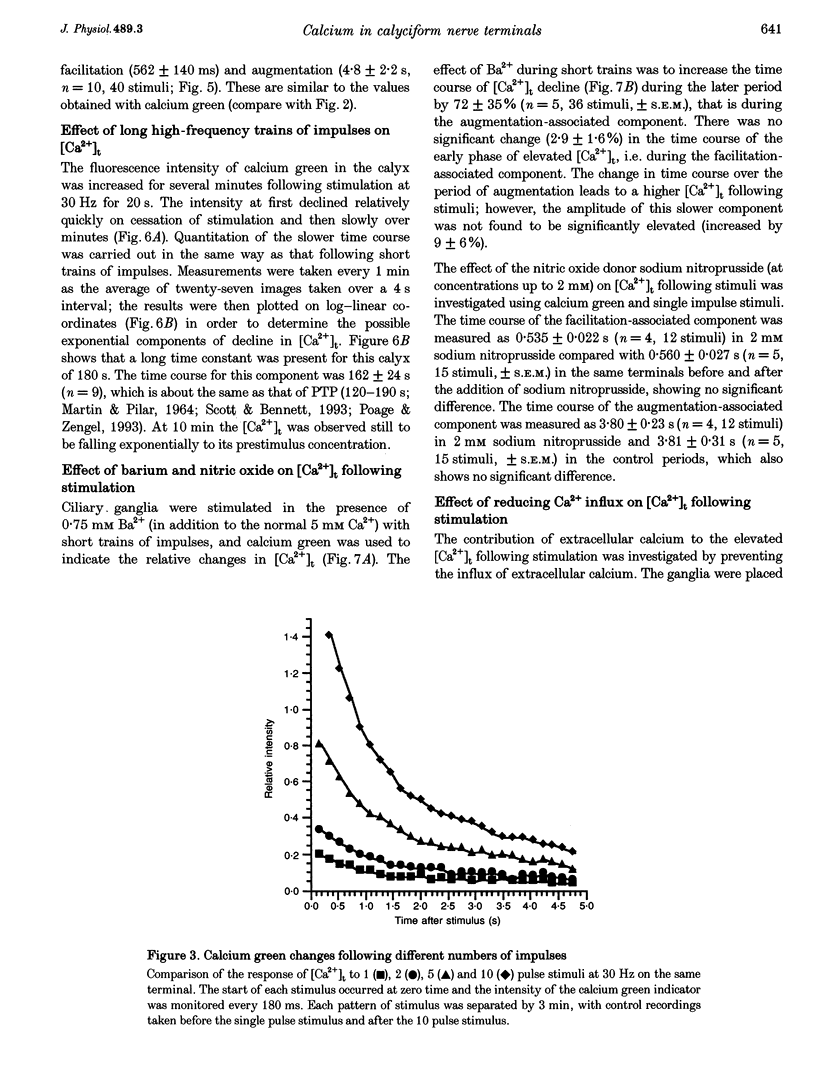
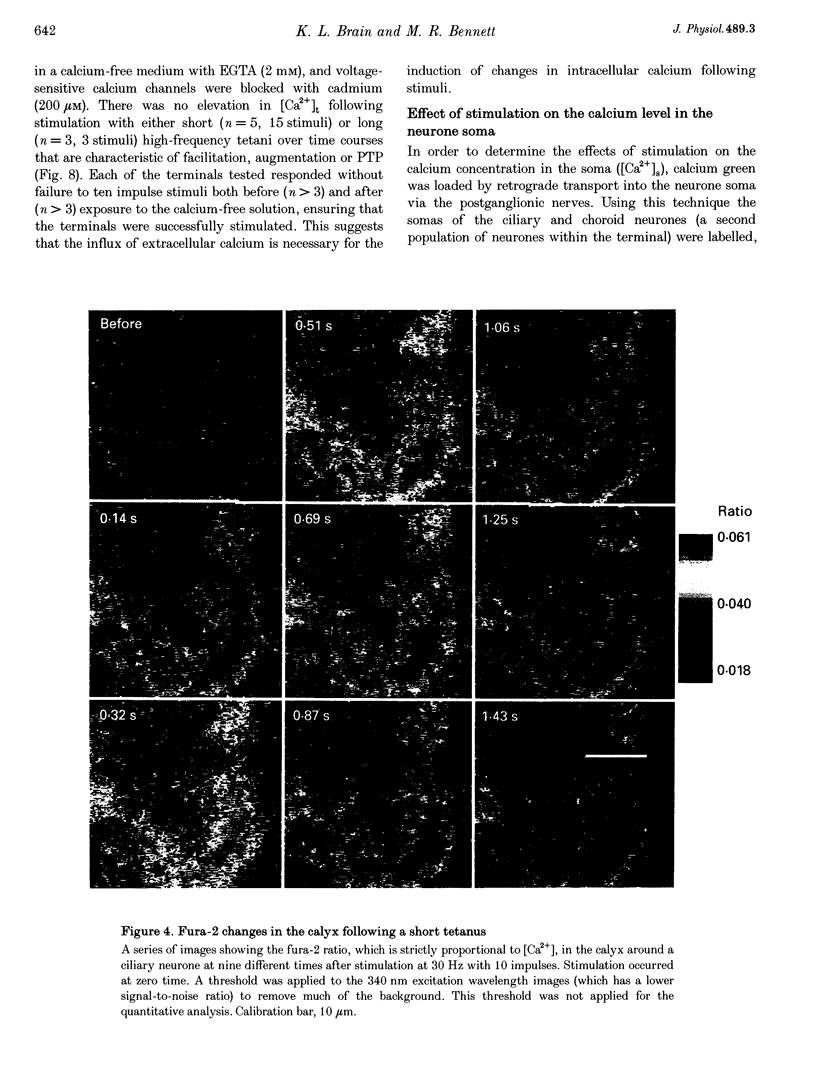
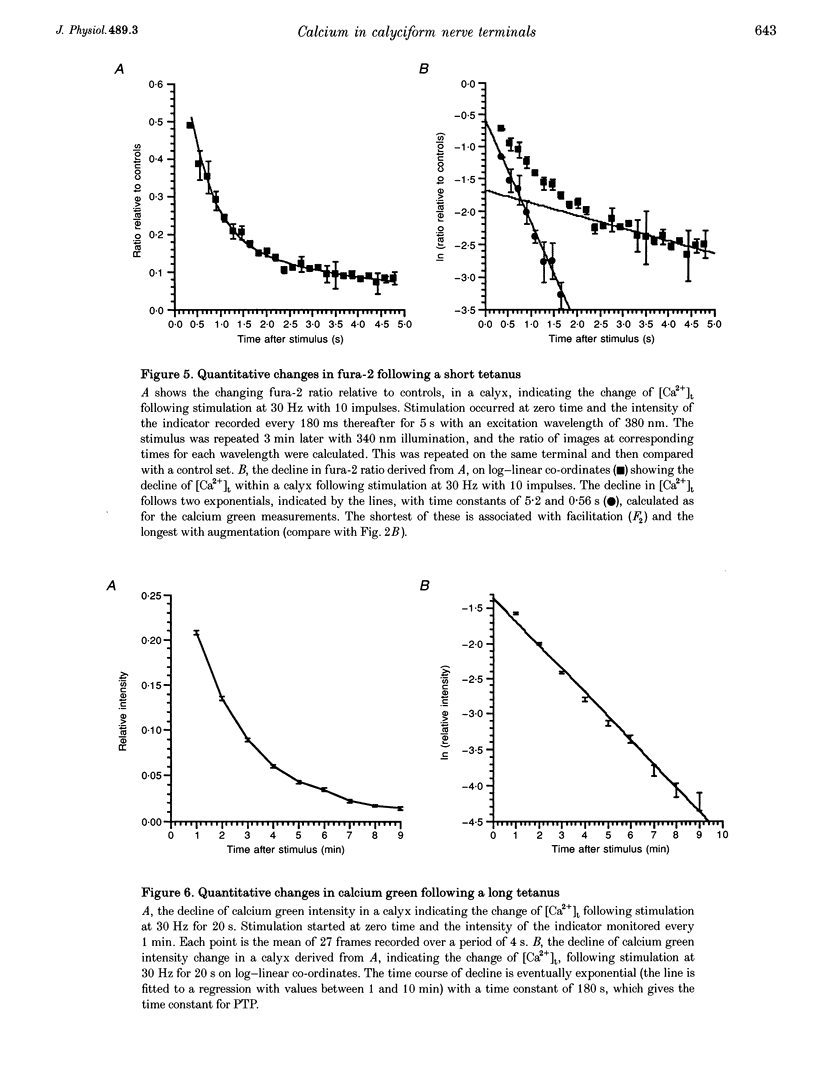
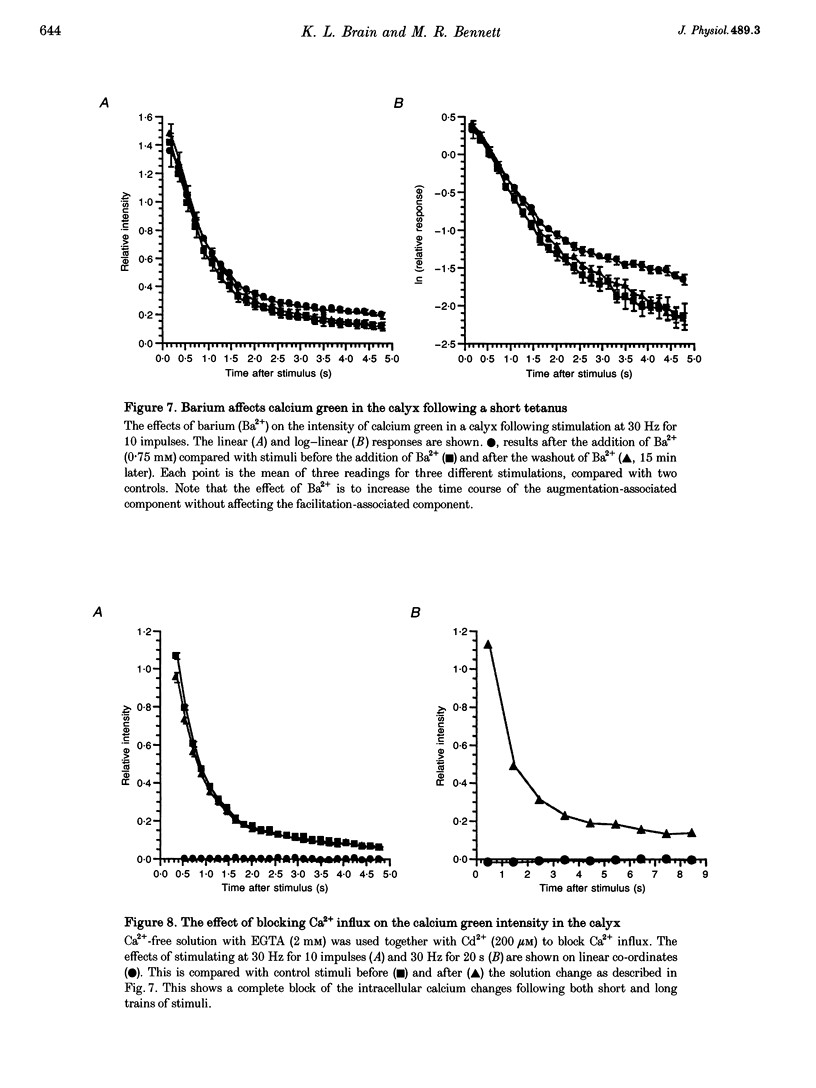
Abstract
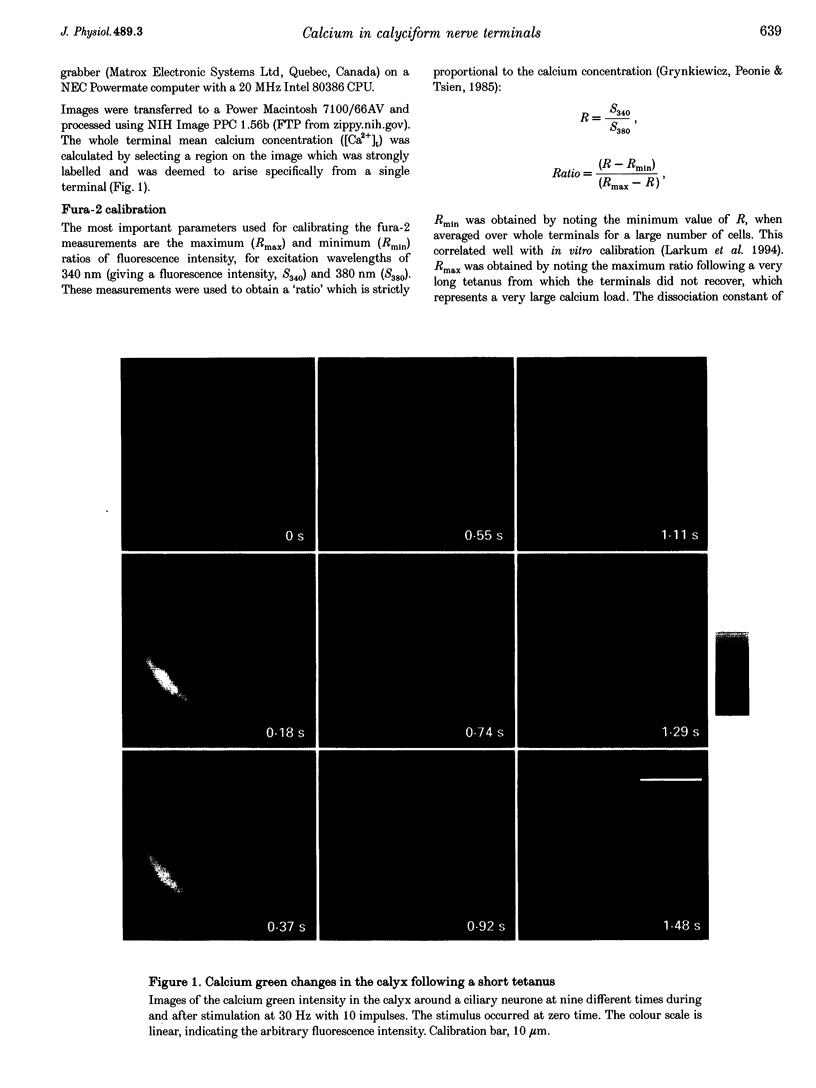
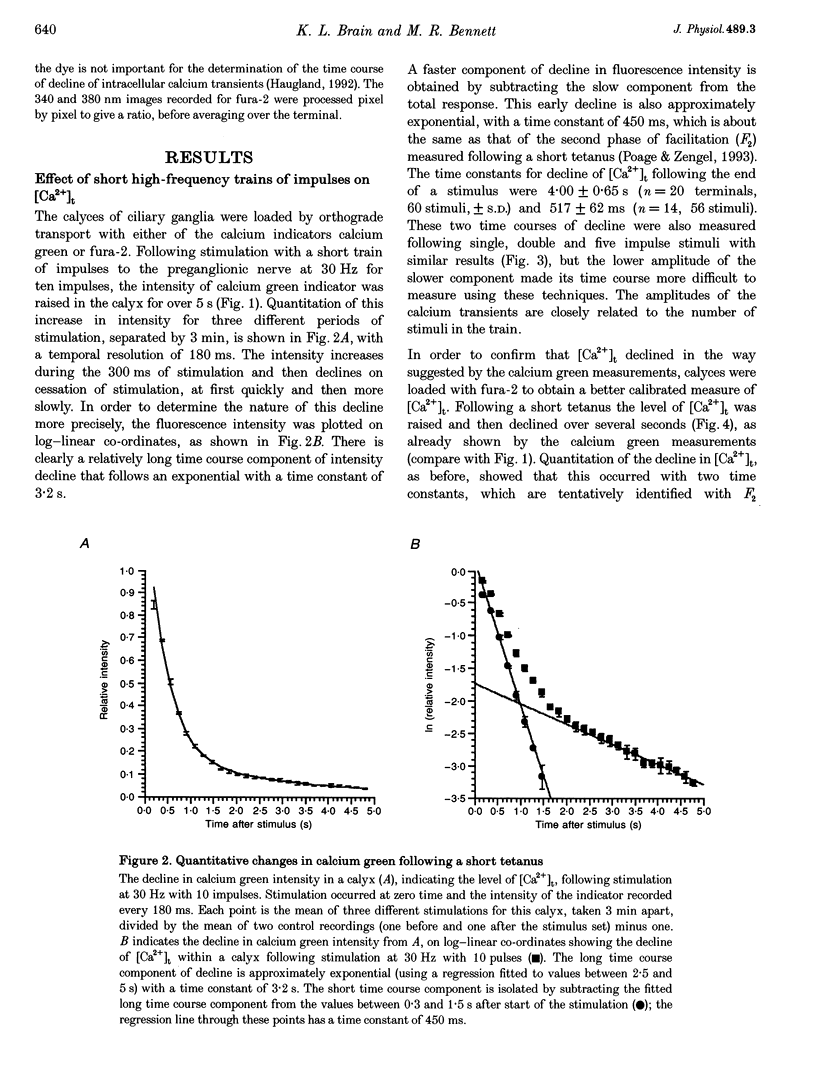
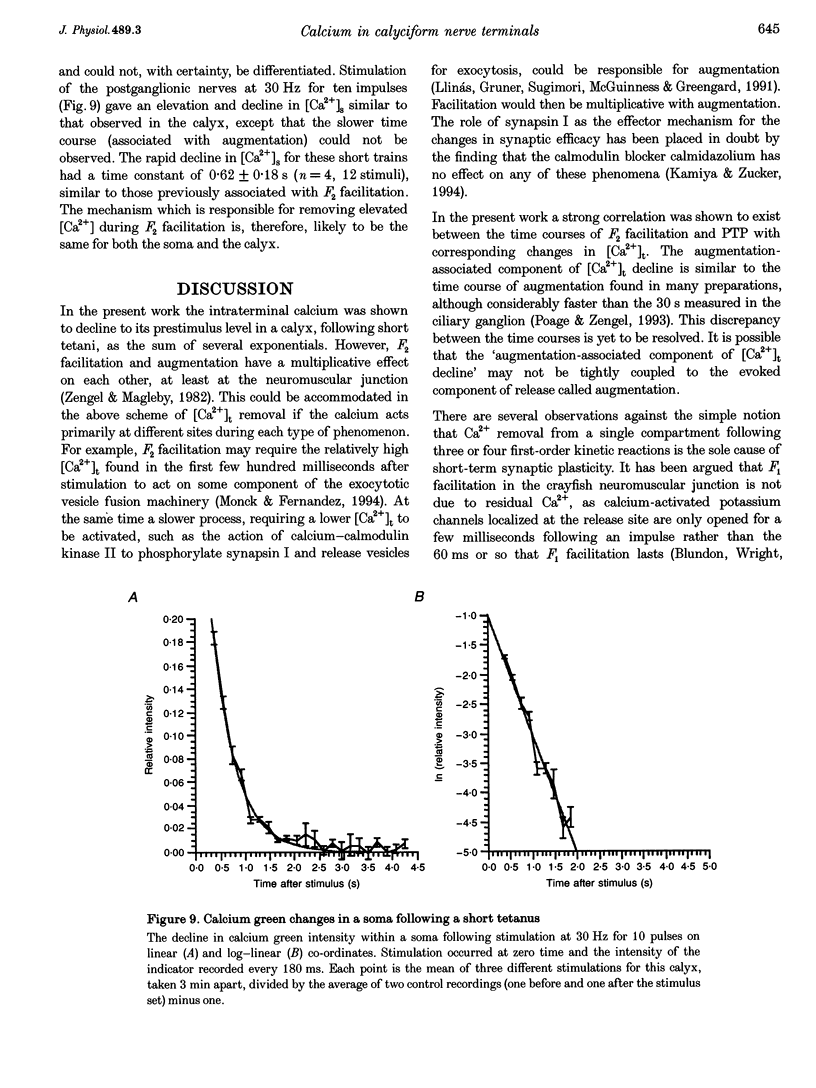
1. The calyciform nerve terminals of chick ciliary ganglia were loaded with the calcium indicators calcium green 1 or fura-2. These were used to determine the change in calcium concentration in the terminal, [Ca2+]t, following short (10 impulses) and long (600 impulses) trains of high-frequency (30 Hz) stimulation. 2. Following a single impulse or a short train, the elevated [Ca2+]t declined along two exponentials with time constants similar to slow (F2) facilitation (0.52 s) and augmentation (4.0 s). After a long train elevated [Ca2+]t declined eventually along a single exponential with the time constant of post-tetanic potentiation (162 s). [Ca2+]t was not elevated through long-term potentiation. 3. Addition of Ba2+ (0.75 mM) to the extracellular solution slowed only the decline of [Ca2+]t associated with augmentation. The addition of the nitric oxide donor sodium nitroprusside did not affect [Ca2+]t following short or long trains. 4. Removal of extracellular calcium (buffered with EGTA) and the blockade of calcium channels with Cd2+ completely prevented the changes in [Ca2+]t. 5. The soma of ciliary ganglion cells were loaded with calcium green and the postganglionic nerves stimulated with a single impulse or a short train of impulses. Following stimuli, the elevated [Ca2+]t declined along a single exponential with a time constant similar to F2 facilitation with no augmentation component evident. 6. The results are discussed in terms of the hypothesis that each impulse in a train gives an equal increment of residual Ca2+ to a compartment for secretion and that Ca2+ is removed from the compartment by three first-order kinetics processes associated with F2 facilitation, augmentation and post-tetanic potentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Augustine G. J., Duffy S. N., Charlton M. P. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991 Jun;11(6):1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain A. I., Quastel D. M. Multiplicative and additive Ca(2+)-dependent components of facilitation at mouse endplates. J Physiol. 1992 Sep;455:383–405. doi: 10.1113/jphysiol.1992.sp019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai A., Spanier R., Rahamimoff H. Isolation, purification, and reconstitution of the Na+ gradient-dependent Ca2+ transporter (Na+-Ca2+ exchanger) from brain synaptic plasma membranes. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6521–6525. doi: 10.1073/pnas.81.20.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R. Nitric oxide release and long term potentiation at synapses in autonomic ganglia. Gen Pharmacol. 1994 Dec;25(8):1541–1551. doi: 10.1016/0306-3623(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Blundon J. A., Wright S. N., Brodwick M. S., Bittner G. D. Residual free calcium is not responsible for facilitation of neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9388–9392. doi: 10.1073/pnas.90.20.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs C. A., Brown T. H., McAfee D. A. Neurophysiology and pharmacology of long-term potentiation in the rat sympathetic ganglion. J Physiol. 1985 Feb;359:503–521. doi: 10.1113/jphysiol.1985.sp015599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney K. R., Zucker R. S., Tank D. W. Calcium in motor nerve terminals associated with posttetanic potentiation. J Neurosci. 1989 Oct;9(10):3558–3567. doi: 10.1523/JNEUROSCI.09-10-03558.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunant Y., Dolivo M. Plasticity of synaptic functions in the exised sympathetic ganglion of the rat. Brain Res. 1968 Aug 26;10(2):271–273. doi: 10.1016/0006-8993(68)90134-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Kamiya H., Zucker R. S. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994 Oct 13;371(6498):603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Kuba K., Kumamoto E. Long-term potentiations in vertebrate synapses: a variety of cascades with common subprocesses. Prog Neurobiol. 1990;34(3):197–269. doi: 10.1016/0301-0082(90)90012-6. [DOI] [PubMed] [Google Scholar]
- Landò L., Zucker R. S. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J Neurophysiol. 1994 Aug;72(2):825–830. doi: 10.1152/jn.1994.72.2.825. [DOI] [PubMed] [Google Scholar]
- Larkum M. E., Warren D. A., Bennett M. R. Calcium concentration changes in the calyciform nerve terminal of the avian ciliary ganglion after tetanic stimulation. J Auton Nerv Syst. 1994 Mar;46(3):175–188. doi: 10.1016/0165-1838(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Lin Y. Q., Bennett M. R. Nitric oxide modulation of quantal secretion in chick ciliary ganglia. J Physiol. 1994 Dec 1;481(Pt 2):385–394. doi: 10.1113/jphysiol.1994.sp020447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Gruner J. A., Sugimori M., McGuinness T. L., Greengard P. Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol. 1991 May;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. PRESYNAPTIC AND POST-SYNAPTIC EVENTS DURING POST-TETANIC POTENTIATION AND FACILITATION IN THE AVIAN CILIARY GANGLION. J Physiol. 1964 Dec;175:17–30. doi: 10.1113/jphysiol.1964.sp007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. A quantitative description of tetanic and post-tetanic potentiation of transmitter release at the frog neuromuscular junction. J Physiol. 1975 Feb;245(1):183–208. doi: 10.1113/jphysiol.1975.sp010840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Zengel J. E. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol. 1976 May;257(2):449–470. doi: 10.1113/jphysiol.1976.sp011378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minota S., Kumamoto E., Kitakoga O., Kuba K. Long-term potentiation induced by a sustained rise in the intraterminal Ca2+ in bull-frog sympathetic ganglia. J Physiol. 1991 Apr;435:421–438. doi: 10.1113/jphysiol.1991.sp018517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monck J. R., Fernandez J. M. The exocytotic fusion pore and neurotransmitter release. Neuron. 1994 Apr;12(4):707–716. doi: 10.1016/0896-6273(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Mulkey R. M., Zucker R. S. Posttetanic potentiation at the crayfish neuromuscular junction is dependent on both intracellular calcium and sodium ion accumulation. J Neurosci. 1992 Nov;12(11):4327–4336. doi: 10.1523/JNEUROSCI.12-11-04327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K. A., Chan N., Davey D. F., Bennett M. R. Location of nitric oxide synthase in the developing avian ciliary ganglion. J Auton Nerv Syst. 1995 Feb 9;51(2):91–102. doi: 10.1016/0165-1838(94)00116-2. [DOI] [PubMed] [Google Scholar]
- Peng Y. Y., Zucker R. S. Release of LHRH is linearly related to the time integral of presynaptic Ca2+ elevation above a threshold level in bullfrog sympathetic ganglia. Neuron. 1993 Mar;10(3):465–473. doi: 10.1016/0896-6273(93)90334-n. [DOI] [PubMed] [Google Scholar]
- Poage R. E., Zengel J. E. Kinetic and pharmacological examination of stimulation-induced increases in synaptic efficacy in the chick ciliary ganglion. Synapse. 1993 May;14(1):81–89. doi: 10.1002/syn.890140111. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Delaney K. R., Tank D. W. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994 Feb;14(2):523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Tank D. W. The maintenance of LTP at hippocampal mossy fiber synapses is independent of sustained presynaptic calcium. Neuron. 1991 Sep;7(3):451–459. doi: 10.1016/0896-6273(91)90297-d. [DOI] [PubMed] [Google Scholar]
- Scott T. R., Bennett M. R. The effect of ions and second messengers on long-term potentiation of chemical transmission in avian ciliary ganglia. Br J Pharmacol. 1993 Sep;110(1):461–469. doi: 10.1111/j.1476-5381.1993.tb13833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swandulla D., Hans M., Zipser K., Augustine G. J. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991 Dec;7(6):915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- Tanabe N., Kijima H. Ca(2+)-dependent and -independent components of transmitter release at the frog neuromuscular junction. J Physiol. 1992 Sep;455:271–289. doi: 10.1113/jphysiol.1992.sp019301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. G., Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994 Feb;14(2):645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H., Chuhma N. Preferential inhibition of omega-conotoxin-sensitive presynaptic Ca2+ channels by adenosine autoreceptors. Nature. 1993 Sep 16;365(6443):256–258. doi: 10.1038/365256a0. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Augmentation and facilitation of transmitter release. A quantitative description at the frog neuromuscular junction. J Gen Physiol. 1982 Oct;80(4):583–611. doi: 10.1085/jgp.80.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L., Horn J. P., McAfee D. A., Yarowsky P. J. Facilitation, augmentation, and potentiation of synaptic transmission at the superior cervical ganglion of the rabbit. J Gen Physiol. 1980 Aug;76(2):213–231. doi: 10.1085/jgp.76.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]