Abstract
Background
The prevalence of eye problems increases with age and, consequently, so does the level of visual impairment. As the incidence of stroke also increases with age, a significant proportion of stroke patients will have age‐related visual problems. It is possible that the effect of interventions for age‐related visual problems may differ in the population of stroke patients compared to the wider population of older people. The interaction between the problems arising directly from stroke and those arising directly from age‐related visual problems will be complex. Interventions for age‐related visual problems may also be affected by the presence of other stroke‐related co‐morbidities. Consequently, the nature and outcome of interventions for age‐related visual problems may be different in patients with stroke.
Objectives
The aim of this review is to determine if interventions for age‐related visual problems improve functional ability following stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (March 2011), the Cochrane Eyes and Vision Group Trials Register (December 2009) and nine electronic bibliographic databases including: the Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), MEDLINE (1950 to February 2011), EMBASE (1980 to February 2011), CINAHL (1982 to February 2011), AMED (1985 to February 2011) and PsycINFO (1967 to February 2011). We also searched reference lists and trials registers, handsearched journals and conference proceedings, and contacted experts.
Selection criteria
Randomised trials in adults after stroke, where the intervention is specifically targeted at assessing, treating or correcting age‐related visual problems, or improving the ability of the patient to cope with visual impairment. Primary outcome was functional ability in activities of daily living and secondary outcomes included functional ability in extended activities of daily living, visual acuity, visual field, visual function, balance, falls, depression and anxiety, discharge destination/residence after stroke, quality of life and social isolation, adverse events and death.
Data collection and analysis
Two review authors independently screened abstracts and planned to extract data and appraise trials. We planned that assessment of methodological quality would be undertaken for allocation concealment, blinding of outcome assessor, method of dealing with missing data and other potential sources of bias.
Main results
We considered 7357 titles, 460 abstracts and 85 full papers. We identified no studies for inclusion in this review.
Authors' conclusions
There are no implications for practice arising from this review. Evidence relating to the management of patients (from the general population) with age‐related visual problems is available from other Cochrane reviews and is likely to be the best evidence available for making treatment decisions about individual patients. Subgroup analyses within these reviews to explore the effect of interventions for age‐related visual problems in patients with stroke are recommended. We recommend that the objectives and selection criteria for this Cochrane review are amended and clarified prior to any future updates.
Keywords: Aged, Humans, Activities of Daily Living, Age Factors, Stroke, Stroke/complications, Vision Disorders, Vision Disorders/therapy
Plain language summary
Interventions for age‐related visual problems in patients with stroke
As people get older they are more likely to develop age‐related visual problems (such as age‐related macular degeneration, cataracts, glaucoma and diabetic retinopathy). As the incidence of stroke increases with age, a significant proportion of stroke patients will have age‐related visual problems. This review aimed to determine if interventions for age‐related visual problems improve functional ability following stroke. We were specifically interested in whether people with stroke responded differently from the general population when treated for age‐related visual problems and also whether assessment and interventions for age‐related visual problems could improve functional ability during stroke rehabilitation. After a complex search we identified no studies for inclusion in this review. Currently best evidence comes from a series of Cochrane reviews which evaluate the effect of specific interventions for different age‐related visual problems (in the general population). We recommend that analyses are carried out within these reviews to explore the impact of these interventions on the subgroup of people with stroke. We encountered a number of methodological problems during this review, relating to selection criteria for including studies; we recommend that this is clarified before future updates of this review are carried out.
Background
The association between visual impairment and disability in activities of daily living is well established (Wolter 2006). The services available to patients with visual problems following stroke are presently inconsistent. We aim to provide an evidence base to facilitate the development of further research and promote best treatments for patients with visual problems following stroke.
We discussed the issues relating to systematic reviews of visual problems after stroke as a multidisciplinary group and formulated the key issues this proposed review seeks to address. We identified these as key issues for two main reasons.
Health professionals see many patients because of age‐related visual problems on a background of stroke but there is little evidence to help them determine whether the treatment options for patients with age‐related visual problems will be effective in patients with stroke. Treatments available for age‐related visual problems potentially have an impact on many of the symptoms arising from stroke. The complexity of the relationship between these symptoms convinced our multidisciplinary group of the need for a systematic review specific to the population of patients with both age‐related visual problems and stroke. Currently health professionals rely on evidence arising from subgroup analyses of systematic reviews of the wider population of patients with age‐related visual problems.
Health professionals see many patients because of their stroke who also have age‐related visual problems. Our multidisciplinary group has anecdotal reports that health professionals often fail to recognise or appreciate age‐related visual problems and that failure to identify and address such problems can have a detrimental effect on stroke rehabilitation. These anecdotal reports suggests that very simple measures, such as ensuring patients wore their correct glasses during rehabilitation, could potentially have a significant impact on the outcome of rehabilitation. A prospective cohort study reported that over 90% of patients admitted to a stroke rehabilitation unit had previously been prescribed glasses; more than 25% of the sample did not have their glasses with them in hospital (Lotery 2000). Prospective cohort studies investigating presence of visual problems after stroke have also demonstrated that a large proportion of patients with stroke may also have ocular pathologies (Lotery 2000; Rowe 2009).
Many people with stroke will have stroke‐related visual problems including visual field defects, eye movement disorders and visual perceptual problems (including visual neglect or inattention). There are a range of specific interventions and management strategies for these stroke‐related visual problems which are the focus of other reviews. The emphasis of this review is on interventions for, and the management of, age‐related visual problems in the population of patients with stroke and not on interventions for stroke‐related visual problems.
The emphasis on age‐related problems is highly relevant as the world population is recognised to be aging, with an increasing proportion of elderly people within the population. Figures from the United Nations (United Nations 2002) suggest that in the year 2000 in developed countries approximately 19.4% of the population was over 60 years (with 3.1% over 80 years). This is predicted to rise to 28.2% over 60 years (5.4% over 80 years) by 2025, and to 33.5% over 60 years (9.6% over 80 years) by 2050.
This review is one of a series of reviews supported by the Royal National Institute for the Blind (RNIB) in the UK. The aim of these reviews was to identify the evidence base for treatments of visual problems following stroke.
Description of the condition
The prevalence of eye problems increases with age and, consequently, so does the level of visual impairment. According to the World Health Organization standard of classification, 6.2% of people in the age group 75 to 79 years are visually impaired. In the age group of 90 years and over, the figure increases to 36.9% (Evans 2004). Almost all age‐related visual impairment is attributable to one of the following five problems (Evans 2004; Reidy 1998):
Age‐related macular degeneration (AMD)
AMD is a degeneration of the central part of the retina. There is impairment of the oxygen and nutrient supply to the retinal cells leading to loss of central vision (dry AMD). Damage to retinal cells allows new vessels to grow in the retina (wet AMD). These vessels haemorrhage and the tissue swells, causing permanent scarring. This occurs at the most sensitive central part of the retina (the macula) resulting in significant decrease in fine or detailed vision. AMD is the most common cause of blind registration in the UK (Bunce 2008). Patients have reduced ability to perform activities of daily living that require good perception of detail: recognising faces, driving, reading, cooking and answering the telephone (Williams 1998). There is a loss of quality of life (Mitchell 2006) and levels of depression are doubled compared with contemporaries (Rovner 2002).
Uncorrected refractive error
The ability of the eye to focus light sharply on the retina alters in the presence of refractive error. The magnitude corresponds to the strength of lens prescription in a patient's glasses. Refractive error increases in irregularity (Asano 2005) as patients become more hyperopic, or long‐sighted, with age (Wang 1994). It causes a general blurring of vision, but is eminently amenable to treatment.
Glaucoma
Damage to the optic nerve at the point where it exits the eye, often because of a rise in pressure within the eye, causes loss of retinal neurons and scotoma, or blind patches in the peripheral field of view. As the disease progresses, these patches, which are often arch‐like in shape, enlarge, join and extend inwards to leave only a very small area of intact vision at the centre of the visual field. This affects patients’ peripheral awareness and makes navigation and unaided mobility more difficult. The most severe functional disabilities occur in the areas of reading and driving (Green 2002), the latter becoming illegal in more severe cases.
Cataracts
This is a loss of transparency of the lens of the eye. The lens becomes progressively thicker, more opaque and less regular in internal structure, so that light travelling through the eye is bent in irregular ways. Cataracts primarily cause a loss of visual acuity and contrast sensitivity. As a result patients have difficulty with fine visual tasks, especially in poor lighting or at night time. They have poorer perception of colour, greater sensitivity to glare and occasionally diplopia (double vision) (Stuen 2003).
Diabetic eye disease
This is a complication of diabetes, especially where blood sugar control is poor or the condition is long standing. Damage to the smallest blood vessels that supply the retina means they leak, haemorrhage and stop supplying blood efficiently, so that the retina is starved of oxygen. This can affect both central and peripheral vision. Vision may fluctuate significantly from day to day (Horowitz 2004). Depending on the type of diabetic eye disease, its effects are similar to those AMD and glaucoma patients experience.
Given that the incidence of stroke increases with age, a significant proportion of stroke patients have age‐related visual problems (Wolter 2006). Poor visual acuity has a negative impact on rehabilitation of older people (Johansen 2003) and people with stroke (Jones 2006; Lotery 2000; Rowe 2009). Uncorrected visual impairment resulting from age‐related visual problems causes difficulties with performing activities of daily living and mobility tasks (Wolter 2006). Untreated age‐related visual problems following stroke can adversely affect quality of life (Jones 2006). Uncorrected refractive error is a common age‐related visual problem. Using glasses addresses the problem, but there is evidence that following stroke this simple measure is often overlooked (Lotery 2000). Importantly, although vision loss is the third most common reason for requiring assistance with activities of daily living for those over 70 years of age, it is often overlooked when treating patients for other conditions (Warnecke 2003).
Description of the intervention
There are a number of different treatment and management approaches available to patients with age‐related visual problems. This review will consider any intervention that is specifically targeted at improving age‐related visual problems, or improving the ability of patients to cope with such problems.
These interventions may include but are not limited to the following:
Environmental modification
This makes it easier for the patient to cope with age‐related visual problems during everyday activities within the home (or immediate) environment. Examples include increased lighting, use of contrast, tactile enhancements and large print devices.
Activities of daily living training
This intervention aims to train a patient to cope with age‐related visual problems. Examples include cooking, personal care and household chores.
Drugs
These generally aim to reduce or alleviate symptoms or slow the progression of a specific age‐related visual problem. Examples include vascular endothelial growth factor (VEGF) inhibitors and intra‐ocular pressure (IOP) lowering medication.
Surgery
Surgery aims to reduce or alleviate a specific age‐related visual problem. Examples include cataract extraction, vitrectomy, laser photocoagulation and photo‐dynamic therapy.
Visual aids and equipment
These work by correcting an age‐related visual problem (e.g. correcting a refractive error), or helping the patient cope with the age‐related visual problem (e.g. magnifying objects). Examples include magnifiers, telescopes, closed‐circuit television (CCTV) and absorptive lenses.
Assessment and screening interventions
These work by identifying a patient's age‐related visual problems and initiating appropriate referral or treatments. These might include standardised visual assessments, screening and referral for visual assessment and intervention.
How the intervention might work
There is substantial evidence of the effect of each of these interventions in populations with age‐related visual problems. It is beyond the scope of this review to address the specific methods by which each of the many individual interventions may work in the population of older people with age‐related visual problems. This review focuses on how specific interventions may work in the population of patients with stroke. It is possible that the effect of interventions for age‐related visual problems may differ in the population of stroke patients compared with the wider population of older people. The interaction between the problems arising directly from stroke and those arising directly from age‐related visual problems will be complex. Interventions for age‐related visual problems may also be affected by the presence of other stroke‐related co‐morbidities. Consequently, the nature and outcome of interventions for age‐related visual problems may be different in patients with stroke.
The aim of this review is to determine the effects and impact of interventions in stroke patients with age‐related visual problems. Determining these effects will help healthcare clinicians make treatment decisions for this patient group with complex needs. It is not the aim of this review to compare the effect of treatments between people with age‐related visual problems and stroke and people with age‐related visual problems only.
We are unaware of any research in this area. However, the following are examples of how stroke may alter intervention for age‐related visual problems and its result.
Environmental modification
Patients with a variety of stroke‐related problems, including motor, cognitive and sensory problems, could potentially benefit from environmental modification in order to improve their functional ability and independence in activities of daily living. Environmental modification may also benefit age‐related visual problems by removing possible trip hazards and aiding safe movement and activity. Environmental modification may therefore have several benefits for stroke patients with age‐related visual problems, improving their function, activities of daily living and mobility, and reducing the risk of adverse events such as falls.
Activities of daily living training
Activities of daily living training is an intervention commonly offered to improve function associated with age‐related loss of vision. Similarly, patients with complex problems following stroke can benefit from such training. It has the potential to be particularly beneficial as it may help address the complex and holistic needs of patients with both stroke and age‐related visual problems.
Pharmacological interventions
Vision has been identified as important to the success of physical rehabilitation. Drugs which preserve the visual field may be of significant benefit when it comes to achieving functioning and independence in activities of daily living goals after stroke. Non‐compliance with drug therapy (failure of a patient to take medication as it is prescribed by a health professional) is known to adversely affect patients with glaucoma in general populations (Patel 1995). Non‐compliance with drug therapy regimes in patients with complex problems and needs after stroke may also adversely affect outcomes. Education and awareness among ward or rehabilitation unit staff must be effective to ensure drug therapy is continued during inpatient episodes.
Surgery
While arguably the physical effects of surgery ought to be similar in the population of older people and the population of stroke patients, there are some specific important issues which may impact on the benefits of a surgical intervention. For example, do patients with mobility or perceptual problems following stroke have the ability to care for themselves adequately post‐surgery? What is the impact of the post‐surgical care on the mobility, functioning and well‐being of a patient with stroke? Such questions pose themselves particularly where recovery from surgery may require bed rest in a prone position. If patients are unable to administer essential eye drops, due to upper limb, cognitive or perceptual problems, surgical intervention may remove the need for eye drops and improve independence, functional ability and quality of life. Indeed, there is a case for surgical intervention with conditions such as glaucoma where compliance with drug therapy is poor (Patel 1995).
Visual aids and equipment
Many visual aids used to assist with age‐related visual problems are hand‐held and may therefore be difficult to handle for those with upper limb problems. Learning to use visual aids and equipment may require intact cognitive processing, and patients with cognitive difficulties following stroke may be unable to acquire such new skills. Thus, while health professionals tend to encourage the use of visual aids and equipment to assist patients with age‐related visual problems, certain subgroups of patients with stroke may not benefit fully from such aids and equipment.
Assessment and screening interventions
Appropriate assessment and screening may help encourage patients with stroke to participate more effectively in rehabilitation after stroke. For example, screening and ensuring that patients wear appropriate glasses for their needs during therapy may affect rehabilitation (Freeman 1988). This may be particularly important for patients with speech or cognitive problems who are unable to ensure that they wear the correct glasses (MacDiarmid 2007). Thus, very simple and low‐cost screening may be particularly effective in improving rehabilitation outcomes in stroke patients with age‐related eye problems.
Why it is important to do this review
Age‐related visual problems in older people are extremely common and diverse in nature. They can result in wide‐ranging functional difficulties that adversely affect rehabilitation should a person also have a history of stroke. There are a wide variety of treatment and rehabilitation options for age‐related visual problems and, while there is a large group of patients who have both stroke and age‐related visual problems, we lack evidence of the effectiveness of interventions on functional ability. There is one recently published review of the literature relating to age‐related visual problems in stroke (Wolter 2006). This review provides a broad overview of the literature relating to visual problems after stroke, but does not provide a rigorous, systematic analysis of outcomes of treatment interventions for age‐related visual problems.
A high‐quality systematic review of the existing evidence base is essential in order to determine the effectiveness of any treatment or management approaches for stroke patients with age‐related visual problems . This will also facilitate appropriate planning and prioritisation of future primary research.
While other reviews address interventions aimed at stroke‐related visual problems, such as visual field defects, eye movement disorders and visual perceptual problems (neglect, inattention), this review may include participants who have co‐existing age‐related and stroke‐related visual problems, but the emphasis of the review is on the effect of interventions targeting the age‐related visual problems of stroke patients.
Objectives
Research question
Do interventions for age‐related visual problems improve functional ability following stroke?
Specific objectives
-
To determine whether, in stroke patients with age‐related visual problems:
environmental modification is more effective than control, placebo or no intervention in improving functional ability in activities of daily living;
activities of daily living training is more effective than control, placebo or no intervention in improving functional ability in activities of daily living;
pharmacological interventions are more effective than control, placebo or no intervention in improving functional ability in activities of daily living;
surgical interventions are more effective than control, placebo or no intervention in improving functional ability in activities of daily living;
vision aids and equipment interventions are more effective than control, placebo or no intervention in improving functional ability in activities of daily living;
assessment and screening interventions are more effective than control, placebo or no intervention in improving functional ability in activities of daily living.
-
To determine whether, in stroke patients with age‐related visual problems:
environmental modification is more effective than control, placebo or no intervention in improving secondary outcomes;
activities of daily living training is more effective than control, placebo or no intervention in improving secondary outcomes;
pharmacological interventions are more effective than control, placebo or no intervention in improving secondary outcomes;
surgical interventions are more effective than control, placebo or no intervention in improving secondary outcomes;
vision aids and equipment are more effective than control, placebo or no intervention in improving secondary outcomes;
assessment and screening are more effective than control, placebo or no intervention in improving secondary outcomes.
To explore the relationship between patient characteristics and the effect of interventions aimed at improving functional abilities in activities of daily living, using subgroup analysis.
To make specific recommendations for future research into the effectiveness of interventions for age‐related visual disorders in patients with stroke, based on a knowledge of the existing evidence base.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT) and randomised controlled cross‐over trials (we planned to analyse the first phase as a parallel‐group trial).
Types of participants
Adult participants (over 18 years of age) after stroke (using the World Health Organization (WHO) definition of stroke or a clinical definition if not specifically stated, i.e. signs and symptoms persisting longer than 24 hours). We did not limit participants to patients who have age‐related visual problems as we anticipated that some interventions such as routine visual assessment may be aimed at the whole population of stroke patients. However, we also planned to include studies that only included stroke patients who have age‐related visual problems such as AMD, diabetic retinopathy, glaucoma, cataracts and vascular problems. We planned to accept a clinical diagnosis of AMD, cataract, glaucoma or diabetic retinopathy. We planned to document the method of diagnosing the condition; the extent of visual acuity, visual field and other visual function loss and planned subgroup analysis to investigate the effect of no loss, partial loss or severe loss in each category. We excluded studies that included participants who have age‐related visual problems but no stroke. We planned to include studies with participants who have stroke‐related visual problems, assuming that the intervention is one that is specifically targeted at age‐related vision problems. We planned to document the presence or absence and extent of stroke‐related visual problems.
Types of interventions
We planned to include any intervention that is specifically targeted at assessing, treating or correcting age‐related visual problems, or improving the ability of the patient to cope with visual impairment. We anticipated that interventions would include: visual assessment and screening, visual aids and equipment (glasses, lighting, magnifiers, CCTV), surgery (e.g. cataract removal), drugs (e.g. for glaucoma and macular degeneration), training in activities of daily living and environmental modifications.
We planned to compare interventions with a no treatment, placebo or control intervention. We anticipated the following specific comparisons.
Environmental modification versus no treatment, placebo, control or usual care.
Activities in daily living training versus no treatment, placebo, control or usual care.
Pharmacological interventions versus no treatment, placebo, control or usual care.
Surgical interventions versus no treatment, placebo, control or usual care.
Visual aids and equipment versus no treatment, placebo, control or usual care.
Assessment and screening intervention versus usual care.
We classified interventions that use laser treatment as surgery for the purposes of this review.
Types of outcome measures
If possible, we planned to assess outcome at the end of the intervention period and at a follow‐up point (ideally six months after the completion of the intervention). Some of the interventions, such as long‐term use of pharmacological interventions or visual aids, will not have an endpoint; in these cases we planned to assess outcome at a follow‐up point after the start of the intervention (ideally six months after the start of the intervention).
Primary outcomes
Functional ability in activities of daily living
We included studies using the following validated scales: Barthel Activities of Daily Living (ADL) Index (Mahoney 1965), Functional Independence Measure (FIM) (Smith 1990), Modified Rankin Scale, Katz Index of Activities of Daily Living (Katz 1963) and Rehabilitation Activities Profile. If a study reported more than one of these functional ability scales, we planned to use the scale listed earliest in this list.
Secondary outcomes
Functional ability in extended activities of daily living
Nottingham Extended Activities of Daily Living scale, Lawton Instrumental Activities of Daily Living, Frenchay Activities Index (Holbrook 1983), Rivermead ADL Score.
Visual acuity
Snellen and LogMAR chart or equivalents.
Visual field
Data from visual field plots: we planned to include measures of the size or depth, or both, of the visual field loss and descriptions of type of visual field loss.
Visual function
We included contrast sensitivity data.
Balance
Berg Balance Scale (Berg 1989)), Functional Reach (Duncan 1990), Get Up and Go Test (Mathias 1986), Standing Balance Test, Step Test or other standardised balance measure. We would not include measures of weight distribution or postural sway during standing, as it is not possible for us to establish the relationship between ability to maintain balance and these outcomes.
Falls
Number of reported falls, Falls Efficacy Scale (Tinetti 1990).
Depression and anxiety
Hospital Anxiety and Depression Scale, Beck Depressive Inventory, General Health Questionnaire, Geriatric Depression Scale.
Discharge destination or residence after stroke
Dichotomous variable: discharged to previous place of residence, i.e. place of residence prior to stroke or discharged to alternative destination.
Quality of life and social isolation
EQ5D, Health‐Related Quality of Life Scale, Quality of Well Being Scale, SF36.
Adverse events
Any reported adverse events excluding falls and deaths.
Death
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (3 March 2011), the Cochrane Eyes and Vision Group Trials Register (December 2009) and the following electronic bibliographic databases:
MEDLINE (1950 to February 2011) (Appendix 1);
The Cochrane Central Register of Controlled Trials (CENTRAL) 2011 Issue 1, part of The Cochrane Library. www.thecochranelibrary.com (accessed February 2011) (Appendix 2);
EMBASE (1980 to February 2011) (Appendix 3);
CINAHL (1982 to February 2011) (Appendix 4);
AMED (1985 to February 2011);
PsycINFO (1967 to February 2011);
Dissertations & Theses (PQDT) database (1861 to February 2011);
British Nursing Index (1985 to February 2011);
PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy, www.psycbite.com) (February 2011).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we:
-
searched the following registers of ongoing trials:
ClinicalTrials.gov (http://clinicaltrials.gov/) (February 2010);
Current Controlled Trials (www.controlled‐trials.com) (February 2010);
Trials Central (www.trialscentral.org) (February 2010);
Stroke Trials Registry (www.strokecenter.org/trials/) (February 2010);
Health Service Research Projects in Progress (wwwcf.nlm.nih.gov/hsr_project/home_proj.cfm) (February 2010);
National Eye Institute Clinical Studies Database (http://clinicalstudies.info.nih.gov/cgi/protinstitute.cgi?NEI.0.html) (February 2010);
-
handsearched the following journals and conference proceedings:
Australian Orthoptic Journal (1959 to 2010);
British Orthoptic Journal (1939 to 2003);
British and Irish Orthoptic Journal (2004 to 2010);
International Strabismological Association (ISA) (1966 to 2010);
International Orthoptic Association (IOA) (www.liverpool.ac.uk/orthoptics/research/search.htm) (1967 to 2008);
Proceedings of Association for Research in Vision and Ophthalmology (www.arvo.org) (1969 to 2010);
Proceedings of the European Strabismological Association (ESA) (1969 to 2009);
performed citation tracking using Web of Science Cited Reference Search for all included studies;
searched the reference lists of included trials and review articles about vision after stroke;
contacted experts in the field (including authors of included trials and excluded studies identified as possible preliminary or pilot work).
We searched for trials in all languages and arranged translation of trials published in languages other than English.
Data collection and analysis
One review author (CH) ran all the electronic searches, downloaded references into bibliographic software and removed duplicates. One review author (CH) excluded any titles which were obviously not related to stroke and vision. We obtained the abstracts for any references related to stroke and vision. Two review authors (CH and AP) independently considered each of these abstracts, excluded any studies which were clearly not RCTs or cross‐over trials, and excluded any studies where the intervention was clearly not aimed at an age‐related visual problem. We resolved any disagreements between review authors through discussion. We obtained the full papers for any studies included at this stage.
Selection of studies
Two review authors (CH and AP) independently applied the selection criteria, considering and documenting the types of studies, types of participants, intervention, comparisons of intervention and outcome measures. Each review author classified each study as one to include or exclude. If there was disagreement between these two review authors, they reached consensus through discussion involving a third review author.
We planned to list any excluded studies that included participants with age‐related visual problems and stroke in the Characteristics of excluded studies table, with the reason for exclusion. We did not list studies that were excluded because they included participants who had stroke‐related visual problems (i.e. visual field defect, eye movement disorders, visual perceptual problems) but not age‐related visual disorders, unless the two review authors agreed that there was a clear reason to do so.
Data extraction and management
We planned to use a pre‐designed form to extract data from the included studies. Two review authors planned to document the following independently.
Methods: study design, method of randomisation.
Participants: number of participants, inclusion criteria. We planned to document the type of age‐related visual problem and the method of diagnosis. We planned to record the country of origin of participants.
Interventions: description of interventions given to each treatment group including, if relevant, the duration, intensity, frequency or dose. We planned to classify the type of intervention as visual aids and equipment, surgery, drugs, activities of daily living training, environmental modification or assessment and screening. We planned to classify the type of control as no treatment, placebo, control or standard care. We planned to document the professional background of the person providing the intervention (e.g. occupational therapist, orthoptist).
Outcomes: we planned to document the primary and secondary outcomes relevant to this review. Where a study had used a number of different methods of measuring the same outcome, we will planned to note the outcome to be used for any subsequent analysis.
Notes: we planned to document any important confounding variables. If a study included more than two intervention groups, we planned to note the method of including these groups in any subsequent analysis.
In addition, the review authors planned to independently document, if data allowed, the following demographics of the included participants: age, gender, place of residence, type of stroke, side of stroke, time since stroke, presence of stroke‐related visual field loss or eye movement disorder, initial functional ability and previous assessments or interventions for age‐related visual problems.
The review authors planned to resolve any data extraction discrepancies through discussion.
Assessment of risk of bias in included studies
We planned to assess risk of bias using The Cochrane Collaboration's 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and by answering the following questions for each included study and documenting this within 'Risk of bias' tables.
Was allocation adequately concealed?
Studies with adequate concealment would include those which have used central randomisation at a site remote from the study; computerised allocation, in which records are in a locked readable file that can be accessible only after entering patient details; and the drawing of opaque envelopes. Studies with inadequate concealment would include those using an open list or table of random numbers, open computer systems or drawing of non‐opaque envelopes. Studies with unclear concealment would include those with no or inadequate information in the report.
Was knowledge of the allocated intervention adequately concealed from the outcome assessor?
We would consider studies adequately concealed if the outcome assessor was masked and the report does not identify any unmasking. We would consider studies inadequately concealed if the outcome assessor was not masked or where the report clearly identifies that unmasking occurred during the study. We would document concealment as unclear if a study does not state whether or not an outcome assessor was masked or there is insufficient information to judge.
Were incomplete outcome data adequately addressed?
Studies adequately addressing incomplete outcome data would have: no missing outcome data; missing outcome data which are unlikely to be related to true outcome; missing outcome data which are balanced in numbers across intervention groups with similar reasons for missing data across groups; a reported effect size (difference in means or standardised difference in means) among missing outcomes which are insufficient to have clinical relevance to observed effect size; or missing data which have been imputed using appropriate methods. Studies inadequately addressing incomplete outcome data would either have: missing outcome data which are likely to be related to true outcome with either imbalance in numbers or reasons for missing data across intervention groups; a reported effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; as‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation. We would document the addressing of incomplete outcome data as unclear if there is insufficient reporting to allow assessment, or if this is not addressed in the report.
Was the study apparently free of other problems that could put it at a high risk of bias?
We would assess a study as not free of bias if it has at least one important risk of bias such as a potential source of bias related to the specific study design used; an extreme baseline imbalance; a claim to have been fraudulent; or some other problem. If there is insufficient information or the information provided is unclear, we would document the risk of other bias as unclear.
We planned to produce a 'Risk of bias' summary figure to illustrate the potential biases within each of the included studies.
Measures of treatment effect
For dichotomous variables, we planned to calculate the treatment effect using a fixed‐effect model and reported as Peto odds ratios (OR) with 95% confidence intervals (CI). For continuous data, we planned to calculate the treatment effect using standardised mean differences (SMD) and 95% CI where studies used different scales for the assessment of the same outcome. We planned to use mean differences (MD) and 95% CI where studies had all used the same method of measuring outcome.
Unit of analysis issues
The primary outcome of functional ability in activities of daily living, and secondary outcomes of functional ability in extended activities of daily living, visual acuity, visual field and visual function data, balance, falls, depression/anxiety, and quality of life and social isolation comprise either ordinal data from measurement scales, count data or continuous data, and we planned to analyse these as continuous variables.
Where reported outcomes have a scale where a lower value is indicative of a better outcome (e.g. count of number of falls, scale of depression/anxiety) we planned to multiple the reported values by ‐1, so that in all analyses a higher value will be indicative of a better outcome.
If studies report change values and the baseline value is available, we planned to calculate the value at follow‐up (change value ‐ baseline value). If studies reported change values and the baseline value is available, we planned to use these data in meta‐analyses, but planned sensitivity analyses to investigate the effect of including these data.
We planned to analyse discharge destination, adverse events and death as dichotomous variables.
Dealing with missing data
If an included study does not report a particular outcome, we planned not to include that study in the analyses of that outcome. If an included study has missing data (e.g. reports means but not standard deviations for the follow‐up data) we planned to take logical steps to enter an assumed value. Such steps may have included estimating a standard deviation based on a reported standard error, or estimating a follow‐up standard deviation based on a baseline value. We planned to do sensitivity analyses to investigate the effect of entering assumed values.
Assessment of heterogeneity
We planned to determine heterogeneity using the I2statistic. If the I2 statistic was greater than 50% we would consider this to be substantial heterogeneity. If the I2 statistic was less than or equal to 50% we would use a fixed‐effect meta‐analysis. If the I2 statistic was greater than 50%, we would explore the individual trial characteristics to identify potential sources of heterogeneity, using pre‐planned subgroup analyses. Where there is substantial heterogeneity, we would perform meta‐analysis using both fixed‐effect and random‐effects modelling to assess sensitivity to the choice of modelling approach. If we find non‐identical results, we would report the most conservative outcome.
Assessment of reporting biases
We attempted to avoid reporting biases by using a comprehensive search strategy which included searching for unpublished studies and searching trials registers. We planned to carry out sensitivity analyses to explore the effect of publication type.
Data synthesis
We planned that two review authors would independently extract data from the included trials. One review author would enter the data into the Review Manager software, RevMan 5.1 (RevMan 2011) and the other review author would check the entries. They would resolve any disagreements through discussion, with reference to the original report.
Subgroup analysis and investigation of heterogeneity
We intended to explore heterogeneity by subgroup analyses to investigate the effect of:
age (under 60 years, 60 years and over);
gender (male, female);
time after stroke (less than three months, less than six months, more than six months);
type of age‐related visual problems (macular degeneration, refractive error, cataracts, glaucoma, diabetic retinopathy);
visual services/intervention received prior to stroke (e.g. regular eye assessment);
severity of visual acuity loss (mild, moderate, severe);
extent of visual field loss (absent, partial, severe);
other visual function loss (presence, absence);
side of stroke (left, right);
presence of visual field loss after stroke (presence, absence);
presence of eye movement disorders (presence/absence);
presence of visual inattention/neglect (presence, absence);
level of motor impairment (mild, moderate, severe);
level of cognitive impairment (mild, moderate, severe);
type of treatment (e.g. for surgery ‐ cataract extraction for cataract, vitrectomy in diabetic retinopathy; for visual aids ‐ stand magnifiers, telescopes; for assessment/screening ‐ by orthoptist, occupational therapist, doctor).
We planned to use an established method for subgroup analyses (Deeks 2001).
Sensitivity analysis
We intended to carry out sensitivity analysis to explore the effect of the following methodological features.
Allocation concealment. We planned to reanalyse data, excluding trials with inadequate or unclear allocation concealment.
Masking of outcome assessor. We planned to reanalyse data, excluding trials without or with unclear masking of outcome assessor.
Missing outcome data. We planned to reanalyse the data, excluding trials with inadequate or unclear methods of dealing with missing outcome data.
Other bias. We planned to reanalyse the data, excluding trials assessed as having other bias, or where it was unclear as to whether they have other bias.
Type of intervention. We planned to reanalyse data, excluding trials where the classification of the type of intervention was uncertain.
Publication type (peer‐reviewed journal, conference abstract/proceedings, doctoral dissertation). We planned to reanalyse data including only those trials from peer‐reviewed journals.
Results
Description of studies
Results of the search
The results of the search are summarised in Figure 1.
1.
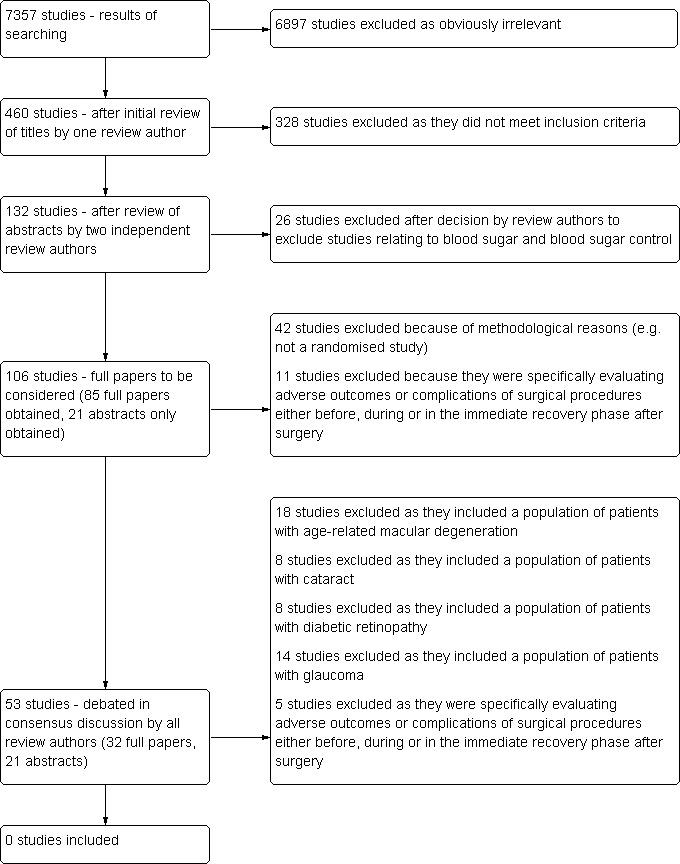
Flow diagram illustrating the results of searching
Our search strategy identified 7357 titles in the main databases. After elimination of duplicates and obviously irrelevant studies we were left with 1034 'possibly relevant' abstracts which covered all topics in this series of reviews. Four hundred and‐sixty related to age‐related visual problems: we obtained these 460 abstracts and two review authors (CH and AP) evaluated their inclusion according to the criteria described in the protocol. At least one of the two review authors assessed 132 abstracts as 'include' or 'unsure'.
Twenty‐six of these 132 abstracts were reports of studies specifically relating to blood sugar or blood sugar control. Discussion amongst the review authors led to the decision to exclude all of these studies, as these were focused on interventions to prevent visual problems which were beyond the scope of this review. This left 106 studies which we aimed to consider after viewing the full paper. We obtained the full paper of 85 of these and two review authors (CH and AP) met to discuss the inclusion of these 85 studies. The remaining 21 of the 106 studies we held abstracts for (and the intention at this stage was to continue to pursue full papers for these studies).
We excluded 53 of the 85 studies (full papers): 42 because of methodological reasons (e.g. not a randomised study) and 11 because they were specifically evaluating adverse outcomes or complications of surgical procedures either before, during or in the immediate recovery phase after surgery, and consequently did not have appropriate outcome measures.
We were unsure about the inclusion or exclusion of the remaining 32 studies (full papers). Twenty‐four of these 32 studies were randomised controlled trials evaluating interventions in populations of people with age‐related macular degeneration (12 studies), cataract (two studies), diabetic retinopathy (six studies) or glaucoma (four studies). We considered it possible that these studies could contain a sub‐population of stroke patients. Discussion amongst the review authors led to the decision to exclude all these studies (see Discussion for further details of this decision). We had questions relating to the methods of the other eight of these 32 studies (e.g. they were not clearly randomised controlled trials) but, as all these studies either included the population of people with age‐related macular degeneration (three studies) or glaucoma (three studies) or were evaluating adverse outcomes or complications of surgery (two studies), we also excluded all eight of these studies (in line with previous decisions made by the review authors).
In light of the decisions made to exclude studies evaluating the adverse outcomes or complications of surgical procedures and studies containing populations of people with age‐related macular degeneration, cataract, diabetic retinopathy or glaucoma, rather than obtain the full papers of the remaining 21 studies which we were still pursuing, we re‐evaluated the abstracts from these studies. We then excluded all 21 of these studies. This decision was made as the studies were evaluating surgical procedures (three studies) or included populations of people with age‐related macular degeneration (three studies), cataract (six studies), diabetic retinopathy (two studies) or glaucoma (seven studies).
Therefore, we identified no studies for inclusion in this review.
Included studies
We did not include any studies in this review.
Excluded studies
We considered 106 studies and discussed them in detail (85 full papers and 21 abstracts). Of the 85 full papers we excluded 42 because of methodological or design reasons (e.g. not a randomised study). These are not listed in the Characteristics of excluded studies table. We excluded 11 studies because they were evaluating adverse outcome or complications of surgical procedures (Ahmed 2002; Akman 2004; Corke 1999; Jacobi 2000; Lira 2001; Morel 2006; Nicholson 2000; Ozdemir 2004; Schein 2000; Thompson 1986; Yuen 2007). Twenty‐four were RCTs which were limited to one specific population of patients with age‐related visual problems:
12 included a population of patients with age‐related macular degeneration (Boyer 2009; Brown 2009; Busbee 2005; Ciulla 2002; Heier 2006; Lai 2009; Michels 2005; Pulido 2006; Regillo 2008; Reichel 2007; Rosenfield 2006; Slakter 2006);
two included a population of patients with cataracts (Harwood 2005; Uusitalo 1999);
six included a population of patients with diabetic retinopathy (DIRECT study 2008a; DIRECT study 2008b; DX‐Retinopathy study 2006; MDR study 2005; PKS‐DRS study 2005; READ‐2 study 2009);
four included a population of patients with glaucoma (Abdollahi 2002; García‐Sánchez 2004; Pfeiffer 2002; Walters 1998).
We excluded eight studies because, although we originally had questions relating to the methods, we later agreed that these were evaluating adverse outcome or complications of surgical procedures or limited to one specific population of patients with age‐related visual problems:
two evaluated surgical procedures (Arraes 2006; Joussen 2009);
three included a population of patients with age‐related macular degeneration (Antoszyk 2008; MPS 1994; Siddiqui 2006);
three included a population of patients with glaucoma (Anderson 2003; Erb 2005; Jampel 2005).
Following decisions made pertaining to the exclusion of studies evaluating adverse outcome or complications of surgical procedures, or limited to one specific population of patients with age‐related visual problems, we reconsidered the abstracts of the 21 studies for which we had not obtained full papers. We excluded all these 21 studies because:
three evaluated surgical procedures (Frezzotti 1982; Maze 1995; Unknown 2003);
three included a population of patients with age‐related macular degeneration (Koester 2006; Unknown 2001; Unknown 2006);
six included a population of patients with cataract (Assia 1998; Davis 1989; Manabe 1994; Manners 1996; Patel 1998; Xu 2009);
two included a population of patients with diabetic retinopathy (Kal 2006; Mikami 1985);
seven included a population of patients with glaucoma (Bojic 1993; Caramazza 1999; Fleck 1991; Geijssen 1990; Kolomoitseva 1994; Ritch 2005; Valimaki 1999).
Note: we have not checked whether any individual references identified in the searches relate to the same study. It is therefore possible that some of our references to 'studies' in fact relate to the same clinical trial. As these are all excluded from the review we did not view this to be important.
Risk of bias in included studies
We did not include any studies in this review.
Effects of interventions
We found no evidence relating to the effect of interventions for age‐related visual problems in the population of people with stroke.
Discussion
Summary of main results
Following extensive and comprehensive searching, we identified no studies which evaluated the effect of interventions for age‐related visual problems in the population of people with stroke. The searching identified:
no studies which only included stroke patients;
no studies which explicitly stated that stroke patients were included as a subgroup of patients with an age‐related problem;
24 studies which might include stroke patients as a subgroup of patients with an age‐related problem (either age‐related macular degeneration, cataract, diabetic retinopathy or glaucoma). Following discussion amongst review authors, we excluded these studies from the review.
We were not explicit in our protocol as to whether or not we would include studies which had a subgroup of stroke patients as part of a larger trial of an intervention for a specific age‐related visual problem. All review authors debated whether to:
exclude all studies which might include stroke patients as a subgroup of patients with age‐related visual problem, or
contact the authors of all the studies which might include stroke patients and ask if they included stroke patients, and whether data were available for this subgroup.
The review authors unanimously agreed that these studies should all be excluded. The key reasons for this were as follows.
Age‐related visual problems (for the general population) are well covered by Cochrane systematic reviews (there are 14 Cochrane reviews relating to age‐related macular degeneration (AMD); eight for cataract; five for diabetic retinopathy; 12 for glaucoma ‐ see Table 1). If there was to be subgroup analysis for stroke patients, the review authors felt that this should most correctly be within these condition‐specific Cochrane reviews (although none of them do currently include subgroup analysis of patients with stroke).
1. Summary of Cochrane reviews of age‐related visual problems.
| Cochrane review | Age‐related visual problem | Intervention studied |
| Eandi 2008 | AMD | Macular translocation |
| Reddy 2006 | AMD | Antiangiogenic therapy with interferon alfa |
| Evans 2008 | AMD | Antioxidant vitamin and mineral supplements for prevention |
| Wormald 2007 | AMD | Photodynamic therapy |
| Gehlbach 2009 | AMD | Statins |
| Vedula 2008 | AMD | Antiangiogenic therapy with anti‐vascular endothelial growth factor |
| Evans 2006 | AMD | Antioxidant vitamin and mineral supplements for slowing the progression |
| Evans 1999 | AMD | Ginkgo biloba extract |
| Virgili 2007 | AMD | Laser photocoagulation |
| Parodi 2009 | AMD | Laser treatment of drusen to prevent progression |
| Giansanti 2009 | AMD | Submacular surgery |
| Casparis 2009 | AMD | Surgery for cataracts |
| Geltzer 2007 | AMD | Surgical implantation of steroids with antiangiogenic characteristics |
| Evans 2010 | AMD | Radiotherapy |
| Fedorowicz 2011 | Cataract | Day care versus in‐patient surgery |
| Leyland 2006 | Cataract | Multifocal versus monofocal intraocular lenses |
| Sivaprasad 2004 | Cataract | Non‐steroidal anti‐inflammatory agents after cataract surgery |
| Alhassan 2008 | Cataract | Peribulbar versus retrobulbar anaesthesia for cataract surgery |
| Keay 2009 | Cataract | Routine preoperative medical testing for cataract surgery |
| Davison 2007 | Cataract | Sub‐tenon's anaesthesia versus topical anaesthesia for cataract surgery |
| Do 2008 | Cataract | Surgery for post‐vitrectomy cataract |
| Riaz 2006 | Cataract | Surgical interventions |
| Smith 2011 | Diabetic retinopathy | Anti‐vascular endothelial growth factor |
| Parravano 2009 | Diabetic retinopathy | Antiangiogenic therapy with anti‐vascular endothelial growth factor |
| Grover 2008 | Diabetic retinopathy | Intravitreal steroids for macular edema |
| Lopes 2008 | Diabetic retinopathy | Pentoxifylline |
| Lopes 2008a | Diabetic retinopathy | Vitamin C and superoxide dismutase |
| Law 2007 | Glaucoma | Acupuncture |
| Minckler 2006 | Glaucoma | Aqueous shunts |
| Kirwan 2009 | Glaucoma | Beta radiation for glaucoma surgery |
| Sycha 2010 | Glaucoma | Interventions for normal tension glaucoma |
| Wilkins 2005 | Glaucoma | Intraoperative mitomycin C for glaucoma surgery |
| Rolim 2007 | Glaucoma | Laser trabeculoplasty |
| Friedman 2006 | Glaucoma | Lens extraction |
| Vass 2007 | Glaucoma | Medical interventions |
| Burr 2004 | Glaucoma | Medical versus surgical interventions |
| Sena 2010 | Glaucoma | Neuroprotection |
| Wormald 2001 | Glaucoma | Post‐operative 5‐fluorouracil |
| Hatt 2006 | Glaucoma | Screening for prevention of optic nerve damage |
AMD: age‐related macular degeneration
Our search strategy was designed to identify studies specific to 'stroke' and 'age‐related visual problems'. This search strategy was not designed to identify all trials to do with AMD, cataract, diabetic retinopathy or glaucoma. Subsequently, further extensive searching would be required if studies which might include stroke patients as a subgroup were to be included.
Managing the subgroup analyses that would arise from the inclusion of condition‐specific randomised controlled trials (RCTs) which had subgroups of stroke patients within this stroke review would be very difficult as there could potentially be a really wide range of interventions and conditions, making the information difficult to synthesise and to access.
To make a clinical decision about the treatment of an age‐related visual problem in a patient who also has stroke, the best ‐ and most comprehensive ‐ evidence to look at is arguably always going to be the Cochrane review specific to that visual problem.
Potential biases in the review process
As there are no studies included in this review, any potential biases arise from the methods of searching and selection of studies. There are two key areas which may potentially have introduced bias into this review.
Decision to exclude RCTs which might include stroke patients as a subgroup of patients with age‐related visual problems
We failed to recognise adequately that our proposed research questions and search strategy would identify RCTs designed to evaluate the effects of interventions to treat or prevent specific age‐related visual problems which may potentially include subgroups of patients with stroke. We failed to develop a protocol designed to explicitly deal with such RCTs. As a result, the decision as to whether to include or exclude RCTs we identified which might include stroke patients as a subgroup had to be made with knowledge of the search results. Knowledge of the potential workload and time implications which would have been associated with inclusion of these studies may have biased the opinions of the review authors who made this decision. However, the decision was unanimously agreed by all 11 review authors, only two of whom (AP and CH) would have been directly affected by the increased workload had the studies been included. We feel that the arguments made for exclusion of this group of studies are strong and valid, and we are confident that this was the correct decision.
Decision not to pursue full papers or further information from authors for some studies
At the time of the decision relating to the exclusion of RCTs which might include stroke patients as a subgroup we were still pursuing full papers to enable us to assess 21 studies, and had questions relating to the design of eight studies. After the decision was made to exclude the RCTs which might include stroke patients, we decided to reappraise the available abstracts or titles, or both, of these 29 studies rather than to continue pursuing full papers. This led to the exclusion of all 29 studies. Arguably we ought to have obtained the full papers for these studies. However, we felt that the selection criteria had been sufficiently clarified to enable us to reapply the selection criteria based on the limited information available on these studies. We are confident that we have not, as a result of lack of information, excluded any studies which met the inclusion criteria.
Clinical relevance of review questions
As this review has resulted in a complex and time‐consuming search process which has not identified any relevant studies we feel it is appropriate to consider the clinical relevance of the questions addressed in this review. This is important in order to make decisions about the future of this Cochrane review ‐ specifically whether time ought to be spent regularly updating the searches.
The issues that this review sought to address arose from discussions amongst a multidisciplinary group of clinicians and are described in the Background section. The first issue related to whether clinicians could apply the evidence base for specific age‐related visual problems to the population of patients who also had stroke. We acknowledged that currently health professionals rely on evidence arising from subgroup analyses of systematic reviews of the wider population of patients with age‐related visual problems, but argued that the complexity of the relationship between these problems justified the need for a systematic review specific to the population of patients with both age‐related visual problems and stroke. In retrospect we now accept that this argument was largely erroneous and we recognise that evidence arising from subgroup analyses of systematic reviews specific to particular age‐related visual problems is likely to provide the most robust evidence. We also recognise that such subgroup analyses will be most effectively synthesised and made accessible within Cochrane reviews relating to those specific age‐related conditions. Nevertheless, our motivation for pursuing this question arose largely out of a clinical interest in the potential impact of interventions for age‐related visual problems on outcomes which are particularly relevant to people with stroke, such as functional activities of daily living and ability to participate successfully in rehabilitation. We believe this remains a clinically relevant and patient‐centred research question, and urge authors of Cochrane reviews relating to age‐related visual problems to consider subgroup analysis to explore the effect of co‐morbid conditions including stroke and to include patient‐centred outcomes such as activities of daily living, depression, anxiety, discharge destination and quality of life.
The second issue which this review sought to address related more specifically to whether health professionals adequately identified and addressed age‐related visual problems in patients with stroke. This included specific issues such as whether health professionals ensured that patients had and used appropriate corrective glasses during rehabilitation. Although we found no randomised clinical trials addressing this issue we believe that this remains a clinically relevant question, which was successfully addressed within the search strategy for this review. However, as the specific clinical relevance of this question arguably relates to interventions which stroke health professionals are likely to deliver (rather than interventions which eye‐care professionals, such as ophthalmologists and optometrists, are qualified to deliver) we propose that the interventions originally identified as relevant to this review are too broad, as they include pharmacological and surgical interventions which would need specialist eye‐care professionals to deliver. Consequently, we propose that for future updates of this review the interventions covered should be reduced to assessment and screening strategies to identify patients with age‐related visual problems and appropriate interventions to compensate or substitute for the presence of age‐related visual problems such as environmental modifications, activities of daily living training, vision aids and equipment interventions. We propose that the review objectives and inclusion criteria are amended accordingly prior to any future updates.
Authors' conclusions
Implications for practice.
There are no implications for practice arising from this review. Evidence relating to the management of patients (from the general population) with age‐related visual problems is available from other Cochrane reviews, and is likely to be the best evidence available for making treatment decisions about individual patients. Health professionals will have to use clinical judgement and expertise to determine the possible impact of treatment for any age‐related visual problem in an individual who has had a stroke.
Implications for research.
Are randomised controlled trials required?
Randomised controlled trials (RCTs) are required to determine the effect of interventions delivered by health professionals working within stroke‐care settings to improve the identification or management of age‐related visual problems. We propose that these should address the impact of interventions including environment modification, activities of daily living training, vision aids and equipment interventions, and assessment and screening interventions, and include outcomes relevant to functional activities of daily living.
Are other primary research studies required?
Other primary research studies may be required in preparation for well‐designed RCTs.
Are further systematic reviews required?
We recommend that future updates of Cochrane systematic reviews addressing specific interventions for age‐related visual problems and low vision rehabilitation should consider subgroup analysis to explore the effect of the intervention on the population of people with stroke. We also recommend that these reviews consider patient‐centred outcomes such as functional activities of daily living and quality of life.
We recommend that the objectives and selection criteria for this Cochrane review are amended and clarified prior to any updates of this review. We also propose that for future updates the searching is, in the first instance, restricted to updates from the Cochrane Central Register of Controlled Trials (CENTRAL), and more extensive searching is only performed when we have evidence of active research in this field.
Acknowledgements
We would like to thank Brenda Thomas and Marion Kelt for their help in developing the search strategy.
Appendices
Appendix 1. MEDLINE search strategy
To avoid duplication of effort we designed broad search strategies for the major databases sensitive enough to cover the scope of a series of three Cochrane reviews of interventions for different visual disorders following stroke. We devised the following search strategy, using a combination of controlled vocabulary (MeSH) and free‐text terms, for MEDLINE and modified it to suit other databases.
MEDLINE (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp eye/ 9. exp visually impaired persons/ 10. exp ocular physiological processes/ or exp diagnostic techniques, ophthalmological/ 11. Optometry/ or Orthoptics/ 12. eye diseases/ or vision disorders/ or eye manifestations/ or blindness/ or diplopia/ 13. vision, binocular/ or vision, monocular/ or exp visual acuity/ or visual fields/ or vision, low/ or perimetry/ or ophthalmology/ or vision screening/ 14. exp eye diseases, hereditary/ or exp eye hemorrhage/ or exp lacrimal apparatus diseases/ or exp lens diseases/ or exp ocular hypertension/ or exp ocular hypotension/ or exp ocular motility disorders/ or exp optic nerve diseases/ or exp orbital diseases/ or exp pupil disorders/ or exp refractive errors/ or exp retinal diseases/ or exp blindness, cortical/ or exp hemianopsia/ or exp vitreoretinopathy, proliferative/ or exp vitreous detachment/ or scotoma/ 15. abducens nerve/ or oculomotor nerve/ or trochlear nerve/ 16. (nystagmus or smooth pursuit or saccades or depth perception or stereopsis or gaze disorder$ or retinal or retinopathy or macular degeneration or glaucoma or cataract$ or ophthalmol$ or optic nerve).tw. 17. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation or (one adj3 half syndrome)).tw 18. ((visual$ or vision or eye or eyes or eyesight or sight) adj5 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw. 19. (hemianop$ or blindness or low vision or refractive errors or vitreoretinopathy or vitreous detachment or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw. 20. (oscillopsia or visual tracking or fresnel prism$).tw 21. ((III or IV or VI or third or fourth or sixth) adj3 nerve palsy).tw 22. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 23. 7 and 22 24. Randomized Controlled Trials as Topic/ 25. random allocation/ 26. Controlled Clinical Trials as Topic/ 27. control groups/ 28. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 29. double‐blind method/ 30. single‐blind method/ 31. Placebos/ 32. placebo effect/ 33. cross‐over studies/ 34. Multicenter Studies as Topic/ 35. Therapies, Investigational/ 36. Drug Evaluation/ 37. Research Design/ 38. Program Evaluation/ 39. evaluation studies as topic/ 40. randomized controlled trial.pt. 41. controlled clinical trial.pt. 42. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 43. multicenter study.pt. 44. (evaluation studies or comparative study).pt. 45. random$.tw. 46. (controlled adj5 (trial$ or stud$)).tw. 47. (clinical$ adj5 trial$).tw. 48. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 49. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 50. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 51. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 52. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 53. (coin adj5 (flip or flipped or toss$)).tw. 54. latin square.tw. 55. versus.tw. 56. (cross‐over or cross over or crossover).tw. 57. placebo$.tw. 58. sham.tw. 59. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 60. controls.tw. 61. (treatment$ adj6 order).tw. 62. or/24‐61 63. 23 and 62 64. exp child/ or exp infant/ 65. (neonat$ or child or children or childhood or juvenile or infant or toddler).tw 66. exp neoplasms/ 67. (cancer$ or carcinoma$ or tumor$ or tumour$ or neoplasm$).tw 68. case reports.pt or case report$.tw 69. 64 or 65 or 66 or 67 or 68 70. 63 not 69 71. limit 70 to humans
Appendix 2. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
MeSH descriptor Cerebrovascular Disorders, this term only
MeSH descriptor Basal Ganglia Cerebrovascular Disease explode all trees
MeSH descriptor Brain Ischemia explode all trees
MeSH descriptor Carotid Artery Diseases explode all trees
MeSH descriptor Intracranial Arterial Diseases explode all trees
MeSH descriptor Intracranial Arteriovenous Malformations explode all trees
MeSH descriptor Intracranial Embolism and Thrombosis explode all trees
MeSH descriptor Intracranial Hemorrhages explode all trees
MeSH descriptor Stroke explode all trees
MeSH descriptor Brain Infarction explode all trees
MeSH descriptor Vasospasm, Intracranial, this term only
MeSH descriptor Vertebral Artery Dissection, this term only
stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplex* or SAH
(brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)
(brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
MeSH descriptor Hemiplegia, this term only
MeSH descriptor Paresis explode all trees
hemipleg* or hemipar* or paresis or paretic 1735
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
MeSH descriptor Eye explode all trees
MeSH descriptor Visually Impaired Persons explode all trees
MeSH descriptor Ocular Physiological Processes explode all trees
MeSH descriptor Diagnostic Techniques, Ophthalmological explode all trees
MeSH descriptor Optometry explode all trees
MeSH descriptor Orthoptics explode all trees
MeSH descriptor Eye Diseases, this term only
MeSH descriptor Vision Disorders, this term only
MeSH descriptor Eye Manifestations, this term only
MeSH descriptor Blindness, this term only
MeSH descriptor Diplopia explode all trees
MeSH descriptor Vision, Binocular, this term only
MeSH descriptor Vision, Monocular, this term only
MeSH descriptor Visual Acuity explode all trees
MeSH descriptor Visual Fields, this term only
MeSH descriptor Vision, Low, this term only
MeSH descriptor Perimetry, this term only
MeSH descriptor Ophthalmology, this term only
MeSH descriptor Vision Screening, this term only
MeSH descriptor Eye Diseases, Hereditary explode all trees
MeSH descriptor Eye Hemorrhage explode all trees
MeSH descriptor Lacrimal Apparatus Diseases explode all trees
MeSH descriptor Lens Diseases explode all trees
MeSH descriptor Ocular Hypertension explode all trees
MeSH descriptor Ocular Hypotension explode all trees
MeSH descriptor Ocular Motility Disorders explode all trees
MeSH descriptor Optic Nerve Diseases explode all trees
MeSH descriptor Orbital Diseases explode all trees
MeSH descriptor Pupil Disorders explode all trees
MeSH descriptor Refractive Errors explode all trees
MeSH descriptor Retinal Diseases explode all trees
MeSH descriptor Blindness, Cortical explode all trees
MeSH descriptor Hemianopsia explode all trees
MeSH descriptor Vitreoretinopathy, Proliferative explode all trees
MeSH descriptor Vitreous Detachment explode all trees
MeSH descriptor Scotoma, this term only
MeSH descriptor Abducens Nerve, this term only
MeSH descriptor Oculomotor Nerve, this term only
MeSH descriptor Trochlear Nerve, this term only
nystagmus or smooth pursuit or saccades or depth perception or stereopsis or gaze disorder* or retinal or retinopathy or macular degeneration or glaucoma or cataract* or ophthalmol* or optic nerve
intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation
one near/3 half syndrome
(visual* or vision or eye or eyes or eyesight or sight) near/5 (problem* or disorder* or impair* or disabilit* or loss or disease* or defect* or manifestation* or screening or test* or examination*)
hemianop* or blindness or low vision or refractive errors or vitreoretinopathy or vitreous detachment or scotoma or diplopia or optometr* or ocular or orthoptic*
oscillopsia or visual tracking or fresnel prism*
III or IV or VI or third or fourth or sixth near/3 nerve palsy
(#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65)
(#19 AND #66)
MeSH descriptor Infant explode all trees
MeSH descriptor Child explode all trees
neonat* or child or children or childhood or juvenile or infan* or toddler
MeSH descriptor Neoplasms explode all trees
cancer* or carcinoma* or tumor* or tumour* or neoplasm*
(#68 OR #69 OR #70 OR #71 OR #72)
(#67 AND NOT #73)
Appendix 3. EMBASE search strategy
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or exp carotid artery disease/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke unit/ or stroke patient.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ or paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp eye/ or exp eye disease/ or exp visual disorder/ 9. exp visual system examination/ or eye examination/ or exp vision test/ 10. exp ophthalmology/ or orthoptics/ or exp visual system/ or exp visual system function/ or depth perception/ 11. exp visual aid/ 12. abducens nerve/ or oculomotor nerve/ or trochlear nerve/ 13. (nystagmus or smooth pursuit or saccades or depth perception or stereopsis or gaze disorder$ or retinal or retinopathy or macular degeneration or glaucoma or cataract$ or ophthalmol$ or optic nerve).tw. 14. (intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation or (one adj3 half syndrome)).tw. 15. ((visual$ or vision or eye or eyes or eyesight or sight) adj5 (problem$ or disorder$ or impair$ or disabilit$ or loss or disease$ or defect$ or manifestation$ or screening or test$ or examination$)).tw. 16. (hemianop$ or blindness or low vision or refractive errors or vitreoretinopathy or vitreous detachment or scotoma or diplopia or optometr$ or ocular or orthoptic$).tw. 17. (oscillopsia or visual tracking or fresnel prism$).tw. 18. ((III or IV or VI or third or fourth or sixth) adj3 nerve palsy).tw. 19. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 20. 7 and 19 21. Randomized Controlled Trial/ 22. Randomization/ 23. Controlled Study/ 24. control group/ 25. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/ 26. Crossover Procedure/ 27. Double Blind Procedure/ 28. Single Blind Procedure/ or triple blind procedure/ 29. latin square design/ 30. Parallel Design/ 31. placebo/ 32. Multicenter Study/ 33. experimental design/ or experimental study/ or quasi experimental study/ 34. experimental therapy/ 35. drug comparison/ or drug dose comparison/ 36. drug screening/ 37. Evaluation/ or "Evaluation and Follow Up"/ or evaluation research/ or clinical evaluation/ 38. Methodology/ 39. "types of study"/ 40. research subject/ 41. Comparative Study/ 42. random$.tw. 43. (controlled adj5 (trial$ or stud$)).tw. 44. (clinical$ adj5 trial$).tw. 45. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 46. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 47. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 48. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 49. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 50. (coin adj5 (flip or flipped or toss$)).tw. 51. latin square.tw. 52. versus.tw. 53. (cross‐over or cross over or crossover).tw. 54. placebo$.tw. 55. sham.tw. 56. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 57. controls.tw. 58. (treatment$ adj6 order).tw. 59. or/21‐58 60. 20 and 59 61. exp child/ or exp newborn/ 62. (neonat$ or child or children or childhood or juvenile or infant or toddler).tw. 63. exp Neoplasm/ 64. (cancer$ or carcinoma$ or tumor$ or tumour$ or neoplasm$).tw. 65. case report/ or case study/ 66. 61 or 62 or 63 or 64 or 65 67. 60 not 66 68. limit 67 to human
Appendix 4. CINAHL search strategy
1. MH "Cerebrovascular Disorders+" or MH "stroke patients" or MH "stroke units" 2. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* ) 3. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) 4. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) 5. S3 and S4 6. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachmoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) 7. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) 8. S6 and S7 9. MH "Hemiplegia" 10. TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic 11. S1 or S2 or S5 or S8 or S9 or S10 12. MH "Eye+" or MH "Rehabilitation of Vision Impaired+" or MH "Optometry" or MH "Eye Diseases+" 13. MH "Visual Acuity+" or MH "Perimetry+" or MH "Ophthalmology+" or MH "Vision Screening+" or MH "Ocular Physiology+" 14. TI ( orthoptics or vision, monocular or vision, binocular ) or AB ( orthoptics or vision, monocular or vision, binocular ) 15. TI ( vitreous detachment or hemianopsia or hemianopia or quadrantanopia ) or AB ( vitreous detachment or hemianopsia or hemianopia or quadrantanopia ) 16. MH "Abducens Nerve" or MH "oculomotor nerve" or MH "troclear nerve" or MH "optic nerve" or MH "nystagmus, pathologic 17. TI ( smooth pursuit or saccades or gaze disorder* or retinal or retinopathy or ophthalmol* ) or AB ( smooth pursuit or saccades or gaze disorder* or retinal or retinopathy or ophthalmol*) 18. TI ( hemianop* or blindness or low vision or refractive errors or vitreoretinopathy or vitreous detachment or scotoma or diplopia or optometry* or ocular or orthoptic* ) or AB ( hemianop* or blindness or low vision or refractive errors or vitreoretinopathy or vitreous detachment or scotoma or diplopia or optometry* or ocular or orthoptic* ) 19. TI ( oscillopsia or visual tracking or fresnel prism* ) or AB ( oscillopsia or visual tracking or fresnel prism* ) 20. TI ( intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation ) or AB ( intranuclear ophthalmoplegia or parinaud's syndrome or weber's syndrome or skew deviation or conjugate deviation ) 21. TI ( visual* or vision or eye or eyes or eyesight or sight ) or AB ( visual* or vision or eye or eyes or eyesight or sight ) 22. TI ( problem* or disorder* or impair* or disability* or loss or disease* or defect* or manifestation* or screening or test* or examination* ) or AB ( problem* or disorder* or impair* or disability* or loss or disease* or defect* or manifestation* or screening or test* or examination* ) 23. 21 and S22 24. TI ( third or fourth or sixth ) or AB ( third or fourth or sixth ) 25. AB nerve palsy or TI nerve palsy 26. S24 and S25 27. S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S23 or S26 28. S11 and S27 29. (MH "Random Assignment") or (MH "Random Sample+") 30. (MH "Crossover Design") or (MH "Clinical Trials+") or (MH "Comparative Studies") 31. (MH "Control (Research)") or (MH "Control Group") 32.(MH "Factorial Design") or (MH "Quasi‐Experimental Studies") or (MH "Nonrandomized Trials") 33. (MH "Placebo Effect") or (MH "Placebos") or (MH "Meta Analysis") 34. (MH "Community Trials") or (MH "Experimental Studies") or (MH "One‐Shot Case Study") or (MH "Pretest‐Posttest Design+") or (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") or (MH "Study Design") 35. (MH "Clinical Research") or (MH "Clinical Nursing Research") 36. PT clinical trial 37. PT systematic review 38. TI random* or AB random* 39. TI ( singl* or doubl* or tripl* or trebl* ) or AB ( singl* or doubl* or tripl* or trebl* ) 40. TI ( blind* or mask* ) or AB ( blind* or mask*) 41. S39 and S40 42. TI ( crossover or cross‐over or placebo* or control* or factorial or sham ) or AB ( crossover or cross‐over or placebo* or control* or factorial or sham ) 43. TI ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) or AB ( clin* or intervention* or compar* or experiment* or preventive or therapeutic ) 44. TI trial* or AB trial* 45. S43 and S44 46. TI ( counterbalance* or multiple baseline* or ABAB design ) or AB ( counterbalance* or multiple baseline* or ABAB design ) 47. TI ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) or AB ( meta analysis* or metaanlaysis or meta‐anlaysis or systematic review* ) 48. S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S41 or S42 or S45 or S46 or S47 49. S28 and S48
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdollahi 2002 | Design: RCT Population: glaucoma |
| Ahmed 2002 | Study evaluates adverse outcome or complications of surgical procedure |
| Akman 2004 | Study evaluates adverse outcome or complications of surgical procedure |
| Anderson 2003 | Design: secondary analysis of RCT Population: glaucoma |
| Antoszyk 2008 | Design: follow‐up data from RCT Population: AMD |
| Arraes 2006 | Design: unclear (translation required) Intervention: surgery Outcomes: safety |
| Assia 1998 | Design: unclear (further information required) Population: cataract Notes: abstract only |
| Bojic 1993 | Design: RCT (more information required) Population: glaucoma Notes: abstract only |
| Boyer 2009 | Design: RCT Population: AMD |
| Brown 2009 | Design: RCT Population: AMD |
| Busbee 2005 | Design: RCT Population: AMD |
| Caramazza 1999 | Design: RCT (more information required) Population: glaucoma Notes: abstract only, translation required |
| Ciulla 2002 | Design: RCT Population: AMD |
| Corke 1999 | Study evaluates adverse outcome or complications of surgical procedure |
| Davis 1989 | Design: RCT Population: cataract Notes: abstract only |
| DIRECT study 2008a | Design: RCT Population: diabetic retinopathy |
| DIRECT study 2008b | Design: RCT Population: diabetic retinopathy |
| DX‐Retinopathy study 2006 | Design: RCT Population: diabetic retinopathy |
| Erb 2005 | Design: unclear (requires translation) Population: glaucoma |
| Fleck 1991 | Design: RCT Population: glaucoma Notes: abstract only |
| Frezzotti 1982 | Design: unsure (more information required) Intervention: surgery Notes: no abstract, more information required |
| García‐Sánchez 2004 | Design: RCT Population: glaucoma |
| Geijssen 1990 | Design: unsure (more information required) Population: glaucoma Notes: no abstract, more information required |
| Harwood 2005 | Design: RCT Population: cataract |
| Heier 2006 | Design: RCT Population: AMD |
| Jacobi 2000 | Study evaluates adverse outcome or complications of surgical procedure |
| Jampel 2005 | Design: review of RCT data Population: glaucoma |
| Joussen 2009 | Design: unclear ‐ does this include a report of a RCT? Intervention: surgery |
| Kal 2006 | Design: unclear (unlikely to be RCT) Population: diabetes Notes: no abstract, more information required |
| Koester 2006 | Design: unclear (more information required) Population: AMD Notes: abstract only |
| Kolomoitseva 1994 | Design: unclear (more information required) Population: glaucoma Notes: abstract only |
| Lai 2009 | Design: RCT Population: AMD |
| Lira 2001 | Study evaluates adverse outcome or complications of surgical procedure |
| Manabe 1994 | Design: unclear (more information required) Population: cataract Notes: abstract only |
| Manners 1996 | Design: RCT Population: cataract Notes: abstract only |
| Maze 1995 | Design: unsure (more information required) Intervention: surgery Notes: no abstract, more information required |
| MDR study 2005 | Design: RCT Population: diabetic retinopathy |
| Michels 2005 | Design: RCT Population: AMD |
| Mikami 1985 | Design: unsure (more information required) Population: diabetic retinopathy Notes: no abstract, more information required |
| Morel 2006 | Study evaluates adverse outcome or complications of surgical procedure |
| MPS 1994 | Design: RCT. Unclear relating to eligibility criteria for people with stroke (were they excluded?) Population: AMD |
| Nicholson 2000 | Study evaluates adverse outcome or complications of surgical procedure |
| Ozdemir 2004 | Study evaluates adverse outcome or complications of surgical procedure |
| Patel 1998 | Design: RCT Population: cataract Notes: abstract only |
| Pfeiffer 2002 | Design: RCT Population: glaucoma |
| PKS‐DRS study 2005 | Design: RCT Population: diabetic retinopathy |
| Pulido 2006 | Design: RCT Population: AMD |
| READ‐2 study 2009 | Design: RCT Population: diabetic retinopathy |
| Regillo 2008 | Design: RCT Population: AMD |
| Reichel 2007 | Design: RCT Population: AMD |
| Ritch 2005 | Design: review Population: glaucoma Notes: abstract only |
| Rosenfield 2006 | Design: RCT Population: AMD |
| Schein 2000 | Study evaluates adverse outcome or complications of surgical procedure |
| Siddiqui 2006 | Design: possibly reports 2 RCTs Population: AMD |
| Slakter 2006 | Design: RCT Population: AMD |
| Thompson 1986 | Study evaluates adverse outcome or complications of surgical procedure |
| Unknown 2001 | Design: report of 2 RCTs (more information required) Population: AMD Notes: abstract only |
| Unknown 2003 | Design: report of RCT (more information required) Intervention: surgery (laser) Notes: abstract only |
| Unknown 2006 | Design: unclear (more information required) Population: AMD Notes: abstract only |
| Uusitalo 1999 | Design: RCT Population: cataract |
| Valimaki 1999 | Design: unsure (more information required) Population: glaucoma Notes: no abstract, more information required |
| Walters 1998 | Design: RCT Population: glaucoma |
| Xu 2009 | Design: unclear Population: cataract Notes: abstract only |
| Yuen 2007 | Study evaluates adverse outcome or complications of surgical procedure |
AMD: age‐related macular degeneration RCT: randomised controlled trial
Differences between protocol and review
The protocol was not clear as to whether RCTs of interventions for specific age‐related visual problems which may potentially include subgroups of patients with stroke should be included or excluded. Review authors made the decision to exclude these studies. This decision is documented and described within the review.
The protocol was not clear as to whether RCTs of interventions relating to blood sugar and blood sugar control, which may prevent the development of age‐related visual problems, should be included or excluded. The review authors made the decision to exclude these studies as they related to the prevention of age‐related visual problems rather than the treatment of age‐related visual problems.
Contributions of authors
Alex Pollock led this review, provided methodological expertise, acted as a second review author and wrote the final review. Christine Hazelton ran searches, identified relevant studies, acted as first review author and provided content expertise.
Clair Henderson, Baljean Dhillon, Heather Orr, Katrina Livingstone, Frank A Munro, Fiona Rowe, Uma Shahani, Jayne Angilley and Peter Langhorne provided additional content expertise, read and commented on drafts, and acted as additional review authors where there was uncertainty or disagreement.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Royal National Institute of Blind People (RNIB, Scotland), UK.
Funded this review, and employs Clair Henderson
-
Chief Scientist Office, Scottish Government, UK.
The Nursing, Midwifery and Allied Health Professions Research Unit, which is funded by the Chief Scientist Office in Scotland employs Alex Pollock
Declarations of interest
Alex Pollock has received project grant funding to support a body of work relating to visual problems after stroke. The grant funding primarily supports the salary for a research assistant, who is working on a series of reviews and surveys related to visual problems after stroke. The grant funding was awarded by the Royal National Institute for Blind people (RNIB).
Christine Hazelton's post as research assistant on this review is funded by RNIB Scotland.
Clair Henderson works for RNIB, which is the leading organisation in the UK supporting blind and partially sighted people.
Fiona Rowe was Chief Investigator for Vision In Stroke (VIS) study which reported data in relation to age‐related visual impairment in stroke. The VIS study was completely unfunded research work.
The work presented here represents the view of the authors and not necessarily those of the funding bodies.
New
References
References to studies excluded from this review
Abdollahi 2002 {published data only}
- Abdollahi A, Naini M‐T, Shams J, Zarei R. Effect of low‐molecular‐weight heparin on postoperative inflammation in phacomorphic glaucoma. Archives of Iranian Medicine 2002;5(4):225‐9. [Google Scholar]
Ahmed 2002 {published data only}
- Ahmed IIK, Zabriskie NA, Crandall AS, Burns TA, Alder SC, Patel BCK. Topical versus retrobulbar anesthesia for combine phacotrabeculectomy. Prospective randomized study. Journal of Cataract and Refractive Surgery 2002;28:631‐8. [DOI] [PubMed] [Google Scholar]
Akman 2004 {published data only}
- Akman A, Yilmaz G, Oto S, Akova Y. Comparison of various pupil dilation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation. Ophthalmology 2004;111:1693‐8. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson DR, Drance SM, Schulzer M on behalf of the Collaborative Normal‐tension Glaucoma Study Group. Factors that predict the benefit of lowering intraocular pressure in normal tension glaucoma. American Journal of Ophthalmology 2003;136:820‐9. [DOI] [PubMed] [Google Scholar]
Antoszyk 2008 {published data only}
- Antoszyk AN, Tuomi L, Chung CY, Singh A on behalf of the FOCUS Study Group. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration (FOCUS): year 2 results. American Journal of Ophthalmology 2008;145:862‐74. [DOI] [PubMed] [Google Scholar]
Arraes 2006 {published data only}
- Arraes J, Diniz JR, Escarião P, Melo C, Arraes T. Clear lens extraction: visual outcomes and vitreoretinopathy frequence [Extraҫão do cristalino translúcido: resultados visuais e freqüência de vítreo‐retinopatias]. Arquivos Brasileiros de Oftalmologia 2006;69(5):671‐4. [DOI] [PubMed] [Google Scholar]
Assia 1998 {published data only}
- Assia EI, Raskin T, Kaiserman I, Rotenstreich Y, Segev F. Effect of aspirin intake on bleeding during cataract surgery. Journal of Cataract and Refractive Surgery 1998;24(9):1243‐6. [DOI] [PubMed] [Google Scholar]
Bojic 1993 {published data only}
- Bojic L, Racic G, Gosovic S, Kovacevic H. The effect of hyperbaric oxygen breathing on the visual field in glaucoma. Acta Ophthalmologica 1993;71(3):315‐9. [DOI] [PubMed] [Google Scholar]
Boyer 2009 {published data only}
- Boyer DS, Heier JS, Brown DM, Francom S, Ianchulev T, Rubio RG. A phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age‐related macular degeneration. Ophthalmology 2009;116:1731‐9. [DOI] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age‐related macular degeneration: two‐year results of the ANCHOR study. Ophthalmology 2009;116:57‐65. [DOI] [PubMed] [Google Scholar]
Busbee 2005 {published data only}
- Busbee BG. Surgery for hemorrhagic choroidal neovascular lesions of age‐related macular degeneration quality‐of‐life findings SST report no. 14. Evidence‐Based Opthalmopathy 2005;6(2):78‐9. [Google Scholar]
Caramazza 1999 {published data only}
- Caramazza N, Damele M, Parente G, Alessandrini A, Cellini M. Use of polyunsaturated fatty acids in the treatment of glaucomatous optic neuropathy (GON). Annali di Ottalmologia e Clinica Oculistica 1999;125:329‐38. [Google Scholar]
Ciulla 2002 {published data only}
- Ciulla TA, Danis RP, Klein SB, Malinovsky VE, Soni PS, Pratt LM, et al. Proton therapy for exudative age‐related macular degeneration: a randomised, sham‐controlled clinical trial. American Journal of Ophthalmology 2002;134:905‐6. [DOI] [PubMed] [Google Scholar]
Corke 1999 {published data only}
- Corke PJ, Baker J, Cammack R. Comparison of 1% ropivacaine and a mixture of 2% lignocaine and 0.5% bupivacaine for peribulbar anaesthesia in cataract surgery. Anaesthesia and Intensive Care 1999;27:249‐52. [DOI] [PubMed] [Google Scholar]
Davis 1989 {published data only}
- Davis PL, O'Connor JP. Peribulbar block for cataract surgery: a prospective double‐blind study of two local anesthetics. Canadian Journal of Ophthalmology 1989;24(4):155‐8. [PubMed] [Google Scholar]
DIRECT study 2008a {published data only}
- Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT‐Prevent 1) and progression (DIRECT‐Protect 1) of retinopathy in type 1 diabetes: randomised, placebo‐controlled trials. Lancet 2008;372:1394‐402. [DOI] [PubMed] [Google Scholar]
DIRECT study 2008b {published data only}
- Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT‐Protect 2): a randomised placebo‐controlled trial. Lancet 2008;372:1385‐93. [DOI] [PubMed] [Google Scholar]
DX‐Retinopathy study 2006 {published data only}
- Ribeiro ML, Seres AI, Carneiro AM, Stur M, Zourdani A, Caillon P, et al. Effect of calcium dobesilate on progression of early diabetic retinopathy: a randomised double‐blind study. Graefe's Archive for Clinical and Experimental Ophthalmology 2006;244:1591‐600. [DOI] [PubMed] [Google Scholar]
Erb 2005 {published data only}
- Erb C. Early manifest glaucoma trial (EMGT). Update 2004. Ophthalmologe 2005;102:219‐21. [DOI] [PubMed] [Google Scholar]
Fleck 1991 {published data only}
- Fleck BW, Dhillon B, Khanna V, Fairley E, McGlynn C. A randomised, prospective comparison of ND:YAG laser iridoctomy and operative peripheral iridectomy in fellow eyes. Eye 1991;5(3):315‐21. [DOI] [PubMed] [Google Scholar]
Frezzotti 1982 {published data only}
- Frezzotti R. The ophthalmologist's approach to orbital surgery. Journal of Neurosurgical Sciences 1982;26(1):1‐10. [PubMed] [Google Scholar]
García‐Sánchez 2004 {published data only}
- García‐Sánchez J, Rouland J‐F, Spiegel D, Pajic B, Cunliffe I, Traverso C, et al. A comparison of the fixed combination of latanoprost and timolol with the unfixed combination of brimonide and timolol in patients with elevated intraocular pressure. A six month, evaluator masked, multicentre study in Europe. British Journal of Ophthalmology 2004;88:877‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geijssen 1990 {published data only}
- Geijssen HC, Greve EL. Focal ischaemic normal pressure glaucoma versus high pressure glaucoma. Documenta Ophthalmologica 1990;75:291‐302. [DOI] [PubMed] [Google Scholar]
Harwood 2005 {published data only}
- Harwood RH, Foss AJE, Osbom F, Gregson RM, Zaman A, Musud T. Falls and health status in elderly women following first cataract surgery: a randomised controlled trial. British Journal of Ophthalmology 2005;89:53‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heier 2006 {published data only}
- Heier JS, Boyer DS, Ciulla TA, Ferrone PJ, Jumper JM, Gentile RC, et al. Ranibizumab combined with Verteporfin photodynamic therapy in neovascular age‐related macular degeneration. Year 1 results of the FOCUS study. Archives of Ophthalmology 2006;124:1532‐42. [DOI] [PubMed] [Google Scholar]
Jacobi 2000 {published data only}
- Jacobi PC, Dietlein TS, Jacobi FK. A comparative study of topical vs retrobulbar anesthesia in complicated cataract surgery. Archives of Ophthalmology 2000;118(8):1037‐43. [DOI] [PubMed] [Google Scholar]
Jampel 2005 {published data only}
- Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). American Journal of Ophthalmology 2005;140:16‐22. [DOI] [PubMed] [Google Scholar]
Joussen 2009 {published data only}
- Joussen AM, Lux A, Kirchhof B. Shifting of the proliferative vitreoretinopathy milieu after tamponade with heavy silicone oil in eyes prone to proliferative vitreoretinopathy and bleeding. British Journal of Ophthalmology 2009;93:128‐9. [DOI] [PubMed] [Google Scholar]
Kal 2006 {published data only}
- Kal O, Gultekin F, Bakici MZ, Kal A. The relation between anticardiolipin antibodies and complications of type 2 diabetes mellitis. Saudi Medical Journal 2006;27(6):902‐4. [PubMed] [Google Scholar]
Koester 2006 {published data only}
- Koester W. Results with ultraviolet irradiation of autologous blood in age‐related macular degeneration. Arztezeitschrift fur Naturheilverfahren und Regulationsmedizin 2006;47(6):412‐4. [Google Scholar]
Kolomoitseva 1994 {published data only}
- Kolomoitseva EM, Ermakova VN, Abdulkadyrova Z. Results of pikamilon therapy of patients with open‐angle glaucoma. Vestnik Oftalmologii 1994;110(4):4‐7. [PubMed] [Google Scholar]
Lai 2009 {published data only}
- Lai TYY, Liu DTL, Chan K‐P, Luk FOJ, Pang C‐P, Lam DSC. Visual outcomes and growth factor changes of two doses of intravitreal bevacizumab for neovascular age‐related macular degeneration. A randomised, controlled trial. Retina 2009;26:1218‐26. [DOI] [PubMed] [Google Scholar]
Lira 2001 {published data only}
- Lira RPC, Nascimento MA, Moreira‐Filho DC, Kara‐José N, Arieta CEL. Are routine preoperative medical tests needed with cataract surgery?. Pan American Journal of Public Health 2001;10(1):13‐7. [DOI] [PubMed] [Google Scholar]
Manabe 1994 {published data only}
- Manabe R, Azuma I, Uyama M, Otori T, Yamamoto M. Clinical evaluation of a balanced hemostatic agent, Ophthalm K tablets, by double‐blind controlled trial. Evaluation of the usefulness of Ophthalm K tablets in cataract operation. Folia Ophthalmologica Japonica 1994;45(9):1003‐12. [Google Scholar]
Manners 1996 {published data only}
- Manners TD. Randomised trial of topical versus sub‐Tenon's local anaesthesia for small‐incision cataract surgery. Eye 1996;10(3):367‐70. [DOI] [PubMed] [Google Scholar]
Maze 1995 {published data only}
- Maze M, Poree L, Rabin BC. Anesthetic and analgesic actions of alpha2 adrenoceptor agonists. Pharmacology Communications 1995;6:175‐82. [Google Scholar]
MDR study 2005 {published data only}
- Macugen Diabeteic Retinopathy study group. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112:1747‐57. [DOI] [PubMed] [Google Scholar]
Michels 2005 {published data only}
- Michels S, Wachtlin J, Gamulescu MA, Heimann H, Prünte C, Inhoffen W, et al. Comparison of early retreatment with the standard regimen of verteporfin therapy for neovascular age‐related macular degeneration. Ophthalmology 2005;112:2070‐5. [DOI] [PubMed] [Google Scholar]
Mikami 1985 {published data only}
- Mikami T, Andoh D, Iwafune Y, Yoshimoto H. The effect of ticlopidine on simple diabetic retinopathy. Folia Ophthalmologica Japonica 1985;36(1):88‐91. [Google Scholar]
Morel 2006 {published data only}
- Morel J, Pascal J, Charier D, Pasquale VD, Gain P, Auboyer C, et al. Preoperative peribulbar block in patients undergoing retinal detachment surgery under general anaesthesia: a randomized double‐blind study. Anaesthesia and Analgesia 2006;102:1082‐7. [DOI] [PubMed] [Google Scholar]
MPS 1994 {published data only}
- Macular Photocoagulation Study (MPS) Group. Evaluation of argon green versus krypton red laser for photocoagulation of subfoveal choroidal neovascularization in the macular photocoagulation study. Archives of Ophthalmology 1994;112:1176‐84. [DOI] [PubMed] [Google Scholar]
Nicholson 2000 {published data only}
- Nicholson G, Sutton B, Hall GM. 1% ropivacaine with 0.75% bupivacaine and 2% lidocaine for peribulbar anaesthesia. British Journal of Anaesthesia 2000;84(1):89‐91. [DOI] [PubMed] [Google Scholar]
Ozdemir 2004 {published data only}
- Ozdemir M, Ozdemir G, Zencirci B, Oksuz H. Articaine versus lidocaine plus bupivacaine for peribulbar anaesthesia in cataract surgery. British Journal of Anaesthesia 2004;92(2):231‐4. [DOI] [PubMed] [Google Scholar]
Patel 1998 {published data only}
- Patel BCK, Clinch TE, Burns TA, Shomaker ST, Jessen R, Crandall AS. Prospective evaluation of topical versus retrobulbar anesthesia: a converting surgeon's experience. Journal of Cataract and Refractive Surgery 1998;24(6):853‐60. [DOI] [PubMed] [Google Scholar]
Pfeiffer 2002 {published data only}
- Pfeiffer N. A comparison of the fixed combination of latranopost and timolol with its individual components. Graefe's Archive for Clinical and Experimental Ophthalmology 2002;240:893‐9. [DOI] [PubMed] [Google Scholar]
PKS‐DRS study 2005 {published data only}
- The PKC‐DRS study group. The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe nonproliferative diabetic retinopathy. Diabetes 2005;54:2188‐97. [DOI] [PubMed] [Google Scholar]
Pulido 2006 {published data only}
- Pulido JS, Winters JL, Boyer D. Preliminary analysis of the final multicentre investigation of rheopheresis for age‐related macular degeneration (AMD) trial (MIRA‐1) results. Transactions of the American Ophthalmological Society 2006;104:221‐31. [PMC free article] [PubMed] [Google Scholar]
READ‐2 study 2009 {published data only}
- Nguyen QD, Shah SM, Heier JS, Do DV, Lin J, Boyer D, et al. Primary end point (six months) results of the ranibizumab of edema of the macula in diabetes (READ‐2) study. Ophthalmology 2009;116:2175‐81. [DOI] [PubMed] [Google Scholar]
Regillo 2008 {published data only}
- Regillo CD, Brown DM, Abraham P, Yue H, Ianachulev T, Schneider S, et al. Randomized, double‐masked, sham‐controlled trial of ranibizumab for neovascular age‐related macular degeneration: PIER study year 1. American Journal of Ophthalmology 2008;145(2):239‐48. [DOI] [PubMed] [Google Scholar]
Reichel 2007 {published data only}
- Reichel E. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age‐related macular degeneration: year results of the focus study ‐ commentary. Evidence‐Based Ophthalmology 2007;8(2):99‐100. [Google Scholar]
Ritch 2005 {published data only}
- Ritch R. Complementary therapy for the treatment of glaucoma: a perspective. Ophthalmology Clinics of North America 2005;18(4):597‐609. [DOI] [PubMed] [Google Scholar]
Rosenfield 2006 {published data only}
- Rosenfield J, Brown DM, Heier JS, Boyer DS, Kaiser K, Chung CY, et al. Ranibizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2006;355(14):1419‐31. [DOI] [PubMed] [Google Scholar]
Schein 2000 {published data only}
- Schein OD, Katz J, Bass EB. The value of routine preoperative medical testing before cataract surgery. The study of medical testing for cataract surgery. New England Journal of Medicine 2000;342:168‐75. [DOI] [PubMed] [Google Scholar]
Siddiqui 2006 {published data only}
- Siddiqui MAA, Keating GM. Pegaptanib in exudative age‐related macular degeneration. Drugs 2005;65(11):1571‐7. [DOI] [PubMed] [Google Scholar]
Slakter 2006 {published data only}
- Slakter JS, Bochow T, D'Amico DJ, Marks B, Jerdan J, Sullican EK, et al. Anercortabe acetate (15 milligrams) versus photodynamic therapy for treatment of subfoveal neovascularization in age‐related macular degeneration. Ophthalmology 2006;113:3‐13. [DOI] [PubMed] [Google Scholar]
Thompson 1986 {published data only}
- Thompson JT, Glaser BM, Michels RG, Bustros SDE. The use of intravitreal thrombin to control haemorrhage during vitrectomy. Ophthalmology 1986;93(3):279‐81. [DOI] [PubMed] [Google Scholar]
Unknown 2001 {published data only}
- Unknown. Verteporfin. In combination with laser therapy: helpful in some forms of age‐related macular degeneration. Prescrire International 2001;10(53):78‐81. [PubMed] [Google Scholar]
Unknown 2003 {published data only}
- Unknown. New indication in complications of high myopia: no sustained benefit. Prescrire International 2003;12(63):5‐8. [PubMed] [Google Scholar]
Unknown 2006 {published data only}
- Unknown. Ranibizumab (Lucentis) for macular degeneration. Medical Letter on Drugs and Therapeutics 2006;48(1246):85‐6. [PubMed] [Google Scholar]
Uusitalo 1999 {published data only}
- Uusitalo RJ, Maunuksela E‐L, Paloheimo M, Kallio H, Laatikainen L. Converting to topic anesthesia in cataract surgery. Journal of Cataract and Refractive surgery 1999;25:432‐40. [DOI] [PubMed] [Google Scholar]
Valimaki 1999 {published data only}
- Valimaki J. Surgical management of refractory glaucoma with Molteno implant. Acta Ophthalmologica Scandinavica 1999;77(6):728. [PubMed] [Google Scholar]
Walters 1998 {published data only}
- Walkers TR, Maloney S, Slater D, Liss C, Wilson H, Hartenbaum D. Efficacy and tolerability of 0.5% timolol maleate ophthalmic gel‐forming solution QD compared with 0.5% levobunolol hydrochloride BID in patients with open‐angular glaucoma or ocular hypertension. Clinical Therapeutics 1998;20(6):1170‐8. [DOI] [PubMed] [Google Scholar]
Xu 2009 {published data only}
- Xu Y‐P, Zhang J, Shi Y‐Y, Keng C‐X, Liu L‐L. Phacoemulsification combined intraocular lens implantation for cataract patients following pars plana vitrectomy. International Journal of Ophthalmology 2009;9(5):953‐5. [Google Scholar]
Yuen 2007 {published data only}
- Yuen JS, Prineas S, Pham T, Liu H. Effectiveness of superior versus inferior subconjunctival anaesthesia for cataract surgery. Anaesthesia and Intensive Care 2007;35:945‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Alhassan 2008
- Alhassan MB, Kyari F, Ejere HOD. Peribulbar versus retrobulbar anaesthesia for cataract surgery. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004083.pub2] [DOI] [PubMed] [Google Scholar]
Asano 2005
- Asano K, Nomura H, Iwano M, Ando F, Niino N, Shimokata H, et al. Relationship between astigmatism and aging in middle‐aged and elderly Japanese. Japanese Journal of Ophthalmology 2005;49(2):127‐33. [DOI] [PubMed] [Google Scholar]
Berg 1989
- Berg KO, Wood‐Dauphinee S, Williams JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada 1989;41:304‐11. [Google Scholar]
Bunce 2008
- Bunce C, Wormald R. Causes of blind certifications in England and Wales: April 1999‐March 2000. Eye 2008;22:905‐11. [DOI] [PubMed] [Google Scholar]
Burr 2004
- Burr J, Azuara‐Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004399.pub2] [DOI] [PubMed] [Google Scholar]
Casparis 2009
- Casparis H, Lindsley K, Bressler NB. Surgery for cataracts in people with age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006757.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davison 2007
- Davison M, Padroni S, Bunce C, Rüschen H. Sub‐Tenon's anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD006291.pub2] [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. 2nd Edition. London, UK: BMJ Books, 2001. [Google Scholar]
Do 2008
- Do DV, Hawkins B, Gichuhi S, Vedula SS. Surgery for post‐vitrectomy cataract. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006366.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duncan 1990
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. Journal of Gerontology 1990;45(6):M192‐7. [DOI] [PubMed] [Google Scholar]
Eandi 2008
- Eandi CM, Giansanti F, Virgili G. Macular translocation for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006928.pub2] [DOI] [PubMed] [Google Scholar]
Evans 1999
- Evans JR. Ginkgo biloba extract for age‐related macular degeneration. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001775] [DOI] [PubMed] [Google Scholar]
Evans 2004
- Evans JE, Fletcher AE, Wormald RPL. Causes of visual Impairment in people aged 75 years and above in Britain: an add‐on study to the MRC Trial of assessment and management of older people in the community. British Journal of Ophthalmology 2004;88:365‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2006
- Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000254.pub2] [DOI] [PubMed] [Google Scholar]
Evans 2008
- Evans JR, Henshaw KS. Antioxidant vitamin and mineral supplements for preventing age‐related macular degeneration. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000253.pub2] [DOI] [PubMed] [Google Scholar]
Evans 2010
- Evans JR, Sivagnanavel V, Chong V. Radiotherapy for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD004004.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fedorowicz 2011
- Fedorowicz Z, Lawrence D, Gutierrez P, Zuuren EJ. Day care versus in‐patient surgery for age‐related cataract. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD004242.pub4] [DOI] [PubMed] [Google Scholar]
Freeman 1988
- Freeman F, Rudge NB. Cerebrovascular accident and the orthoptist. British Orthoptic Journal 1988;45:8‐18. [Google Scholar]
Friedman 2006
- Friedman D, Vedula SS. Lens extraction for chronic angle‐closure glaucoma. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005555.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gehlbach 2009
- Gehlbach P, Li T, Hatef E. Statins for age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006927.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geltzer 2007
- Geltzer A, Turalba A, Vedula SS. Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005022.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giansanti 2009
- Giansanti F, Eandi CM, Virgili G. Submacular surgery for choroidal neovascularisation secondary to age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006931.pub2] [DOI] [PubMed] [Google Scholar]
Green 2002
- Green J, Siddall H, Murdoch I. Learning to live with glaucoma: a qualitative study of diagnosis and the impact of sight loss. Social Science and Medicine 2002;55(2):257‐67. [DOI] [PubMed] [Google Scholar]
Grover 2008
- Grover D, Li T, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD005656.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatt 2006
- Hatt S, Wormald R, Burr J. Screening for prevention of optic nerve damage due to chronic open angle glaucoma. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006129.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holbrook 1983
- Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age and Ageing 1983;12:166‐70. [DOI] [PubMed] [Google Scholar]
Horowitz 2004
- Horowitz A. The prevalence and consequences of vision impairment in later life. Topics in Geriatric Rehabilitation 2004;20(3):185‐95. [Google Scholar]
Johansen 2003
- Johansen A, White S, Waraisch P. Screening for visual impairment in older people. Archives of Gerontology and Geriatrics 2003;36:289‐93. [DOI] [PubMed] [Google Scholar]
Jones 2006
- Jones SA, Shinton RA. Improving outcome in stroke patients with visual problems. Age and Aging 2006;35:560‐5. [DOI] [PubMed] [Google Scholar]
Katz 1963
- Katz S, Ford AB. Studies of illness in the aged. The Index of ADL: a standardised measure of biological and psychosocial function. JAMA 1963;185:914‐9. [DOI] [PubMed] [Google Scholar]
Keay 2009
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007293.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirwan 2009
- Kirwan JF, Rennie C, Evans JR. Beta radiation for glaucoma surgery. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003433.pub2] [DOI] [PubMed] [Google Scholar]
Law 2007
- Law SK, Li T. Acupuncture for glaucoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyland 2006
- Leyland M, Pringle E. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003169.pub2] [DOI] [PubMed] [Google Scholar]
Lopes 2008
- Lopes de Jesus CC, Atallah AN, Valente O, Moça Trevisani VF. Pentoxifylline for diabetic retinopathy. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006693.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopes 2008a
- Lopes de Jesus CC, Atallah AN, Valente O, Moça Trevisani VF. Vitamin C and superoxide dismutase (SOD) for diabetic retinopathy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006695.pub2] [DOI] [PubMed] [Google Scholar]
Lotery 2000
- Lotery AJ, Wiggam MI, Jackson AJ, Silvestri G, Refson K, Fullerton KJ, et al. Correctable visual impairment in stroke rehabilitation patients. Age and Aging 2000;29:221‐2. [DOI] [PubMed] [Google Scholar]
MacDiarmid 2007
- MacDiarmid S, Rowe FJ, Parsons F. Interdisciplinary aspects of vision and communication deficits following stroke. British and Irish Orthoptic Journal 2007;4:21‐6. [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Mathias 1986
- Mathias S, Nayak U, Isaacs B. Balance in elderly patients: the "Get‐up and Go" test. Archives of Physical Medicine and Rehabilitation 1986;67:387‐9. [PubMed] [Google Scholar]
Minckler 2006
- Minckler DS, Vedula SS, Li TJ, Mathew MC, Ayyala RS, Francis BA. Aqueous shunts for glaucoma. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004918.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitchell 2006
- Mitchell J, Bradley C. Quality of life in age‐related macular degeneration: a review of literature. Health and Quality of Life Outcomes 2006;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parodi 2009
- Parodi MB, Virgili G, Evans JR. Laser treatment of drusen to prevent progression to advanced age‐related macular degeneration. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006537.pub2] [DOI] [PubMed] [Google Scholar]
Parravano 2009
- Parravano M, Menchini F, Virgili G. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007419.pub2] [DOI] [PubMed] [Google Scholar]
Patel 1995
- Patel SC, Spaeth GL. Compliance in patients prescribed eyedrops for glaucoma. Ophthalmic Surgery 1995;26:233‐6. [PubMed] [Google Scholar]
Reddy 2006
- Reddy U, Krzystolik M. Antiangiogenic therapy with interferon alfa for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005138.pub2] [DOI] [PubMed] [Google Scholar]
Reidy 1998
- Reidy A, Minassian DC, Vafidis G, Joseph J, Farrow S, Wu J, et al. Prevalence of serious eye disease and visual impairment in a north London population: population based, cross sectional study. BMJ 1998;316:1643‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2011.
Riaz 2006
- Riaz Y, Mehta JS, Wormald R, Evans JR, Foster A, Ravilla T, et al. Surgical interventions for age‐related cataract. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001323.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rolim 2007
- Rolim de Moura C, Paranhos Jr A, Wormald R. Laser trabeculoplasty for open angle glaucoma. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003919.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovner 2002
- Rovner BW, Casten RJ, Tasman WS. Effect of depression on visual function in age‐related macular degeneration. Archives of Ophthalmology 2002;120(8):1041‐4. [DOI] [PubMed] [Google Scholar]
Rowe 2009
- Rowe F, Brand D, Jackson CA, Price A, Walker L, Harrison S, et al. Visual impairment following stroke: do stroke patients require vision assessment?. Age and Ageing 2009;38:188‐93. [DOI] [PubMed] [Google Scholar]
Sena 2010
- Sena DF, Ramchand K, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD006539.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivaprasad 2004
- Sivaprasad S, Bunce C, Jyothi S. Non‐steroidal anti‐inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004239.pub2] [DOI] [PubMed] [Google Scholar]
Smith 1990
- Smith P, Hamilton BB, Granger CV. The fone FIM. Research Foundation of the State University of New York 1990.
Smith 2011
- Smith JM, Steel DHW. Anti‐vascular endothelial growth factor for prevention of postoperative vitreous cavity haemorrhage after vitrectomy for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD008214.pub2] [DOI] [PubMed] [Google Scholar]
Stuen 2003
- Stuen C, Faye EE. Vision loss: normal and not normal changes among older adults. Generations 2003;27(1):8‐14. [Google Scholar]
Sycha 2010
- Sycha T, Vass C, Findl O, Bauer P, Groke I, Schmetterer L, et al. Interventions for normal tension glaucoma. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD002222.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinetti 1990
- Tinetti ME, Richman D, Powell L. Falls efficacy as a measure of falling. Journal of Gerontology 1990;45(6):239‐43. [DOI] [PubMed] [Google Scholar]
United Nations 2002
- United Nations. World Population Ageing: 1950‐2050. Report to World Assembly on Ageing. Available from: http://www.un.org/esa/population/publications/worldageing19502050/ 2002 (last accessed 7 December 2011).
Vass 2007
- Vass C, Hirn C, Sycha T, Findl O, Sacu S, Bauer P, et al. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003167.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedula 2008
- Vedula SS, Krzystolik M. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD005139.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Virgili 2007
- Virgili G, Bini A. Laser photocoagulation for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004763.pub2] [DOI] [PubMed] [Google Scholar]
Wang 1994
- Wang Q, Klein BEK, Klein R, Moss SE. Refractive status in the Beaver Dam Eye Study. Investigative Ophthalmology and Visual Science 35;13:4344‐7. [PubMed] [Google Scholar]
Warnecke 2003
- Warnecke P. A caregiver's eye on elders with low vision. Caring 2003;22(1):12‐5. [PubMed] [Google Scholar]
Wilkins 2005
- Wilkins M, Indar A, Wormald R. Intraoperative mitomycin C for glaucoma surgery. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD002897.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1998
- Williams RA, Brody BL, Thomas RG, Kaplan RM, Brown SI. The psychosocial impact of macular degeneration. Archives of Ophthalmology 1998;116(4):514‐20. [DOI] [PubMed] [Google Scholar]
Wolter 2006
- Wolter M, Preda S. Visual deficits following stroke: maximising participation in rehabilitation. Topics in Stroke Rehabilitation 2006;13(3):12‐21. [DOI] [PubMed] [Google Scholar]
Wormald 2001
- Wormald R, Wilkins M, Bunce C. Postoperative 5‐Fluorouracil for glaucoma surgery. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001132] [DOI] [PubMed] [Google Scholar]
Wormald 2007
- Wormald R, Evans JR, Smeeth LL, Henshaw KS. Photodynamic therapy for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002030.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pollock 2010
- Pollock A, Hazelton C, Henderson CA, Angilley J, Dhillon B, Langhorne P, et al. Interventions for age‐related visual problems in patients with stroke. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008390] [DOI] [PMC free article] [PubMed] [Google Scholar]


