Abstract
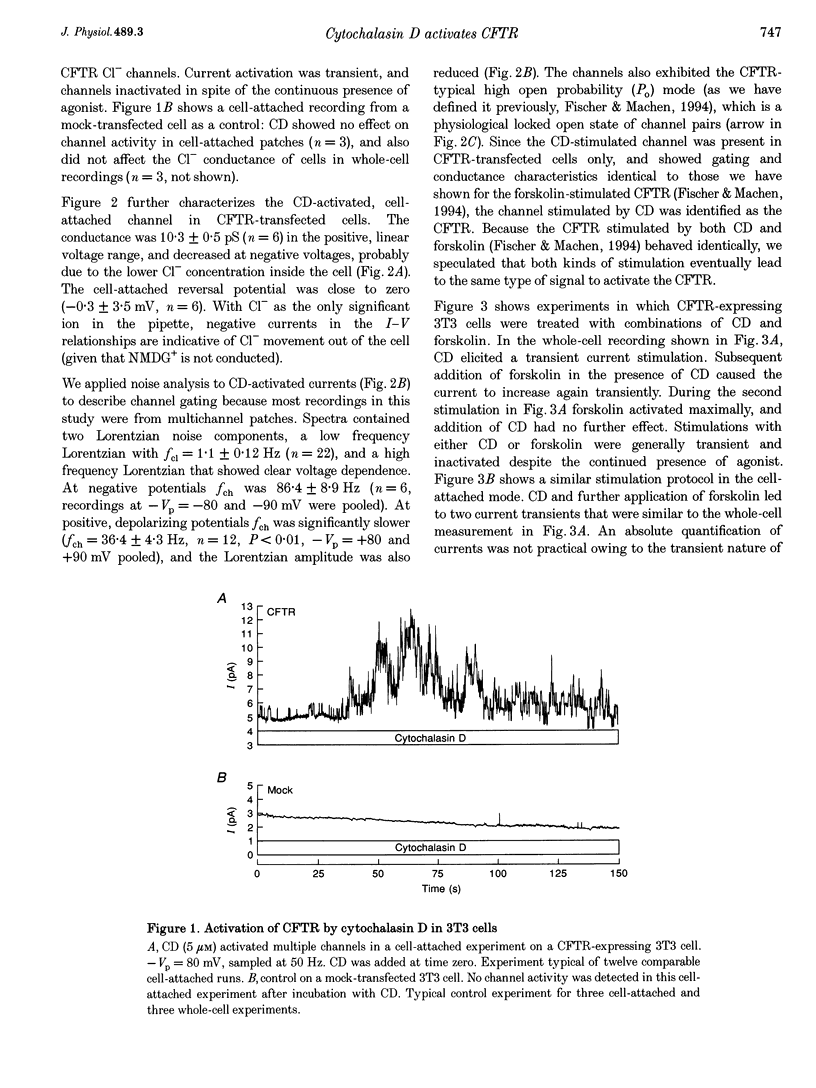
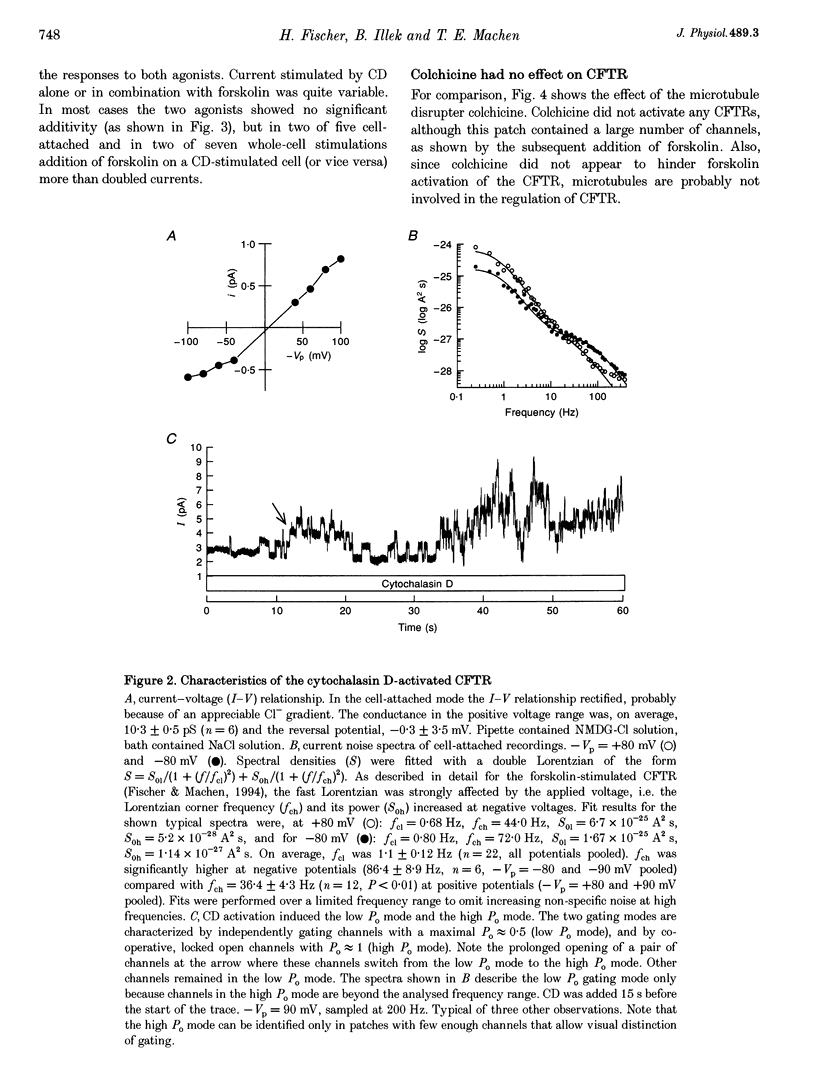
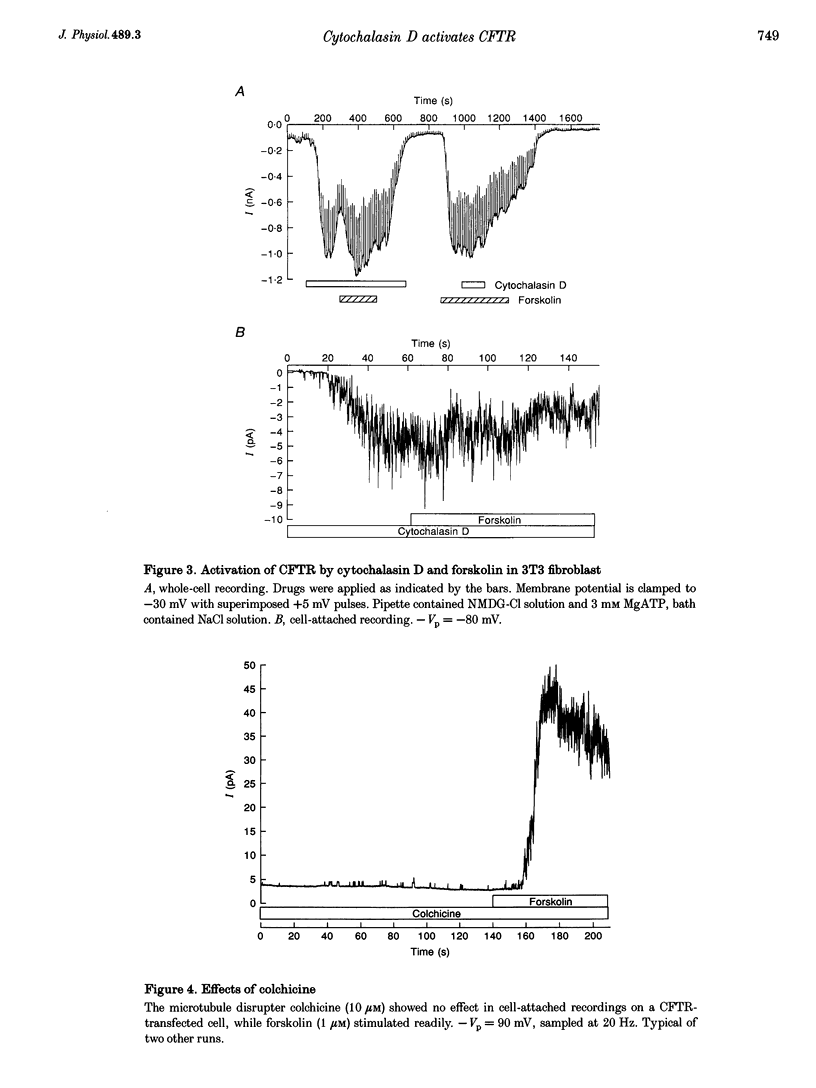
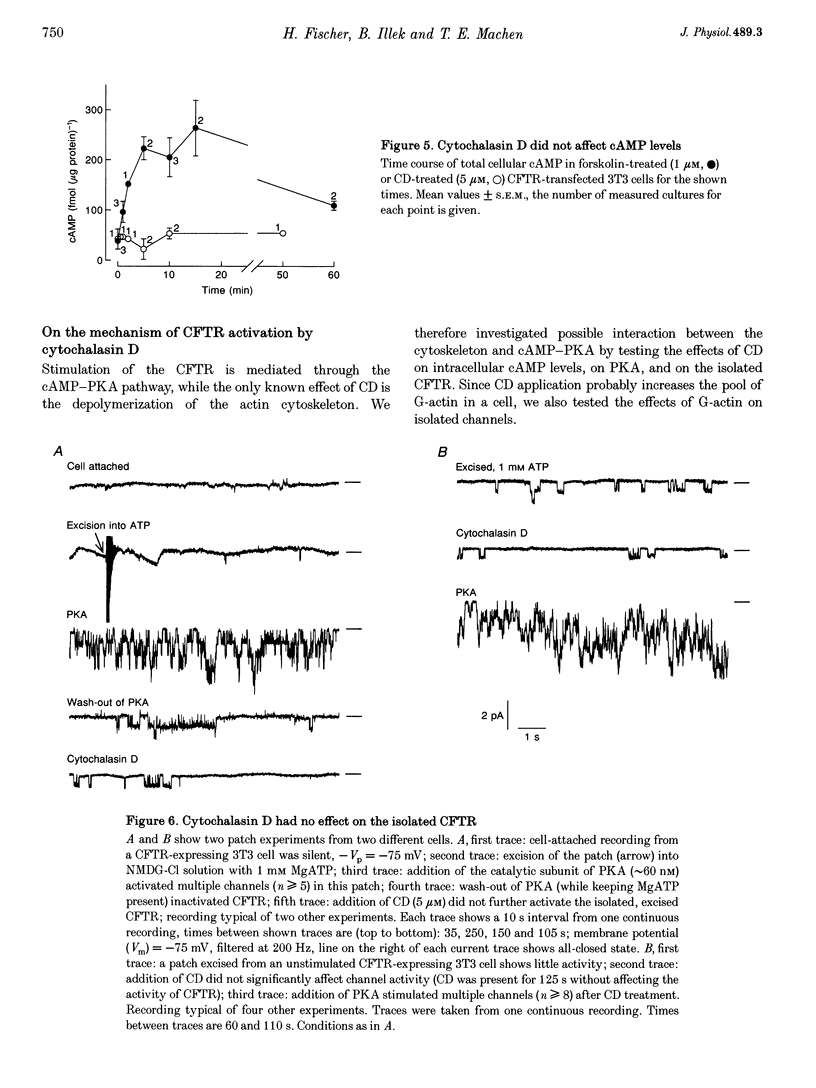
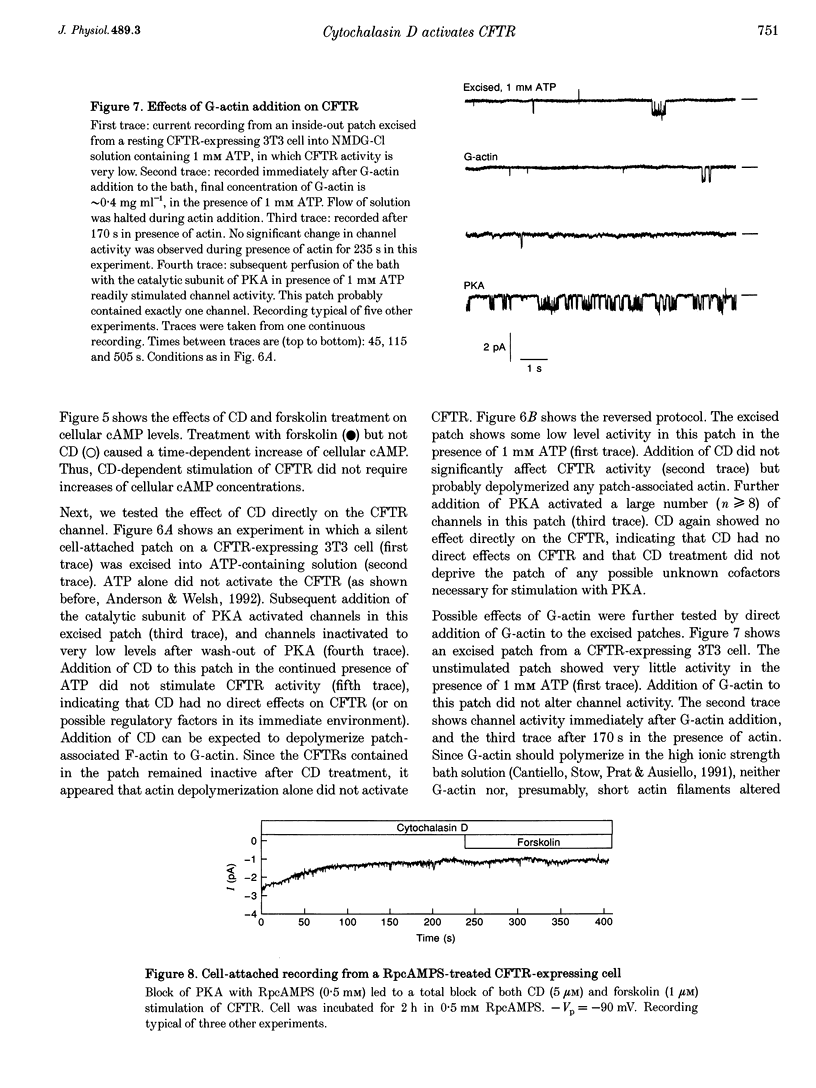
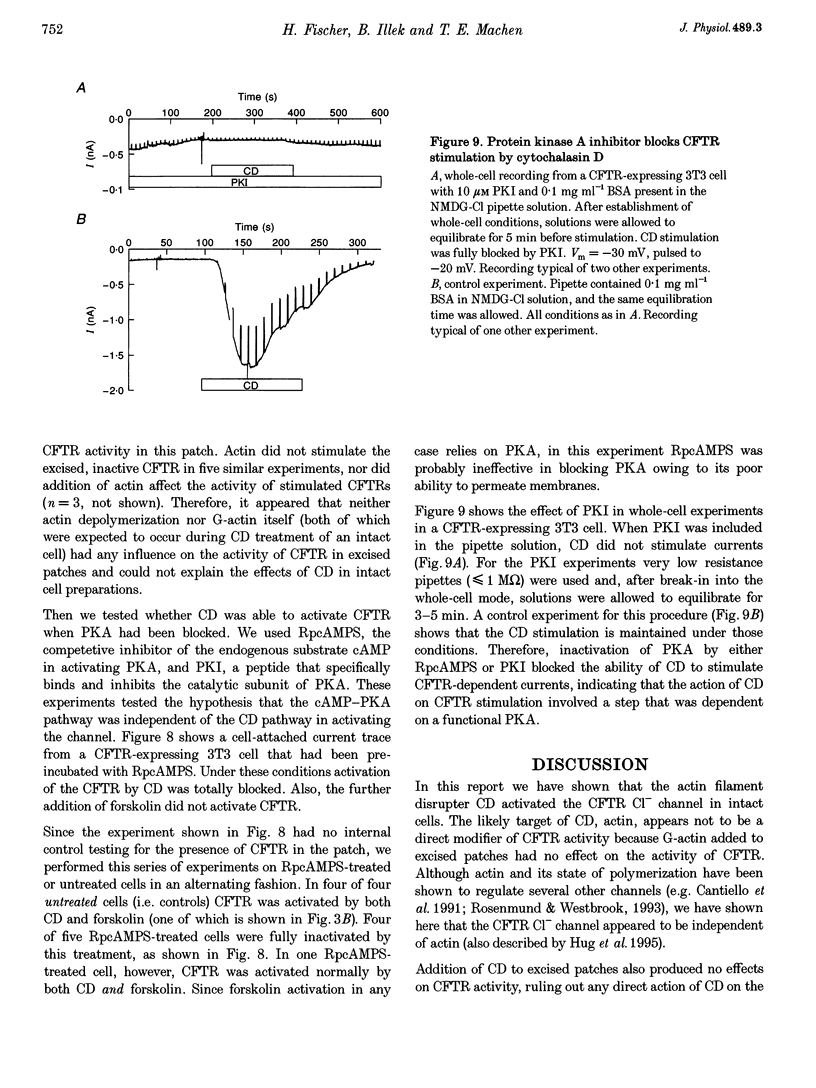
1. Cytochalasin D (CD; 5 microM) readily stimulated cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel activity in cell-attached and whole-cell patch recordings from 3T3 fibroblasts expressing recombinant CFTR but not in mock-transfected cells. CD-stimulated currents were indistinguishable from those evoked by forskolin stimulation. Kinetic analysis of CFTR gating showed identical channel behaviour independent of the agonist used. 2. To elucidate the mechanism of action of CD we tested its effects on cAMP, protein kinase A, and the CFTR itself during CD stimulation. In contrast to forskolin treatment, CD did not increase cellular cAMP content. 3. A direct interaction of CD with the CFTR was ruled out because CD showed no effect on CFTR in excised inside-out patches. 4. CD effects were fully blocked when the cellular protein kinase A was inhibited by treatment of cells with RpcAMPS in cell-attached patches or when protein kinase inhibitor peptide was dialysed into cells in whole-cell experiments. 5. Addition of G-actin to excised patches had no effects on CFTR. 6. We conclude that the stimulatory effect of CD is cAMP independent, but needs a functional protein kinase A in order to activate the CFTR. We propose that cytochalasin D activates CFTR by releasing a cellular inhibitor, e.g. a phosphatase, that is held in place by F-actin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Gregory R. J., Thompson S., Souza D. W., Paul S., Mulligan R. C., Smith A. E., Welsh M. J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991 Jul 12;253(5016):202–205. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science. 1992 Sep 18;257(5077):1701–1704. doi: 10.1126/science.1382316. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T., Hwang T. C., Nairn A. C., Gadsby D. C. Coupling of CFTR Cl- channel gating to an ATP hydrolysis cycle. Neuron. 1994 Mar;12(3):473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Becq F., Jensen T. J., Chang X. B., Savoia A., Rommens J. M., Tsui L. C., Buchwald M., Riordan J. R., Hanrahan J. W. Phosphatase inhibitors activate normal and defective CFTR chloride channels. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):9160–9164. doi: 10.1073/pnas.91.19.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. A., Travis S. M., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993 Jan 25;268(3):2037–2047. [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992 Apr 24;256(5056):530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Cantiello H. F., Stow J. L., Prat A. G., Ausiello D. A. Actin filaments regulate epithelial Na+ channel activity. Am J Physiol. 1991 Nov;261(5 Pt 1):C882–C888. doi: 10.1152/ajpcell.1991.261.5.C882. [DOI] [PubMed] [Google Scholar]
- Cheng S. H., Rich D. P., Marshall J., Gregory R. J., Welsh M. J., Smith A. E. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991 Sep 6;66(5):1027–1036. doi: 10.1016/0092-8674(91)90446-6. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H., Machen T. E. CFTR displays voltage dependence and two gating modes during stimulation. J Gen Physiol. 1994 Sep;104(3):541–566. doi: 10.1085/jgp.104.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T., Kondo M., Mochizuki H., Verkman A. S., Widdicombe J. H. Calcium-dependent regulation of Cl secretion in tracheal epithelium. Am J Physiol. 1992 Feb;262(2 Pt 1):L163–L168. doi: 10.1152/ajplung.1992.262.2.L163. [DOI] [PubMed] [Google Scholar]
- Hug T., Koslowsky T., Ecke D., Greger R., Kunzelmann K. Actin-dependent activation of ion conductances in bronchial epithelial cells. Pflugers Arch. 1995 Mar;429(5):682–690. doi: 10.1007/BF00373989. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Gadsby D. C. Functionally distinct phospho-forms underlie incremental activation of protein kinase-regulated Cl- conductance in mammalian heart. J Gen Physiol. 1993 May;101(5):629–650. doi: 10.1085/jgp.101.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illek B., Fischer H., Santos G. F., Widdicombe J. H., Machen T. E., Reenstra W. W. cAMP-independent activation of CFTR Cl channels by the tyrosine kinase inhibitor genistein. Am J Physiol. 1995 Apr;268(4 Pt 1):C886–C893. doi: 10.1152/ajpcell.1995.268.4.C886. [DOI] [PubMed] [Google Scholar]
- Prat A. G., Xiao Y. F., Ausiello D. A., Cantiello H. F. cAMP-independent regulation of CFTR by the actin cytoskeleton. Am J Physiol. 1995 Jun;268(6 Pt 1):C1552–C1561. doi: 10.1152/ajpcell.1995.268.6.C1552. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Westbrook G. L. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993 May;10(5):805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Schultz B. D., Frizzell R. A., Bridges R. J. ATP alters current fluctuations of cystic fibrosis transmembrane conductance regulator: evidence for a three-state activation mechanism. J Gen Physiol. 1994 Jul;104(1):123–146. doi: 10.1085/jgp.104.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]


