Abstract
The present investigation aimed to assess the safety of photobiomodulation (PBM) on the oral carcinogenesis process induced by 4NQO, focusing on cell proliferation and apoptosis. Sixty-six Wistar rats received systemic 4NQO for 12 (n = 33) and 20 weeks (n = 33), divided into Control group, PBM 0.3 J, and PBM 1 J. Applications for PBM occurred three times a week. At weeks 12 and 20, the animals were euthanized. The immunoreactivity for anti-ROS1 and anti-p53 antibodies was also assessed. Statistical analysis was assessed by multiple t-tests, Kruskal-Wallis, and Spearman’s correlation. At 12 weeks, PBM 1 J group had nodular lesions, distinct from control and PBM 0.3 J groups (p = 0.005). At 20 weeks, nodular lesions were common in control and PBM 0.3 J groups. Histopathological characteristics did not significantly differ between groups at 12 (p = 0.30) and 20 weeks (p = 0.58). Epithelial dysplasia (n = 21) was common at 12 weeks. After 20 weeks, most of the cases revealed squamous cell carcinoma (n = 24). No differences were observed in the immunostaining of p53 and ROS1 among the control and experimental groups and there was no correlation of these proteins with clinicopathological data. During the carcinogenesis process, the PBM did not modify the development of oral lesions and the expression of proliferative and apoptosis proteins.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78763-y.
Keywords: Oral cancer, Oral potentially malignant disorder, Carcinogenesis, Leukoplakia, Photobiomodulation therapy, Laser therapy
Subject terms: Cancer models, Oral cancer
Introduction
Oral carcinogenesis is a multifactorial process where mutations build up over time until a malignant tumor is developed1. Neoplastic cells differ from normal cells in a variety of ways, including resistance to anti-growth signals, evasion of apoptosis, self-sufficiency in growth signals, limitless potential for replication, encouragement of angiogenesis, capacity for tissue invasion and metastasis, altered metabolic pathways, and immunity evasion2,3. These features can enable cancerous cells to endure, multiply, and disseminate outside of their original site. The high death rate, destructive nature of conventional treatments, frequent comorbidities, and frequent oral squamous cell carcinoma (OSCC) diagnoses necessitate a deeper comprehension of the carcinogenic process and the development of novel therapeutic approaches.
Photobiomodulation (PBM) therapy is a non-invasive modality that uses non-ionizing forms of light sources, including lasers and LEDs for producing photophysical and photochemical events. In general, the effects of PBM are generally attributed to enhanced respiratory metabolism and the activation of cell-signaling pathways, which promote cell proliferation, prevent cell death, restore cellular function, and reduce pain and inflammation4–6. In oral medicine, compelling evidence indicates the use of PBM, including, but not limited to, facial pain and neuromuscular disorders (e.g., orofacial pain and temporomandibular disorders), dermatologic diseases (e.g., lichen planus and pemphigus vulgaris), burning mouth syndrome, xerostomia/hyposalivation, recurrent herpes simplex lesions, and recurrent aphthous ulcerations/stomatitis7–12. Also, the guidelines from the Multinational Association of Supportive Care in Cancer (MASCC) recommend the use of PBM for management of oral mucositis in oncology patients undergoing hematopoietic stem cell transplantation, as well as in patients with head and neck cancer undergoing chemotherapy and radiotherapy13.
Despite the well-documented benefits of PBM, concerns about its safety remain, particularly for individuals undergoing oral carcinogenesis or those experiencing field cancerization. Sonis14emphasized that a fundamental requirement for any intervention aimed at mitigating cancer treatment-related complications is ensuring that it does not adversely impact tumor development, behavior, or treatment response. Given the complex biological effects of PBM, further research and robust evidence are essential. Although former studies have sought to elucidate the effects of PBM within oral carcinogenic contexts, most have been confined to in vitro models. While these studies provide valuable contributions, their results remain inconclusive15. As far as we know, only four rodents’ studies have evaluated hitherto the effects of PBM in chemically induced oral carcinogenesis: two using DMBA-induced carcinogenesis in hamsters16,17, one using 4NQO-induced carcinogenesis in mice18, and another using 4NQO-induced carcinogenesis in hamsters19. In all these studies, however, PBM was initiated after several weeks of carcinogen exposure (8–16 weeks), at which point oral lesions were already established. Consequently, none of these studies assessed the potential effects of PBM during the early stages of oral carcinogenesis. Understanding the influence of PBM on field cancerization and its potential impact on malignant transformation is crucial. To address this gap, the current study employed the well-established 4NQO-induced oral carcinogenesis model in rodents to evaluate whether PBM could modify the progression of dysplastic lesions and cancer. This assessment was based on macroscopic and histopathological outcomes, supplemented by analyses of cell proliferation, invasion, and apoptosis.
Results
Establishment of oral carcinogenesis animal model
The model’s validity was confirmed once all 66 animals developed lesions, both macroscopically and histopathologically. The rats performed well throughout the experiment. Only in the first week after ingestion of 4NQO were adverse effects such as chromodacryorrhea, hypersalivation, and acute ulcers covered by a white membrane noticed in some animals. After adapting to 4NQO, all animals were energetic and interactive, and the negative effects were swiftly resolved. The animal’s coat deteriorated with time, becoming more unkempt than usual. Two animals in the 20-week group wheezed at the end of the experiment. However, there was no evidence of chronic weight loss. No animals were lost during the study.
Macroscopical analysis of oral lesions
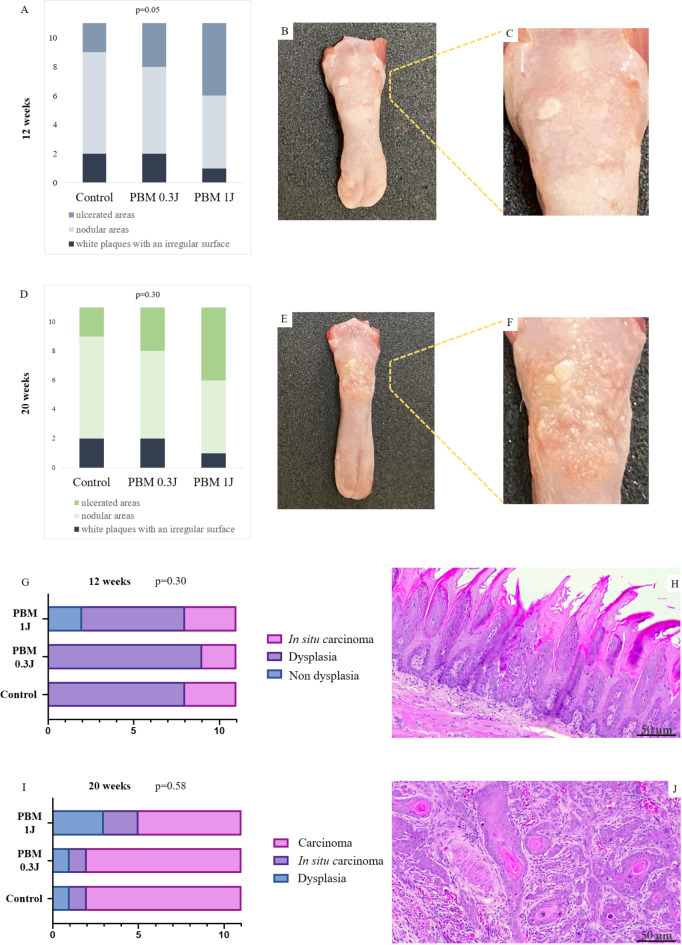
Oral lesions could be observed since week 5 as plaques. In the 12-week analysis, animals in the PBM 1 J group primarily exhibited nodular lesions (Score 4) with a verrucous aspect, which significantly differed from the control and PBM 0.3 J groups that showed more erosive lesions (Score 3) (p = 0.005) (Figs. 1, 2A-C; Table 1).
Fig. 1.
Final macroscopy appearance of each case, organized by number of treatment weeks (i.e., 12 and 20 weeks) and experimental group.
Fig. 2.
Clinical and histopathological features of our sample. (A), distribution of the cases according to clinical presentation in the 12 weeks; (B and C), white plaques with an irregular surface of one case at the end of 12 weeks; (D), distribution of the cases according to clinical presentation in the 20 weeks; (E) and (F), nodular areas with erythematous surface of one case at the end of 20 weeks; (G), distribution of the cases according to histopathological diagnosis in the 12 weeks; (H), epithelial dysplasia; (I), distribution of the cases according to histopathological diagnosis in the 20 weeks; (J), squamous cell carcinoma.
Table 1.
Summarizing data regarding lesion type, histopathological diagnosis, and protein expression.
| Experimental groups | Macroscopy lesion type at the end of the experiment | Histopathological diagnosis | Mean immunoreactive score (min-max) | ||
|---|---|---|---|---|---|
| p53 | ROS1 | ||||
| 12 weeks | Control |
- Areas of white spot/plaque with a smooth surface = 4 - White plaques with an irregular surface = 7 |
- Epithelial dysplasia = 7 - In situ carcinoma = 4 |
8.1 (3–15) |
7.1 (1–15) |
| PBM 0.3 J |
- Areas of white spot/plaque with a smooth surface = 1 - White plaques with an irregular surface = 10 |
- Epithelial dysplasia = 8 - In situ carcinoma = 3 |
6.5 (3–12) |
8 (2–12) |
|
| PBM 1 J |
- Areas of white spot/plaque with a smooth surface = 1 - White plaques with an irregular surface = 5 - Nodular areas = 5 |
- Hyperplasia, acanthosis and hyperkeratosis = 2 - Epithelial dysplasia = 6 - In situ carcinoma = 3 |
6.6 (3–10) |
8.5 (0–15) |
|
| 20 weeks | Control |
- White plaques with an irregular surface = 2 - Nodular areas = 7 - Ulcer = 2 |
- Epithelial dysplasia = 1 - In situ carcinoma = 1 - OSCC = 9 |
8.5 (5–15) |
11.9 (6–15) |
| PBM 0.3 J |
- White plaques with an irregular surface = 2 - Nodular areas = 6 - Ulcer = 3 |
- Epithelial dysplasia = 1 - In situ carcinoma = 1 - OSCC = 9 |
7.8 (2–10) |
11.3 (6–15) |
|
| PBM 1 J |
- White plaques with an irregular surface = 2 - Nodular areas = 5 - Ulcer = 5 |
- Epithelial dysplasia = 3 - In situ carcinoma = 2 - OSCC = 6 |
8.1 (2–12) |
11.4 (8–15) |
|
OSCC, oral squamous cell carcinoma.
In the 20-week evaluation, the nodular lesions (Score 4) with a verrucous aspect were more common in both control (n = 7) and PBM 0.3 J (n = 6) groups. Five rats in the PBM 1 J group had nodular (Score 4), and five had ulcerated lesions (Score 5). No significant difference between groups was found (p = 0.05) (Fig. 2D-F and Table 1).
Figure 1 illustrates the macroscopic appearance of all animals euthanized at 12 and 20 weeks across all experimental groups. The macroscopic aspects of cases diagnosed as in situ carcinoma and oral squamous cell carcinoma are provided in Supplementary Figs. 1 and 2, respectively.
PBM does not affect tongue morphological modifications
No significant difference in histopathological characteristics was observed among the groups of 12 (p = 0.30) and 20 weeks (p = 0.58) (Fig. 2G-J). At 12 weeks, epithelial dysplasia was the most common histological diagnosis across all groups. After 20 weeks, the majority of cases revealed squamous cell carcinoma (Table 1), all graded as well-differentiated. Regarding the pattern of invasion, 16 animals showed neoplastic cells superficially located, while in 8 animals, a deep tissue invasion pattern was observed (Supplementary Table 1).
PBM does not influence cell proliferation and cell apoptosis profile
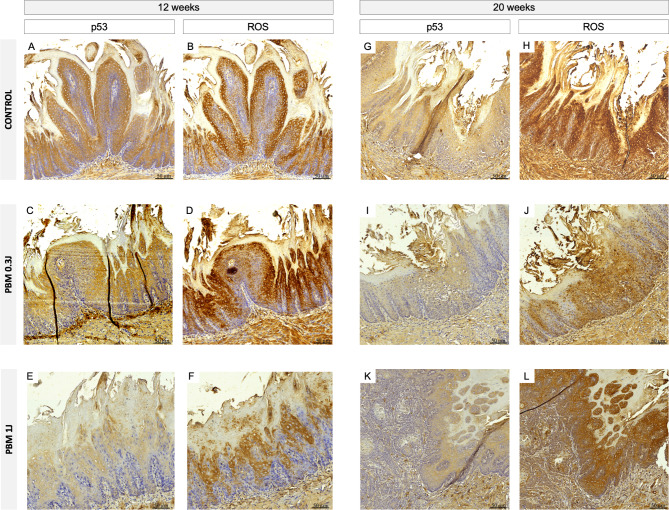
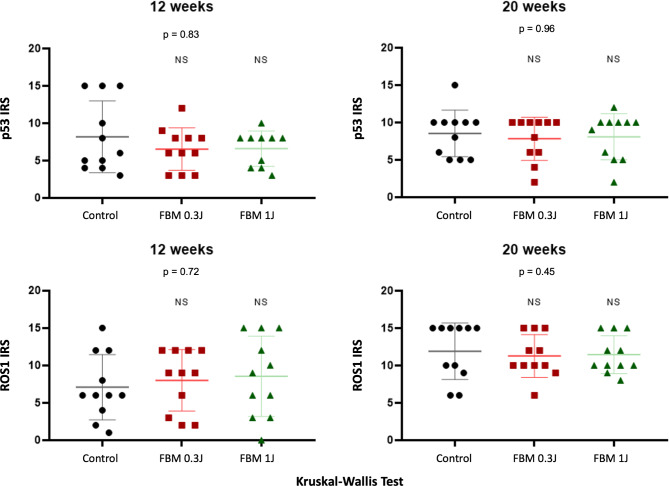
p53 typically exhibited moderate to strong diffuse nuclear immunostaining, while ROS1 staining was evident in both membrane and cytoplasmic distribution, with no nuclear staining observed (Fig. 3). Statistical analysis indicated no significant differences in the immunoreactive scores (IRS) of ROS1 and p53 between the control and PBM groups in the two endpoints (i.e., 12 and 20 weeks) (Fig. 4). Supplementary Table 1 provides the intensity, frequency, and IRS of each case. Table 1 presents the mean IRS for both markers across all groups and time points.
Fig. 3.
Immunohistochemical expression in 12 and 20 weeks of p53 and ROS1 in control and PBM groups. p53 shows moderate to strong diffuse nuclear staining, while ROS1 staining is predominantly localized in the membrane and cytoplasm, with no nuclear staining observed.
Fig. 4.
Statistical analysis in 12 and 20 weeks indicated no significant differences in the immunoreactive scores of p53 and ROS1 between the control and PBM groups.
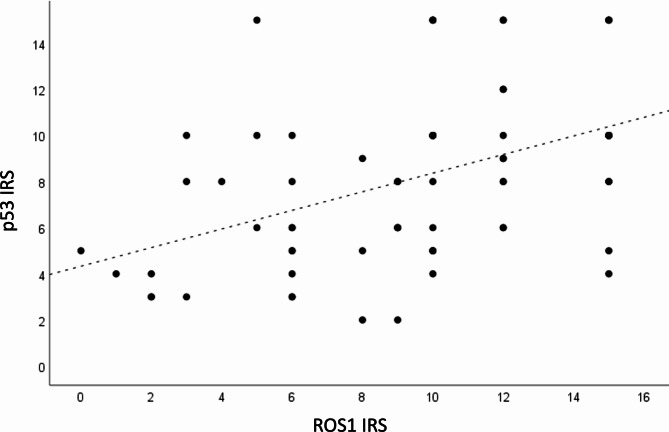
A Spearman’s correlation test - based on the combined data from the control, PBM 0.3 J, and PBM 1 J groups (at 12 and 20 weeks) - revealed a significant association (p < 0.001) between markers, with a coefficient of 0.49, indicating a moderate positive correlation between p53 and ROS1 (Fig. 5).
Fig. 5.
Combined data analysis of Control, PBM 0.3 J, and PBM 1 J groups (12 and 20 Weeks): Spearman’s Correlation Coefficient 0.49, p < 0.001.
Discussion
The use of PBM for therapeutic purposes has been investigated for years. This therapy represents a promising therapeutic approach, leveraging the ability of laser light to modulate biochemical and molecular processes in living cells without generating heat20,21. In the present study, we evaluated the safety of PBM in rat’s oral carcinogenesis process, induced by long-term administration of 4NQO in drinking water. This is a classic animal model that involves the administration of 4NQO, a chemical carcinogen, to induce epithelial hyperplasia, dysplasia and OSCC in animals, mimicking the stages of human oral cancer progression22,23. We tested two different PBM protocols, commonly used for oral mucositis management. Our results demonstrated that the PBM groups were similar to the control group in terms of the number of lesions, occurrence of dysplastic lesions and oral cancer, with neither protocol promoting nor preventing the progression of these lesions.
The oral carcinogenesis model has been widely employed to explore new approaches for both the prevention and treatment of dysplastic and oral cancer lesions. However, only four studies have specifically investigated the effects of PBM in this context16–19. Similiar to our study, Ottaviani18and Neculqueo19used the 4NQO model, which has been recognized as a preferred carcinogen over DMBA for the development of experimental oral carcinogenesis24. Besides that, our study was the first to investigate the effects of PBM from the onset of carcinogenesis, starting PBM at the beginning of the carcinogenic challenge (since day 1) and using clinical parameters recommended for oral mucositis (0.3 J and 1 J). In contrast, all the four previous studies applied PBM only after the lesions were macroscopically established (8–16 weeks) and then assessed whether PBM altered lesion behavior. Our innovative approach allows us to evaluate PBM’s potential not only to modify lesion behavior but also to prevent or stimulate lesion formation from the earliest stages.
Neculqueo et al19. evaluated the effects of PBM in a 4NQO mouse model using two different energy doses (1.5 J and 9 J) in the context of leukoplakia and oral cancer. The animals were exposed to the carcinogen for 16 weeks and then treated over the next two weeks, receiving a total of seven laser sessions across two protocols. However, neither PBM protocol affected the clinical or histological characteristics. These findings align with our results, which showed that the PBM groups were similar to the control group in terms of the number of lesions and the microscopic characteristics of dysplastic lesions at 12 weeks, as well as oral cancer after 20 weeks of 4NQO induction. Previous studies by our group also evaluated the effects of PBM therapy in an oral cancer patient-derived xenograft model, applying 0.2 J/point daily for 12 weeks directly at the tumor implantation site. The treatment showed no impact on tumor growth, morphological characteristics, proliferation rates, or the epigenetic and stem cell profiles25. Similarly, a study using an orthotopic mouse model with a human oral cancer cell line (Cal-33) and PBM at 5.6 J demonstrated no significant effect on tumor growth26. On the other hand, conflicting results have been reported. Monteiro et al16,17. tested the effects of PBM (4 J and 6.65 J, respectively) in hamsters exposed to DMBA for 8 weeks, followed by PBM treatment for an additional 4 weeks. They observed that 100% of the control and PBM-treated animals developed oral cancer, but noted that the PBM group exhibited more cases of poorly differentiated oral cancer compared to the control group. This suggests that PBM did not influence lesion formation since all control animals developed oral cancer. Ottaviani et al18. investigated the effects of PBM on oral carcinogenesis in C57BL/6 mice exposed to 4NQO for 16 weeks, followed by PBM treatment in the 20th week. The study found that PBM treatment inhibited tumor progression and enhanced functional vessel maturation. These discrepancies across studies can be attributed to several factors, including different PBM protocols, cellular contexts, tumor microenvironments, timing of PBM application relative to the stages of carcinogenesis, variations in molecular responses to PBM, and differences in the animal models used.
Importantly, among several characteristics, cancer cells exhibit the hallmark ability to proliferate continuously and escape of apoptosis, disregarding external growth signals27. So, we decided to evaluate p53 and ROS1 markers that allows us to explore the interaction between these factors during carcinogenesis. p53 was chosen for its key role in regulating apoptosis and preventing tumor progression28, while ROS1, typically linked to oncogenic signaling, was included due to emerging evidence of its involvement in cellular stress responses and tumor microenvironment modulation29. Our results suggest that control and PBM groups presented similar results concerning cell proliferation, invasion, and apoptosis in this carcinogenic setting, consistent with previous studies supporting the safety of PBM25,30. In addition, our results demonstrated a moderate positive correlation between p53 and ROS1 (r = 0.49), indicating that as p53 levels increase, ROS1 levels also tend to rise. This indicates a potential relationship between the two markers in influencing cellular behavior, such as proliferation and apoptosis, though other factors may also modulate this interaction.
The 4NQO model has limitations due to biological differences between rodents and humans, which may affect the translatability of findings to clinical settings. Additionally, the prolonged induction period and variability in lesion development make the model time-consuming and less consistent. Also, it is impossible to diligently monitored weekly consumption of 4NQO for each animal. They could not be housed in isolation, leading us to record the total consumption per cage (which contained 2 or 3 animals). Consequently, determining individual consumption in this type of study proves to be unfeasible.
In summary, the present research highlights the safety of PBM in the context of oral carcinogenesis. Notably, previous studies have not explored the potential impacts of PBM during the early stages of oral carcinogenesis, making our investigation particularly relevant. These findings contribute to the ongoing discussion on PBM’s role in cancer biology.
Conclusion
PBM protocols commonly used for managing oral mucositis in oncological patients appear to be safe in the context of 4NQO induced oral carcinogenesis. PBM and control groups were similar in terms of lesion progression, histopathological features, or the expression of key markers such as p53 and ROS1.
Materials and methods
Animal model
Eight-week-old male Wistar rats were used in this present study. All animals were maintained in a room with 12-hour light and 12-hour dark cycle (lights on from 7 a.m. to 7 p.m.). They were housed in air-conditioned quarters. The rats were given a standard pellet diet (Oriental Yeast Co., Ltd., Tokyo, Japan) and tap water ad libitum. During the first five weeks of the study, the animal also received hypercaloric supplementation once a day (2 g per animal) and 5% glucose solution once a week (100 ml per animal).
This study was conducted under the Guide for the Care and Use of Laboratory Animals and was approved by the Porto Alegre University Hospital’s (HCPA, Brazil) Ethics Committee on Animal Use under protocol number 2021 − 0614. The experiment is reported in compliance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Sample size calculation
Each sample size calculation for the experimental groups was performed based on previous studies with a similar methodology. The groups comprised 11 animals to account for the expected mortality rate of ~ 30%31 due to 4NQO-related side effects during the 12 and 20 weeks of carcinogen administration.
Experimental design
The experimental animals were divided into three groups, as follows:
Control Group (n = 22)
Animals receiving 4NQO administration and being handled three times per week while not receiving PBM for 12 weeks (n = 11) and 20 weeks (n = 11).
PBM 0.3 J (n = 22)
Animals that, starting on the first day of 4NQO administration, will receive PBM with a protocol of 0.3 J, three times per week for 12 weeks (n = 11) and 20 weeks (n = 11).
PBM 1 J (n = 22)
Animals that, starting on the first day of 4NQO administration, will receive PBM with a protocol of 1 J, three times per week for 12 weeks (n = 11) and 20 weeks (n = 11).
Oral carcinogenesis model
For oral carcinogenesis induction, all rats were treated with 25 ppm of 4NQO (LOT 711645086-1-1, Toronto Research Chemicals Inc., Toronto, Canada) solution diluted in the drinking water. The 25 mg of carcinogen was diluted in 1 ml of the dimethylsulfoxide solvent (DMSO - Sigma, St. Louis, MO, USA) and then in 1 L of autoclaved water.
Due to photolysis and the danger of the carcinogen, fresh solutions were prepared twice a week and stored in amber bottles that were clearly labeled. Consumption was controlled by overfilling the solution between exchanges. The animals were given pure water twice per week23.
Photobiomodulation protocol
PBM was performed three times a week under inhalational anesthesia with isoflurane, diluted in oxygen, and supplied by a vaporizer at a concentration of 5% for induction and 1–2% for maintenance of anesthesia. An InGaAlP diode laser (660 nm; MMOptics, São Carlos, São Paulo, Brazil) was used in continuous mode and in contact using the punctual technique in the parameters described in Table 2. A point in the posterior region of the dorsum of the tongue was irradiated. Two different protocols were tested (Total radiant energy of 0.3 J and 1 J) to verify if there is a difference in safety during the carcinogenesis process. The 0.3 J and 1 J parameters were chosen for PBM application in this study based on the MASCC/ISOO guidelines for the prevention of oral mucositis in head and neck cancer patients. These parameters are well-established for their efficacy in managing oral mucositis, a common side effect of cancer treatment13. Given that our study focuses on the safety of PBM in the context of oral carcinogenesis, the use of these parameters allowed us to test their impact on a carcinogenically altered mucosa. Our goal was to determine whether PBM, applied under conditions similar to those in clinical settings, could be safely used without stimulating the proliferation of neoplastic cells or affecting the carcinogenic process.
Table 2.
Irradiation parameters applied in the PBM.
| PBM 0.3 J | PBM 1 J | |
|---|---|---|
| Center wavelengths (nm) | 660±10 | |
| Operating mode | Continuous | |
| Peak power (W) | 0.01 | |
| Average power (mW) | 100 | |
| Spot size (cm2) | 0.03 | |
| Beam shape | Round | |
| Irradiance at target (mW/cm2) | 3.333 | |
| Fluence (J/cm2) | 10 | 33.3 |
| Exposure duration (s) | 3 | 10 |
| Total radiant energy (J) | 0.3 | 1 |
| Photon Fluence (p.J/cm2) | 19 | 63 |
| Photon Fluence (Einstein) | 4.2 | 14 |
| Number of points irradiated | 1 | |
| Application technique | Contact | |
| Frequency of sessions | 3 times/week for 12 weeks | |
nm, nanometer; mW, miliwatt; W, watt; J, Jaule; s, second; p.J, photon fluence.
Animal monitoring
The animals were monitored daily for general health and weighed three times a week with an electronic scale. Oral examinations were performed three times a week to monitor changes in the lingual mucosa. Once a week, following isoflurane inhalation sedation, the animals’ tongues were pulled, and the posterior region of the tongue was photographed.
All rats were euthanized by isoflurane inhalation overdose after 12 weeks (n = 33) and 20 weeks (n= 33). The two euthanasia time points were justified to track different stages of carcinogenesis32. At the end of the experiment, a calibrated researcher who was blind to the groups was responsible for clinical classifying the lesions based on the following parameters: Score 1, normal mucosa; Score 2, areas of white spot/plaque with a smooth surface; Score 3, areas of plaques with an irregular surface; Score 4, nodular areas; and Score 5, ulcerated areas (Supplementary Fig. 3 exemplify each clinical score).
Histopathological analysis
All the tongues were then fixed in 10% buffered formaldehyde for 24 h, embedded in paraffin blocks, sectioned at 3 μm, and stained with hematoxylin and eosin (H&E). The histopathological diagnosis was made by an experienced oral pathologist blinded for the groups. The microscopy features were based on no dysplasia (atrophy of the epithelium, hyperkeratosis, hyperplasia), epithelial dysplasia, in situ carcinoma, and squamous cell carcinoma. The cases diagnosed as carcinoma were classified as well-differentiated, moderately differentiated, or poorly differentiated based on the criteria established by the World Health Organization33. Additionally, the lesions were categorized as superficial (when not invading muscle or glandular regions) (Supplementary Fig. 4A) or invasive (Supplementary Fig. 4B).
Immunohistochemistry stain
For the immunohistochemical study, 3-µm thick sections were obtained from paraffin-embedded tissue blocks and mounted on polarized slides. The cases were treated with a heat retrieval solution (Reveal Decloaker, RTU; Biocare Medical) to expose the antigenic epitopes. The endogenous peroxidases were blocked with 0.9% hydrogen peroxide for 5 min each. Polyclonal anti-ROS1 (Cat. No. GTX25512, Rabbit; GeneTex, Irvine, CA, USA; 1:100) and monoclonal anti-p53 (clone DO7, Mouse; BioSB, Santa Barbara, CA, USA; 1:50) were used. The tissue samples were incubated overnight and then incubated with a biotinylated anti-mouse/anti-rabbit antibody and a streptavidin-horseradish peroxidase complex for 40 min each (mouse/rabbit ImmunoDetector Biotin Link and HRP Label; Bio SB). For the negative control samples, the primary antibody was omitted, while breast cancer tissues were utilized as positive controls. 3.3’-Diaminobenzidine was used as the chromogen (Biocare Medical), and the sections were counterstained with Harris hematoxylin.
Immunohistochemical assessment
All cases were evaluated by two independent observers, and any disparities were deliberated until agreement was achieved. The slides were scanned and photographed (20x magnification), in the posterior region of the tongue, where PBM was applied. The signal intensity was assessed according to the following criteria: 0, no staining; 1, weak; 2, moderate; and 3, strong. The percentage of stained cells was categorized into 0, negative; 1, 1–10%; 2, 11–25%; 3, 26–50%; 4, 51–75%; and 5, more than 75%. The intensity and quantity scores were multiplied to give the IRS. This results in a final score ranging from 0 to 15.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA). Clinical scores and histopathological diagnoses were compared between groups using the chi-square test. The IRS values were compared across groups using the Kruskal-Wallis test, with post-hoc analysis applied when necessary. Spearman’s correlation test was used to assess the correlation between p53 and ROS1 IRS. A p-value of < 0.05 was considered statistically significant for all analyses.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The present project received financial support from the Research Incentive Foundation of Hospital de Clínicas de Porto Alegre (FIPE/HCPA). This manuscript is part of the PhD thesis of co-author L.F.S., which is published and available in the library of the University of Campinas (UNICAMP). The authors thank the Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code 001), Brazil. L.F.S., T.R.S., C.H.T.M. are the recipients of fellowships. We also acknowledge the Brazilian National Council for Scientific and Technological Development (CNPq). M.D.M., A.R.S.S., P.A.V are research fellows of CNPq. L.F.S., F.M.S, R.B.M, are research fellow supported by the Agencia Nacional de Investigación e Innovación / Sistema Nacional de Investigadores (ANII/SNI), Uruguay. This study underwent revisions with the assistance of ChatGPT to improve the clarity and adequacy of the English language.
Author contributions
LFS: Methodology, Investigation, Data curation, Writing – Original Draft preparation; TRS, CHT, VRV, FMS: Methodology, Investigation; DC and TNAG: Supervision; VPV: Formal Analysis; CKD: Methodology; RMC, MATM, PAV, ARSS, RBM: Visualization, Reviewing and editing; MDM: Conceptualization, Investigation, Writing- Reviewing and Editing and Project administration.
Data availability
The data supporting the findings of this study are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tanaka, T. & Ishigamori, R. Understanding carcinogenesis for fighting oral cancer. J. Oncol.2011 (603740). 10.1155/2011/603740 (2011). [DOI] [PMC free article] [PubMed]
- 2.Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell. 100, 57–70. 10.1016/s0092-8674(00)81683-9 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Fouad, Y. A. & Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res.7, 1016–1036 (2017). [PMC free article] [PubMed] [Google Scholar]
- 4.Anders, J. J., Lanzafame, R. J. & Arany, P. R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg.33, 183–184. 10.1089/pho.2015.9848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Freitas, L. F. & Hamblin, M. R. Proposed mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron.22, 7000417. 10.1109/JSTQE.2016.2561201 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass, G. E. & Photobiomodulation A review of the molecular evidence for low level light therapy. J. Plast. Reconstr. Aesthet. Surg.74, 1050–1060. 10.1016/j.bjps.2020.12.059 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Pandeshwar, P. et al. Photobiomodulation in oral medicine: a review. J. Investig Clin. Dent.7, 114–126. 10.1111/jicd.12148 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Al-Maweri, S. A. et al. Efficacy of low level laser therapy in the treatment of burning mouth syndrome: a systematic review. Photodiagn Photodyn Ther.17, 188–193. 10.1016/j.pdpdt.2016.11.017 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Al-Maweri, S. A. et al. Efficacy of low-level laser therapy in management of recurrent herpes labialis: a systematic review. Lasers Med. Sci.33, 1423–1430. 10.1007/s10103-018-2542-5 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Munguia, F. M., Jang, J., Salem, M., Clark, G. T. & Enciso, R. Efficacy of low-level laser therapy in the treatment of Temporomandibular Myofascial Pain: a systematic review and Meta-analysis. J. Oral Facial Pain Headache. 32, 287–297. 10.11607/ofph.2032 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Amorim, D. et al. Laser therapy for recurrent aphthous stomatitis: an overview. Clin. Oral Investig. 24, 37–45. 10.1007/s00784-019-03144-z (2020). [DOI] [PubMed] [Google Scholar]
- 12.Golež, A. et al. Effects of low-level light therapy on xerostomia related to hyposalivation: a systematic review and meta-analysis of clinical trials. Lasers Med. Sci.37, 745–758. 10.1007/s10103-021-03392-0 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Zadik, Y. et al. Systematic review of photobiomodulation for the management of oral mucositis in cancer patients and clinical practice guidelines. Support Care Cancer. 27, 3969–3983. 10.1007/s00520-019-04890-2 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Sonis (2006).
- 15.Silveira, F. M. et al. Examining tumor modulating effects of photobiomodulation therapy on head and neck squamous cell carcinomas. Photochem. Photobiol Sci.18, 1621–1637. 10.1039/c9pp00120d (2019). [DOI] [PubMed] [Google Scholar]
- 16.de Monteiro, C. Influence of laser phototherapy (λ660 nm) on the outcome of oral chemical carcinogenesis on the hamster cheek pouch model: histological study. Photomed. Laser Surg.29, 741–745. 10.1089/pho.2010.2896 (2011). [DOI] [PubMed] [Google Scholar]
- 17.de Monteiro, C. et al. J. N. Effects of imiquimod and low-intensity laser (λ660 nm) in chemically induced oral carcinomas in hamster buccal pouch mucosa. Lasers in medical science, 28, 1017–1024. (2013). 10.1007/s10103-012-1192-2). [DOI] [PubMed]
- 18.Ottaviani, G. et al. Laser therapy inhibits Tumor Growth in mice by promoting Immune Surveillance and Vessel normalization. EBioMedicine. 11, 165–172. 10.1016/j.ebiom.2016.07.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neculqueo, G. W. et al. Laser photobiomodulation does not alter clinical and histological characteristics of 4-NQO-induced oral carcinomas and leukoplakia in mice. J. Photochem. Photobiol B. 237, 112597. 10.1016/j.jphotobiol.2022.112597 (2022). [DOI] [PubMed] [Google Scholar]
- 20.Levchenko, S. M. et al. Cellular transformations in near-infrared light-induced apoptosis in cancer cells revealed by label-free CARS imaging. J. Biophotonics. 12, e201900179. 10.1002/jbio.201900179 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Dompe, C. et al. Photobiomodulation-underlying mechanism and clinical applications. J. Clin. Med.9, 1724. 10.3390/jcm9061724 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zigmundo, G. C. O. et al. 4-nitroquinoline-1-oxide (4NQO) induced oral carcinogenesis: a systematic literature review. Pathol. Res. Pract.236, 153970. 10.1016/j.prp.2022.153970 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Schuch, L. F. et al. Proposal of a secure and efficient protocol for a murine oral carcinogenesis model induced by 4-nitroquinoline-1-oxide (4NQO). Pathol. Res. Pract.247, 154547. 10.1016/j.prp.2023.154547 (2023). [DOI] [PubMed] [Google Scholar]
- 24.Kanojia, D. & Vaidya, M. M. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol.42, 655–667. 10.1016/j.oraloncology.2005.10.013 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Silveira, F. M. et al. Impact of photobiomodulation in a patient-derived xenograft model of oral squamous cell carcinoma. Oral Dis.29, 547–556. 10.1111/odi.13967 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Barasch, A., Raber-Durlacher, J., Epstein, J. B. & Carroll, J. Effects of pre-radiation exposure to LLLT of normal and malignant cells. Support Care Cancer. 24, 2497–2501. 10.1007/s00520-015-3051-8 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell. 144, 646–674. 10.1016/j.cell.2011.02.013 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Sinevici, N. & O’sullivan, J. Oral cancer: deregulated molecular events and their use as biomarkers. Oral Oncol.61, 12–18. 10.1016/j.oraloncology.2016.07.013 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Cordani, M. et al. Mutant p53-Associated Molecular mechanisms of ROS Regulation in Cancer cells. Biomol. 10, 361. 10.3390/biom10030361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martins, M. D. et al. The impact of photobiomodulation therapy on the biology and behavior of head and neck squamous cell carcinoma cell lines. J. Photochem. Photobiol B. 209, 111924. 10.1016/j.jphotobiol.2020.111924 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Wagner, V. P. et al. Can propranolol act as a chemopreventive agent during oral carcinogenesis? An experimental animal study. Eur. J. Cancer Prev.30, 315–321. 10.1097/CEJ.0000000000000626 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro, D. A. & Salvadori, D. M. Gingival changes in Wistar rats after oral treatment with 4-nitroquinoline 1-oxide. Eur. J. Dent.1, 152–157 (2007). [PMC free article] [PubMed] [Google Scholar]
- 33.Bryne, M., Koppang, H. S., Lilleng, R. & Kjaerheim, A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J. Pathol.166, 375–381. 10.1002/path.1711660409 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon request.