duwelz); the Schools of Parents and Educators of Haute-Savoie and Calvados, France, for their participation in this study; as well as the VINCI foundation for the financial support to the present study.
Author contributions
RS initiated the study; ER and CMS designed the study, co-authored the study protocol and led the literature review, ER planned and conducted the analysis and wrote the first draft of the manuscript and CMS reviewed it; JB participated to the literature review, analyzed the actigraphy data, and reviewed the manuscript, EL and EE recruited participants, implemented the study protocol with the quantitative data collection and conducted the semi-structured interviews in Haute-Savoie and Normandie respectively, and reviewed the manuscript.
Data availability
The data can be made available upon request to the first author (ER) provided a written agreement of the study funder (the VINCI foundation).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
R. Scelles is deceased.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-76597-2.
References
- 1.Feinberg, I., Davis, N. M., de Bie, E., Grimm, K. J. & Campbell, I. G. The maturational trajectories of NREM and REM sleep durations differ across adolescence on both school-night and extended sleep. Am. J. Physiol.-Regul. Integr. Comp. Physiol.302, R533–R540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohayon, M. M., Carskadon, M. A., Guilleminault, C. & Vitiello, M. V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep27, 1255–1273 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Campbell, I. et al. Adolescent changes in homeostatic regulation of EEG activity in the delta and theta frequency bands during NREM sleep. Sleep34, 83–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley, S. J. et al. A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PloS One9, e112199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carskadon, M. A. Sleep in adolescents: The perfect storm. Pediatr. Clin. North Am.58, 637–647 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hale, L. & Guan, S. Screen time and sleep among school-aged children and adolescents: A systematic literature review. Sleep Med. Rev.21, 50–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowley, S. J., Cain, S. W., Burns, A. C., Acebo, C. & Carskadon, M. A. Increased sensitivity of the circadian system to light in early/mid-puberty. J. Clin. Endocrinol. Metab.100, 4067–4073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Sleep Foundation. Sleep in the Modern Family. https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2014-sleep-and-family (2014).
- 9.Sadeh, A., Dahl, R. E., Shahar, G. & Rosenblat-Stein, S. Sleep and the transition to adolescence: A longitudinal study. Sleep32, 1602–1609 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowley, S. J., Acebo, C. & Carskadon, M. A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med.8, 602–612 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Owens, J., Adolescent Sleep Working Group, Committee on Adolescence. Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics134, e921-932 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winsler, A., Deutsch, A., Vorona, R. D., Payne, P. A. & Szklo-Coxe, M. Sleepless in Fairfax: The difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. J. Youth Adolesc.44, 362–378 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Bartel, K. A., Gradisar, M. & Williamson, P. Protective and risk factors for adolescent sleep: A meta-analytic review. Sleep Med. Rev.21, 72–85 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Brand, S., Hatzinger, M., Beck, J. & Holsboer-Trachsler, E. Perceived parenting styles, personality traits and sleep patterns in adolescents. J. Adolesc.32, 1189–1207 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Khor, S. P. H., McClure, A., Aldridge, G., Bei, B. & Yap, M. B. H. Modifiable parental factors in adolescent sleep: A systematic review and meta-analysis. Sleep Med. Rev.56, 101408 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Li, S. et al. Risk factors associated with short sleep duration among Chinese school-aged children. Sleep Med.11, 907–916 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Meltzer, L. J. & Mindell, J. A. Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. J. Fam. Psychol.21, 67–73 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Brand, S., Gerber, M., Hatzinger, M., Beck, J. & Holsboer-Trachsler, E. Evidence for similarities between adolescents and parents in sleep patterns. Sleep Med.10, 1124–1131 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Fuligni, A. J., Tsai, K. M., Krull, J. L. & Gonzales, N. A. Daily concordance between parent and adolescent sleep habits. J. Adolescent Health56, 244–250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouros, C. D. & El-Sheikh, M. Within-family relations in objective sleep duration, quality, and schedule. Child Dev.88, 1983–2000 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalak, N. et al. The relation of objective sleep patterns, depressive symptoms, and sleep disturbances in adolescent children and their parents: A sleep-EEG study with 47 families. J. Psychiatr. Res.46, 1374–1382 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Gradisar, M. et al. Sleep’s role in the development and resolution of adolescent depression. Nat. Rev. Psychol.1, 512–523 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short, M. A., Gradisar, M., Lack, L. C., Wright, H. & Carskadon, M. A. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med.13, 378–384 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Nilsen, S. A., Bergström, M., Sivertsen, B., Stormark, K. M. & Hysing, M. Sleep in adolescence: Considering family structure and family complexity. J. Marriage Family84, 1152–1174 (2022). [Google Scholar]
- 25.Johns, M. W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep14, 540–545 (1991). [DOI] [PubMed] [Google Scholar]
- 26.Lecendreux, M., Gamble, H., Sanchez-Garrido, L., Giordanella, J.-P. & Konofal, E. P03–389—An evaluation study of the Epworth sleepiness scale adapted for children and adolescents: A tool for case finding of pediatric excessive daytime sleepiness (EDS). Eur. Psychiatry26, 1559 (2011). [Google Scholar]
- 27.Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res.28, 193–213 (1989). [DOI] [PubMed] [Google Scholar]
- 28.Owens, J. A., Spirito, A. & McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep23, 1043–1051 (2000). [PubMed] [Google Scholar]
- 29.Radloff, L. S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas.1, 385–401 (1977). [Google Scholar]
- 30.Chabrol, H., Montovany, A., Chouicha, K. & Duconge, E. Study of the CES-D on a sample of 1,953 adolescent students. Encephale28, 429–432 (2002). [PubMed] [Google Scholar]
- 31.Meltzer, L. J., Walsh, C. M., Traylor, J. & Westin, A. M. L. Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep35, 159–166 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing (2023).
- 33.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 1–48 (2015). [Google Scholar]
- 34.Jeon, E. & Kim, N. Correspondence between parents’ and adolescents’ sleep duration. Int. J. Environ. Res. Public Health19, 1034 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai, S., Buxton, O. M., Master, L. & Hale, L. Daily associations between family interaction quality, stress, and objective sleep in adolescents. Sleep Health8, 69–72 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasser, J., Lecarie, E. K., Park, H. & Doane, L. D. Daily family connection and objective sleep in latinx adolescents: The moderating role of familism values and family communication. J. Youth Adolesc.50, 506–520 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauducco, S. V., Gardner, L. A., Champion, K., Newton, N. & Gradisar, M. It’s past your bedtime, but does it matter anymore? How longitudinal changes in bedtime rules relate to adolescents’ sleep. J. Sleep Res. n/a, e13940. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data can be made available upon request to the first author (ER) provided a written agreement of the study funder (the VINCI foundation).