Abstract
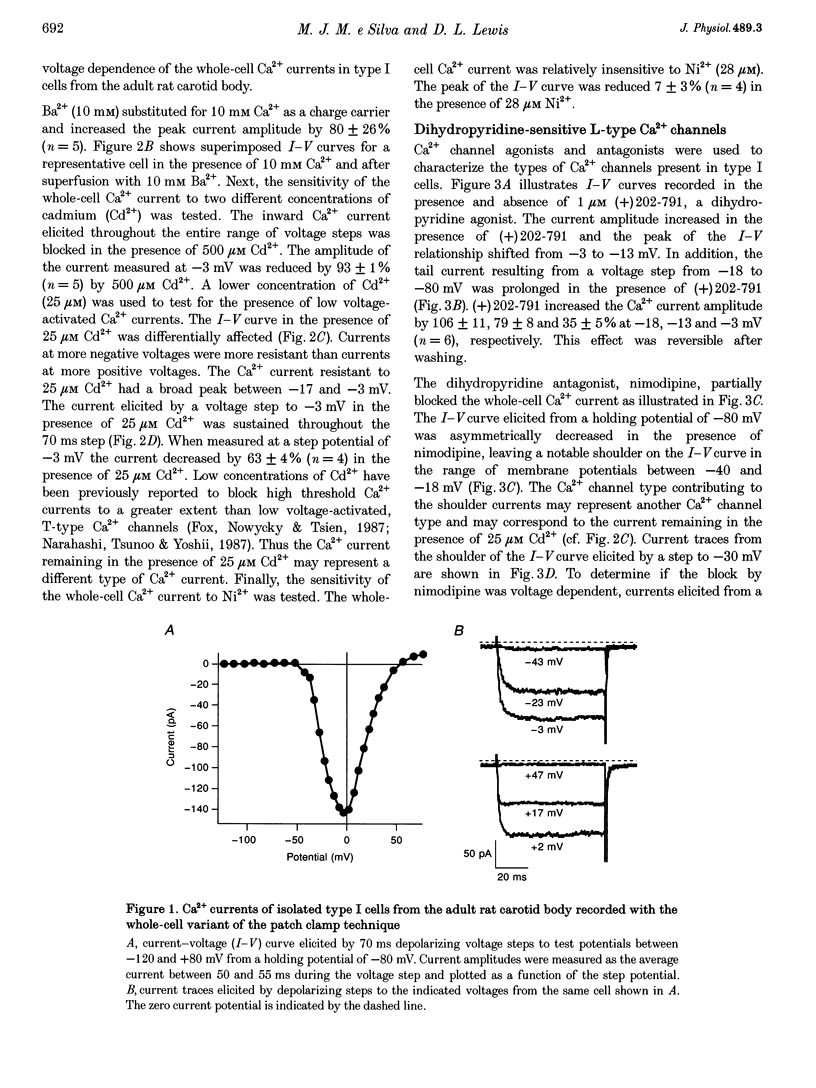
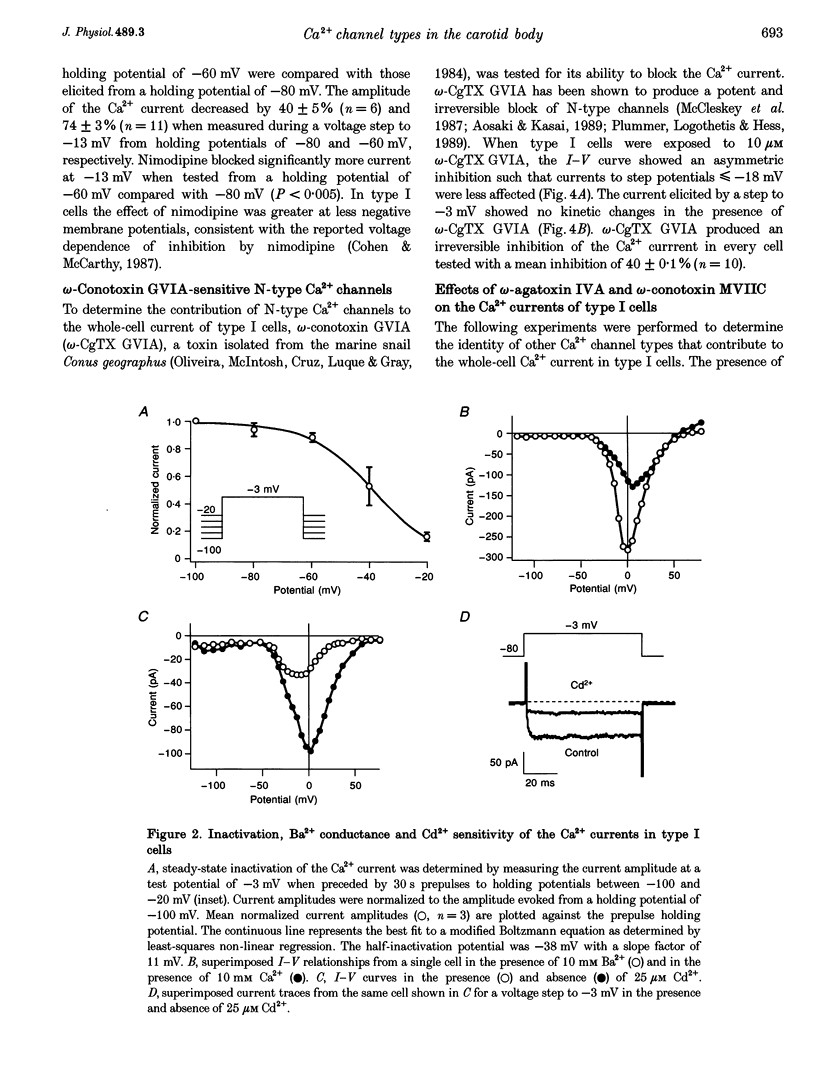
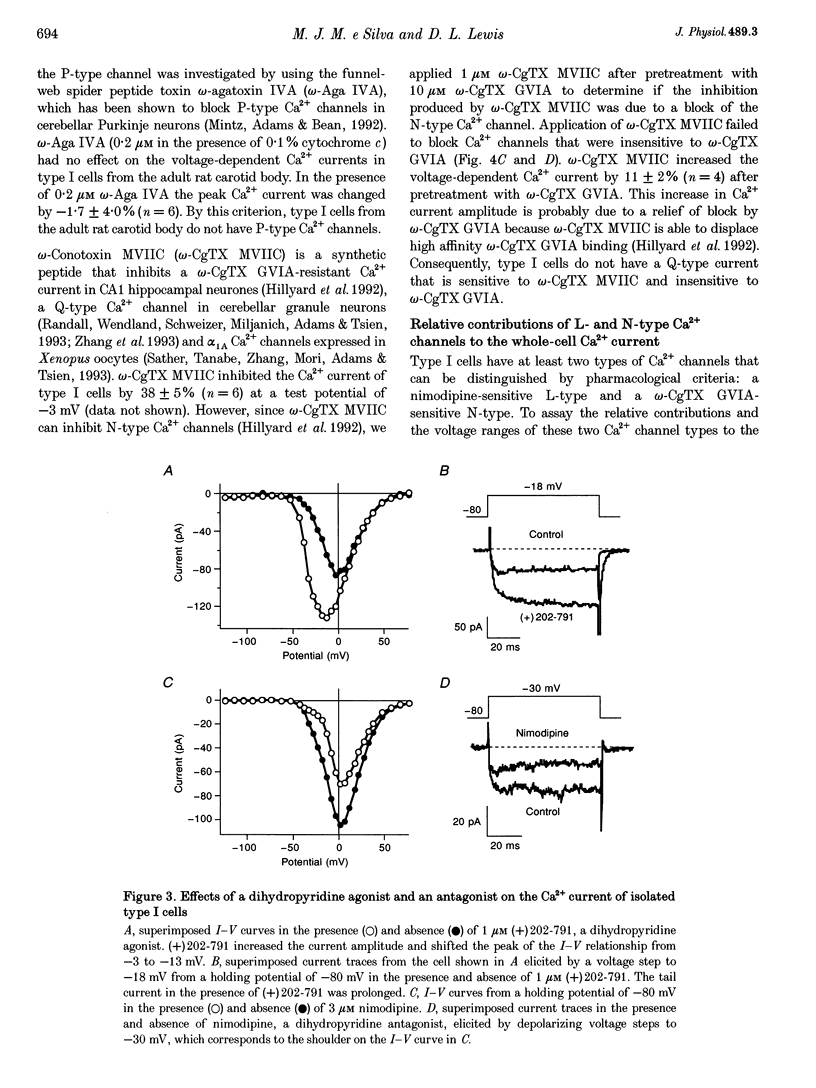
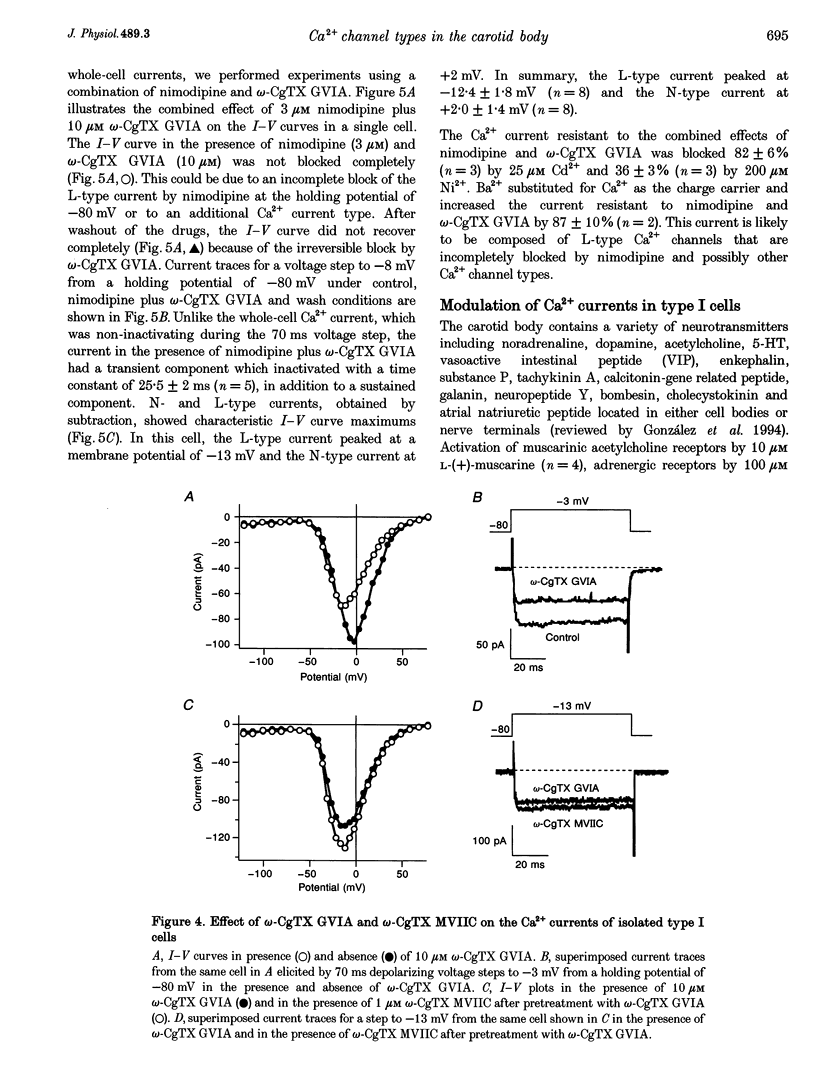
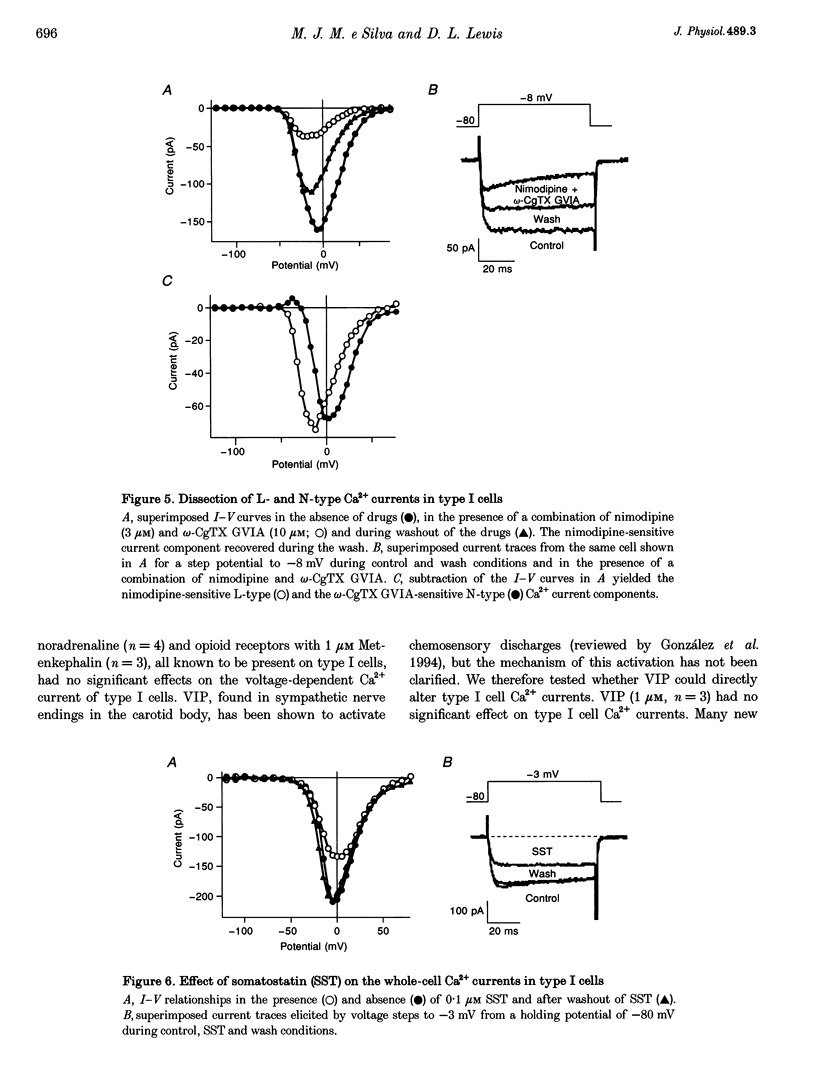
1. Whole-cell voltage-dependent Ca2+ currents recorded from chemoreceptor type I cells of the adult rat carotid body had maximum amplitudes of -94 pA in 10 mM Ca2+ and were half-inactivated at a holding potential of -38 mV. Somatostatin and dopamine inhibited whole-cell Ca2+ current in type I cells. 2. The dihydropyridine agonist (+)202-791 increased the Ca2+ current amplitude by 106% at a step potential of -18 mV. The dihydropyridine antagonist nimodipine decreased the Ca2+ current amplitude by 40% from a holding potential of -80 mV, and by 74% from a holding potential of -60 mV. The nimodipine-sensitive current had a maximum amplitude at a membrane potential of -12 mV. omega-Conotoxin GVIA (omega-CgTX GVIA) blocked the whole-cell Ca2+ current by 40%. The omega-CgTX GVIA-sensitive current had a maximum amplitude at a membrane potential of +2 mV. 3. In summary, type I cells of the adult rat carotid body have dihydropyridine-sensitive L-type and omega-conotoxin GVIA-sensitive N-type voltage-dependent Ca2+ channels. These channels may play a role in the voltage-gated entry of Ca2+ necessary for stimulus-secretion coupling in response to changes in arterial PO2, PCO2 and pH. Inhibition of the Ca2+ currents by somatostatin and dopamine may alter the chemotransduction signal in type I cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aosaki T., Kasai H. Characterization of two kinds of high-voltage-activated Ca-channel currents in chick sensory neurons. Differential sensitivity to dihydropyridines and omega-conotoxin GVIA. Pflugers Arch. 1989 Jun;414(2):150–156. doi: 10.1007/BF00580957. [DOI] [PubMed] [Google Scholar]
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Benot A. R., López-Barneo J. Feedback Inhibition of Ca2+ Currents by Dopamine in Glomus Cells of the Carotid Body. Eur J Neurosci. 1990;2(9):809–812. doi: 10.1111/j.1460-9568.1990.tb00473.x. [DOI] [PubMed] [Google Scholar]
- Biscoe T. J. Carotid body: structure and function. Physiol Rev. 1971 Jul;51(3):437–495. doi: 10.1152/physrev.1971.51.3.437. [DOI] [PubMed] [Google Scholar]
- Buckler K. J., Vaughan-Jones R. D. Effects of hypercapnia on membrane potential and intracellular calcium in rat carotid body type I cells. J Physiol. 1994 Jul 1;478(Pt 1):157–171. doi: 10.1113/jphysiol.1994.sp020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., McCarthy R. T. Nimodipine block of calcium channels in rat anterior pituitary cells. J Physiol. 1987 Jun;387:195–225. doi: 10.1113/jphysiol.1987.sp016570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpiano M. A., Hescheler J. Evidence for a PO2-sensitive K+ channel in the type-I cell of the rabbit carotid body. FEBS Lett. 1989 Jun 5;249(2):195–198. doi: 10.1016/0014-5793(89)80623-4. [DOI] [PubMed] [Google Scholar]
- Duchen M. R., Caddy K. W., Kirby G. C., Patterson D. L., Ponte J., Biscoe T. J. Biophysical studies of the cellular elements of the rabbit carotid body. Neuroscience. 1988 Jul;26(1):291–311. doi: 10.1016/0306-4522(88)90146-7. [DOI] [PubMed] [Google Scholar]
- Fidone S., Gonzalez C., Yoshizaki K. Effects of low oxygen on the release of dopamine from the rabbit carotid body in vitro. J Physiol. 1982 Dec;333:93–110. doi: 10.1113/jphysiol.1982.sp014441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieber L. A., McCleskey E. W. L-type calcium channels in type I cells of the rat carotid body. J Neurophysiol. 1993 Oct;70(4):1378–1384. doi: 10.1152/jn.1993.70.4.1378. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M. D., López-Barneo J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2927–2930. doi: 10.1073/pnas.88.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C., Almaraz L., Obeso A., Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994 Oct;74(4):829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Grönblad M., Akerman K. E., Eränkö O. Induction of exocytosis from glomus cells by incubation of the carotid body of the rat with calcium and ionophore A23187. Anat Rec. 1979 Oct;195(2):387–395. doi: 10.1002/ar.1091950211. [DOI] [PubMed] [Google Scholar]
- Gómez-Niño A., López-López J. R., Almaraz L., González C. Inhibition of [3H]catecholamine release and Ca2+ currents by prostaglandin E2 in rabbit carotid body chemoreceptor cells. J Physiol. 1994 Apr 15;476(2):269–277. doi: 10.1113/jphysiol.1994.sp020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Delpiano M. A., Acker H., Pietruschka F. Ionic currents on type-I cells of the rabbit carotid body measured by voltage-clamp experiments and the effect of hypoxia. Brain Res. 1989 May 1;486(1):79–88. doi: 10.1016/0006-8993(89)91280-8. [DOI] [PubMed] [Google Scholar]
- Hillyard D. R., Monje V. D., Mintz I. M., Bean B. P., Nadasdi L., Ramachandran J., Miljanich G., Azimi-Zoonooz A., McIntosh J. M., Cruz L. J. A new Conus peptide ligand for mammalian presynaptic Ca2+ channels. Neuron. 1992 Jul;9(1):69–77. doi: 10.1016/0896-6273(92)90221-x. [DOI] [PubMed] [Google Scholar]
- Ikeda S. R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurones. J Physiol. 1991 Aug;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Barneo J., López-López J. R., Ureña J., González C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science. 1988 Jul 29;241(4865):580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- López-López J., González C., Ureña J., López-Barneo J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):1001–1015. doi: 10.1085/jgp.93.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey E. W., Fox A. P., Feldman D. H., Cruz L. J., Olivera B. M., Tsien R. W., Yoshikami D. Omega-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Monti-Bloch L., Eyzaguirre C. A comparative physiological and pharmacological study of cat and rabbit carotid body chemoreceptors. Brain Res. 1980 Jul 14;193(2):449–470. doi: 10.1016/0006-8993(80)90177-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Tsunoo A., Yoshii M. Characterization of two types of calcium channels in mouse neuroblastoma cells. J Physiol. 1987 Feb;383:231–249. doi: 10.1113/jphysiol.1987.sp016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse C. A. Carbonic anhydrase and neuronal enzymes in cultured glomus cells of the carotid body of the rat. Cell Tissue Res. 1990 Jul;261(1):65–71. doi: 10.1007/BF00329439. [DOI] [PubMed] [Google Scholar]
- Obeso A., Rocher A., Fidone S., Gonzalez C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience. 1992;47(2):463–472. doi: 10.1016/0306-4522(92)90260-9. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Plummer M. R., Logothetis D. E., Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989 May;2(5):1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. T., Obeso A., López-López J. R., Herreros B., González C. Characterization of cultured chemoreceptor cells dissociated from adult rabbit carotid body. Am J Physiol. 1992 Dec;263(6 Pt 1):C1152–C1159. doi: 10.1152/ajpcell.1992.263.6.C1152. [DOI] [PubMed] [Google Scholar]
- Sather W. A., Tanabe T., Zhang J. F., Mori Y., Adams M. E., Tsien R. W. Distinctive biophysical and pharmacological properties of class A (BI) calcium channel alpha 1 subunits. Neuron. 1993 Aug;11(2):291–303. doi: 10.1016/0896-6273(93)90185-t. [DOI] [PubMed] [Google Scholar]
- Southam E., Garthwaite J. Comparative effects of some nitric oxide donors on cyclic GMP levels in rat cerebellar slices. Neurosci Lett. 1991 Sep 2;130(1):107–111. doi: 10.1016/0304-3940(91)90239-p. [DOI] [PubMed] [Google Scholar]
- Ureña J., López-López J., González C., López-Barneo J. Ionic currents in dispersed chemoreceptor cells of the mammalian carotid body. J Gen Physiol. 1989 May;93(5):979–999. doi: 10.1085/jgp.93.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. R. Nitric oxide: a radical neurotransmitter in the central nervous system. Prog Neurobiol. 1994 Jan;42(1):129–160. doi: 10.1016/0301-0082(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wang Z. Z., Stensaas L. J., Bredt D. S., Dinger B., Fidone S. J. Localization and actions of nitric oxide in the cat carotid body. Neuroscience. 1994 May;60(1):275–286. doi: 10.1016/0306-4522(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]


