Abstract
Perceptual learning is an improvement in one's sensory abilities after training and is thought to help us to better adapt to the sensory environment. Here, we show that perceptual learning also can lead to misperceptions, such that subjects actually perceive stimuli when none are physically presented. After learning, subjects not only showed enhanced performance when tested with the motion direction of the trained stimulus but also often reported seeing dots moving in the trained direction when no stimulus was displayed. We further show that these misperceptions are not attributable to a response bias. These results show that there are costs as well as benefits to perceptual learning and that performance enhancements for a specific feature also can be accompanied by misperceptions of the visual environment.
Keywords: motion, plasticity vision, learning, psychophysics
A central issue in neuroscience is how the adult brain selectively adapts to important environmental changes. Although the brain needs to adapt to new environments, its architecture must protect itself from modification from the continual bombardment of undesirable information. How the brain solves this so-called stability–plasticity dilemma (1, 2) in its sensory areas is largely unresolved.
Plasticity in the early sensory systems has traditionally been thought to occur only during early development and then to be hard-wired in adults (3). This view has been substantiated by studies of critical period development in which gross plasticity of early sensory areas only occurs for a brief period after birth (4, 5). These data were used to support the hypothesis that as opposed to higher-level perceptual stages, the low-level sensory stages need to consistently process primitive sensory features, such as in vision orientation, spatial frequency, and local motion. The stabilization of sensory systems after birth is important because plasticity in sensory areas would alter the input to higher-level processing areas and fundamentally affect our perceptions as well as the comparison between new perceptions and learned templates.
Recently, the view that once sensory systems are stabilized they never change has been challenged by studies of perceptual learning (6–11), which show that even in adults, perceptual abilities can be sharpened with repeated exposure or training. For example, experts such as radiologists develop with training refined abilities to distinguish subtle patterns of tumors in images that show no pattern to the untrained eye (12). Psychophysical studies of visual plasticity have demonstrated that detection or discrimination thresholds can be reduced and usually show a high degree of specificity with respect to the orientation (9, 13, 14), direction (15, 16), retinotopic location (9, 13, 17), and ocularity (13) of the trained visual stimuli. The specificity of perceptual learning has been regarded as a manifestation of plasticity in sensory cortical processes including very low-level stages of processing (18–21), although this does not exclude the involvement of higher-level processing stages (6, 7, 22).
This low-level plasticity has been confirmed by studies of electrophysiology in animals and functional imaging in humans. In the case of vision, single-unit recording studies have shown activity changes of cells in the early visual cortex of monkeys (11, 23–26), and functional imaging in humans shows changes in V1 (27, 28) and the human middle temporal (MT) homologue (29), in correlation with perceptual learning. Learning of features in other modalities such as audition (30–32), somatosensation (33, 34), and motor functions (35, 36) also implicates neural changes in the primary cortical areas for these modalities.
The utility of this sensory plasticity is that it allows our sensory systems to adapt and reflect changes in the surrounding environment. To prevent this plasticity from leading to instability, it has been proposed that focused attention is required gate-learning and thereby selects which features are to be learned. Because attention is usually directed to important features in the environment, it has been presumed that sensory plasticity is a well regulated and controlled process. However, recently it has been found that perceptual learning does not always require attention (16, 18) and can occur from unattended, task-irrelevant stimuli if they are temporally correlated with task-relevant stimuli (37). This finding suggests that perceptual learning may arise through an automatic reinforcement process similar to conditioning.
A natural question to ask when evaluating studies of perceptual learning is, what are the costs of perceptual learning? If there are no costs, why wouldn't sensory processing already be at its maximal level of performance? The standard answers to this question are based on concepts of limited resources. For instance, plasticity that occurs to best fit a given environment may be at the expense of performance in different environments. To date, however, studies of perceptual learning have concentrated only on its benefits, and the possible costs of such learning are ignored.
Here, we report the result that a sensitivity increase for a visual stimulus is associated with a cost (in this case, a misperception) for that same stimulus. We temporally paired a subliminal motion stimulus, which was too dim to be detected by the subjects, with the targets of an unrelated letter task. We made the discovery that, after training, subjects reported seeing motion in the same direction as the subliminally paired motion when presented with blank displays. A careful control experiment indicates that this result is not due to a response bias. We conclude that perceptual learning, in some cases, has a cost in the sense that improvements of task performance for a particular feature can lead to perceptual biases by which subjects misperceive of the visual environment.
Methods
Subjects. Subjects were 18–35 years old and had normal or corrected-to-normal vision. All subjects were naïve as to the purpose of the experiments. A group of eight subjects participated in a pilot study, and a separate group of eight subjects participated in the main experiment. Informed consent was obtained from all participants, and the study conformed to the tenants of the Declaration of Helsinki.
Stimuli. Stimuli were presented on 19-inch cathode-ray tube monitors at a resolution of 1,280 × 768 at 75 Hz controlled by Macintosh G4 computers running os 9.2.2. Experiments were run using custom software. Subjects viewed the display at a distance of 3 feet, and their movements were constrained with a chin rest. Motion stimuli consisted of 200 dots that moved with a speed of 12°/sec. Each dot had a three-frame lifetime. At each frame transition, a new subset of dots was chosen to move in the coherent direction while the rest of the dots moved in random directions.
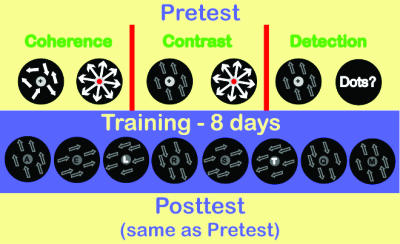
Design. The pilot study and each of the main experiments consisted of three phases (Fig. 1). In phase 1, pretests were conducted in which each subject's performance on low-luminance contrast displays was evaluated. This phase was done for 1 day in the pilot study and for 2 days in the main experiment. In phase 2, the training phase, subjects completed 8–10 sessions in the pilot study and 8 sessions in the main experiment of the letter-pairing task. Each session was conducted on a separate day, and the entire training phase lasted 4 weeks in the pilot study and 2 weeks in the main experiment. In phase 3, posttests were conducted in which each subject's performance was reevaluated with tests that were identical to those used in the pretest phase. Within the test phases, each test type was run in a separate block, and the order of the test types was counterbalanced across subjects (with the exception that warm-up was always run first). Within each test, all conditions (direction and contrast or coherence) were randomly interleaved.
Fig. 1.
Schematic of experimental procedure. All subjects conducted pretests, eight training sessions, and then posttests. In the contrast task, subjects were presented with motion stimuli and reported direction of each stimulus. In the detection task, subjects were presented with the identical stimuli as in the contrast task but reported whether or not they saw the motion stimulus. In RSVP sessions, a sequence of eight foveally (central 1°) presented letters was displayed, and the subject reported the two target letters after the sequence. Subliminal motion stimuli were presented in an annulus (see Methods), one motion direction temporally overlapped each target letter (paired direction), and other directions temporally overlapped the distractors (nonpaired directions).
Pilot Study. For testing sessions, subjects' performance on eight directions (25°, 70°, 115°, 160°, 205°, 250°, 295°, and 340°) of motion was evaluated. In each trial, for all of the three tasks (detection, contrast, and coherence), a fixation point first appeared, and then a motion stimulus was presented for 500 ms, with the fixation point on. Subjects were then cued with a response screen to report their answer. The order of tasks within each testing phase was randomized across subjects.
In the detection task, subjects were presented with 100% coherence motion at 10 randomly interleaved contrasts (chosen to straddle the average threshold on the detection task of a separate group of subjects) and were asked to report, with a key press, the presence or absence of the motion stimulus. Each contrast level was presented 20 times, and thus each subject completed 300 trials for each session.
In the contrast task, subjects were presented with 100% coherence motion at 10 randomly interleaved contrasts (chosen to straddle the average threshold on the detection task of a separate group of subjects) and were asked to choose, with a mouse click, one of eight arrows that corresponded to the direction of the motion stimulus. Each direction at each contrast level was presented 20 times, and thus each subject completed 1,600 trials for each session.
In the coherence task, subjects were presented with 10% coherence motion at root mean squared (RMS) contrast of ≈50 cd/m2 and were asked to choose, with a mouse click, one of eight arrows that corresponded to the direction of the motion stimulus. Each direction was presented 30 times, and thus each subject completed 240 trials for each session.
In the training sessions, a rapid serial visual presentation (RSVP) sequence of eight letters was presented in a central (1°) circle, after which the subject reported the two target letters. The target letters were either light letters in a series of dark distractors or dark letters in a series of light distractors. Letter presentation time was 375 ms temporally centered in a 500-ms motion presentation. Light letters had 5% Michelson contrast, and dark letters had –5% contrast. While letters were presented, 100% coherent motion was presented in a peripheral annulus (1–10°). One motion direction temporally overlapped each target letter (paired direction), and the other directions temporally overlapped the distractors (nonpaired directions). For each subject, four different directions were presented an equal number of times. The paired direction was randomly chosen, from the testing set, for each subject. Each direction was presented for 500 ms at each time. The moving dots were at a contrast where subjects performed at chance level in the contrast task and reported motion on <10% of trials on the detection task (same rate as for 0 contrast).
Main Experiment. For testing sessions, subjects' performance on four directions (70°, 160°, 250°, and 340°) of motion was evaluated. In each trial, for all of the tasks (detection, contrast, and warm-up), a fixation point first appeared, and then a motion stimulus was presented for 500 ms, with the fixation point on. Subjects were then cued with a response screen to report their answer. The order of detection and contrast tasks was randomized across subjects, but the warm-up task was always run first. The detection, contrast, and RSVP tasks were the same as those used in the pilot study, but a different set of parameters was chosen.
In the detection tasks, subjects were presented with 100% coherent motion at 10 randomly interleaved contrasts (0, 0.14, 0.2, 0.28, 0.42, 0.6, 0.9, 1, 1.9, and 11.8 RMS contrast in cd/m2). Each contrast level was presented 30 times, and thus each subject completed 300 trials for each session.
In the contrast tasks, subjects were presented with 100% coherent motion at 10 randomly interleaved contrasts (0, 0.14, 0.2, 0.28, 0.42, 0.6, 0.9, 1, 1.9, and 11.8 RMS contrast in cd/m2). Each direction at each contrast level was presented 30 times, and thus each subject completed 1,200 trials for each session.
In the warm-up task, subjects were presented with the same trial structure as in the contrast task, but no motion stimuli were presented. In each trial, they were asked to choose, with a mouse click, one of four arrows that corresponded to the direction of the perceived motion stimulus. Subjects were instructed that the stimuli would be difficult to see because the task was conducted while they were still adjusting to the dim lighting conditions. Each subject completed 160 trials for each session.
All subjects conducted a practice session with the contrast and detection tasks. This session was conducted on a separate day before the pretests and also to minimize the impact of the testing effects that resulted in the large directionally independent effect found in the contrast task of the pilot study. These sessions also allowed us to choose an appropriate set of contrast levels to use for each pretest by which to capture the dynamic range of all of the subjects.
In the RSVP task, the moving dots had 0.14 cd/m2 RMS contrast. At this contrast, subjects showed chance-level performance in the contrast task and reported motion on <10% of trials on the detection task (same rate as for 0 contrast).
Contrast. For motion stimuli, RMS contrast for the motion stimuli was calculated as the standard deviation of the mean luminance of the stimulus (38, 39): sum[p(i) × (L(i) – Lm)2]1/2, where p(i) is the proportion of pixels with luminance L(i), and Lm is the mean luminance of the stimulus. Lm is sum[p(i) × L(i)].
Analysis. To test for learning of each direction in the coherence task and the biases in the contrast and warm-up tasks, we compared the performance difference between the pretest and posttest with a null learning rate of 0 by using one-tailed t tests. For the contrast psychometric curves, a two-way ANOVA was used to compare performance between the pretest and posttest.
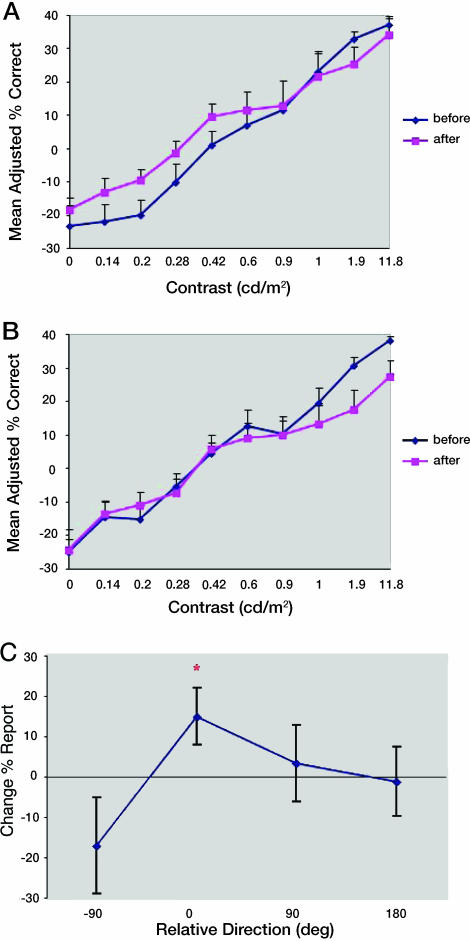
It is important to note that for the 0-contrast trials in the various tests, there is no correct answer. Thus, instead of calculating a value of performance, the bias was established by counting the number of choices made of each direction. For comparison with conditions where a percent-correct value could be calculated, the number of choices for each direction was divided by the total number of trials and then multiplied by the number of direction choices. The 0-contrast value plotted in the contrast psychometric curve (Fig. 3) thus represents a bias, not an actual percent-correct value.
Fig. 3.
Contrast tasks results from the main experiment. (A and B) Psychometric functions for paired (A) and nonpaired (B) directions before and after learning. The daily mean across all conditions was subtracted to adjust for baseline changes. The 0-contrast value represents a bias and not an actual percent correct (see Methods). Contrast is the standard deviation of the mean luminance of the stimulus (see Methods). (C) Directional bias in contrast task. The graph represents the percent change in the number of reports on the 0-contrast trials for each direction between the first and second tests aligned on the paired direction for each subject (0 is the paired direction). Error bars represent standard errors.
Results
Pilot Study. The results of the RSVP task used in the training phase indicate that this task was attentionally demanding. Although the stimulus onset asychrony of 500 ms between the letters in the RSVP task was rather slow, the low (5%) contrast of the letters made the task difficult. Initial performance on the letter task averaged 69.5% (standard deviation, 18.5%) and improved to 77% (standard deviation, 21%) for the final session.
To ensure that the motion stimuli were presented subliminally during the training, the contrast of the dots for the RSVP task was chosen to match a value where subjects performed at chance level in the contrast task and reported motion on <10% of trials on the detection task (for the pilot study, this luminance was below the 2 cd/m2 floor that could be detected by the available spectrophotometer).
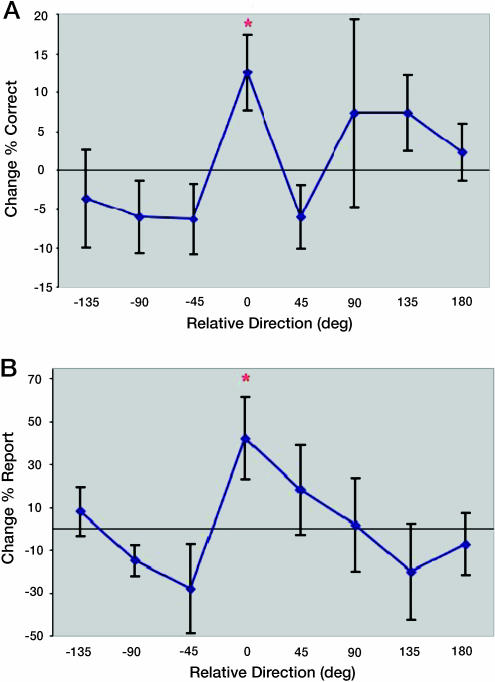
The results of the tests in the pilot study indicate that a subliminal, low-luminance motion stimulus can result in specific learning for the motion direction that was paired with the targets of the RSVP task. In the coherence task, there was a statistically significant improvement for the paired direction of motion (P < 0.05 t test vs. 0; P < 0.01 paired t test vs. other directions) but not for the other motion directions (Fig. 2A). One aspect of this finding is that even though the exposed stimulus was at 100% motion coherence, an improvement can be seen in a task by using bright dots with low motion coherence. Although the results of the contrast task show the same tendency, most subjects showed improvement of performance not only for the paired direction but also for the other directions (data not shown). This change in performance of 14.4% (average across directions and subjects) largely obscured the slightly greater directionally specific improvement for the paired direction.
Fig. 2.
Results from the pilot study. (A) Coherence task results. The graph represents the percent of responses for each direction that were correct on the second test minus those from the first test aligned on the paired direction for each subject (0 is the paired direction). (B) Directional bias in contrast task. The graph represents the percent change in the number of reports on the 0-contrast trials for each direction between the first and second tests aligned on the paired direction for each subject (0 is the paired direction). Error bars represent standard errors.
However, a surprising result of the contrast task was that improved performance was shown for the paired direction, even on the 0-contrast condition, in which no motion stimulus was displayed. To better understand this result, we checked how the bias changed between the pretest and the posttest. We found a dramatic increase of 43% in the number of reports for the paired direction from the pretest to the posttests. This result was significant for the paired direction (P < 0.05 t test vs. 0; P < 0.05 paired t test vs. other directions) but not for the nonpaired directions (Fig. 2B). This effect was highly consistent across subjects, in that seven of eight subjects chose the paired direction more times on the posttest than the pretest.
The results of the contrast task from the pilot study show a clear bias for subjects to report the paired direction when no stimulus is presented but do not rule out the possibility that the subjects' improved performance on the suprathreshold contrast trials resulted in a response bias to report that same direction on the subthreshold trials of that task. To control for this possibility, we ran a new experiment using a different set of subjects. For this experiment, we added a task (warm-up) in which no stimuli were presented, and subjects were asked to make their best guess of the direction they perceived. Subjects were informed that the stimuli would be difficult to see because the task was conducted while they were still adjusting to the dim lighting conditions. The warm-up task was always run first. This order prevents from the warm-up tasks from being influenced by any response bias that could have developed through performing the other tasks. In addition, to minimize directionally nonspecific baseline changes, such as those found in the contrast task of the pilot study, a practice session for the contrast and detection tasks was given to the subjects on a day before the pretests so that subjects would be familiarized with the experimental procedure.
Main Experiment. Performance during the training phase was comparable in the main experiment to that found in the pilot. Initial performance on the RSVP averaged 43% (standard deviation, 27%) and improved to 62% (standard deviation, 19%) for the final session.
In this experiment, we introduced a practice session for the contrast task. Between the practice session and the pretest subjects showed an average increase in performance of 11.8% [practice mean performance, 46.7% (standard deviation, 13%); pretest mean performance, 58.5% (standard deviation, 9.6%)]. This baseline performance change is similar in magnitude to the 14.4% change found between the pretest and posttest of the pilot, and thus much of the expected baseline shift was accounted for before the pretest of the main experiment was conducted.
To ensure that the motion stimuli were presented subliminally during the training, the contrast of the dots for the RSVP task was chosen to be 0.14 cd/m2 RMS contrast, at which level subjects performed at chance level in the practice contrast task [mean, 23% (standard deviation, 3%)] and reported motion on <10% of trials on the practice detection task.
The results of the contrast task, from the main experiment, for the paired direction and the nonpaired directions before and after training are shown in Fig. 3 A and B. Although there were still some baseline changes in this experiment, they were rather small (on average 2.2% improvement across conditions), and we obtained nice psychometric curves by subtracting out each subject's average daily performance across all contrast levels (this is why performance starts off below 0). Significantly improved performance was observed for the paired direction (Fig. 3A) after training (P < 0.01, ANOVA) but not for the nonpaired directions (P = 0.92, ANOVA; see Fig. 3B). Moreover, the change of performance for the paired direction was significantly different from that of the other directions (P < 0.0005, ANOVA). Additionally, we calculated d′ and confirmed that sensitivity improved significantly for the paired direction (P < 0.005, ANOVA).
In the 0-contrast condition of the contrast task, for which no motion stimulus was displayed, subjects in the main experiment also showed an increase in bias to report the paired direction after training. This can be seen in the significant increase (P < 0.05 t test vs. 0; P < 0.05 paired t test vs. other directions) in the number of reports for the paired direction but not for the nonpaired directions (Fig. 3C). This effect was consistent across subjects, in that six of eight subjects chose the paired direction fewer times on the posttest than the pretest.
To better understand the nature of the bias, we examined how subjects' bias changed between the pretest and posttest of the warm-up task. The average result across subjects shows a trend favoring the paired direction, but this was not significant. A problem with this task was revealed through subject interviews showing that some subjects were very uncomfortable reporting the direction of dots that they could not see. It is likely that these subjects, who did not believe our manipulation, resorted to guessing during this task. Thus, to obtain an independent assessment of whether subjects believed the warm-up manipulation, we examined the data from the detection task. Our logic is that if subjects did develop a perceptual bias, then they also would report the presence of dots in the 0-contrast condition in the detection task. This bias would manifest as an increased proportion of false positives in the detection task.
Interestingly, a clear bimodal distribution was observed in the number of false-positive reports for the 0-contrast condition in the detection posttest (Fig. 5A, which is published as supporting information on the PNAS web site). Whereas half (four of eight) of the subjects reported ≈10% false positives for the 0-contrast trials (low-false-positive subjects), the other half reported >30% false positives for this condition (high-false-positive subjects). Thus, we divided the subjects evenly into two categories; high- and low-false-positive subjects. Although all subjects showed an improvement in the detection task between the pretest and posttest (Fig. 5 B and C), most interestingly, the propensity for the high-false-positive subjects to report dots when none were present (0 contrast) changed dramatically between the pretest and posttest of the detection task (Fig. 5B).
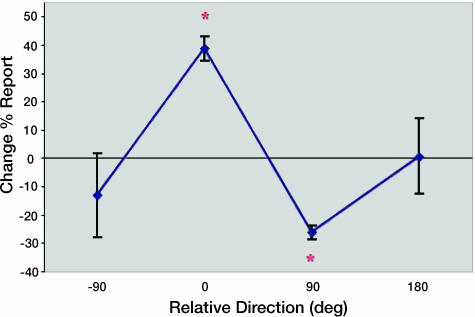
We reanalyzed the data from the warm-up task for these high-false-positive subjects who consistently reported seeing dots when none were presented in the detection task. In the warm-up task these subjects had a significant change in bias (P < 0.001 t test vs. 0; P < 0.001 paired t test vs. other directions) favoring the paired direction (Fig. 4). This change of 39% for the paired direction means that of 160 possible responses, subjects on average chose the paired direction 15.5 more times on the second test than the first (the smallest effect was an increase of 11 reports of the paired direction in one subject). There also was a significant reduction of number of reports for the direction 90° counterclockwise to the paired direction (P < 0.005 t test vs. 0). This reduction of bias for the nonpaired directions is due to the fact that in a forced-choice task, the change in number of reports must sum to zero across conditions, and thus the positive change in the number of reports for the paired direction must be reflected by a negative change for the nonpaired directions.
Fig. 4.
Warm-up task results. The graph represents percent change in the number of reports on the 0-contrast trials for each direction between the first and second tests aligned on the paired direction for each subject (0 is the paired direction). Data represent results from four subjects who reported seeing motion on >30% of the 0-contrast trials in the second detection test. Error bars represent standard errors.
The low-false-positive subjects from the detection task did not show a significant change in bias for any direction (data not shown). The likely reason for this is that these low-false-positive subjects were not confident of their percept in the warm-up task and resorted to guessing. Importantly, the bias for the paired direction was significant across all subjects on the 0-contrast condition in the contrast task (see Figs. 2B and 3C). We suggest that when the 0-contast trials were interleaved with trials in which motion stimuli were actually displayed, subjects had a lower criterion to report a motion direction and thus were less likely to resort to outright guessing, although we cannot rule out the possibility that a response bias influenced the results of the contrast task.
The results of the warm-up task indicate that subjects had indeed developed a perceptual, rather than response, bias to see moving dots when no physical stimulus was presented. In particular, the conjunction of the results from the detection task and the warm-up task demonstrates that the subjects who were more likely to report seeing motion stimuli when none were present also were more likely to report them moving as the paired direction. These results would not be expected to be obtained from a response bias, in which a particular motor response or response object would be favored over the others. In this light, it should be emphasized that while in the RSVP task subjects pressed letter keys, in the detection task subjects pressed number keys, and in the warm-up and detection tasks subjects moved a mouse to respond. Moreover, in informal interviews conducted with a subset of subjects, subjects said that they saw moving dots when presented with a blank display in the absence of a task. Thus, a bias toward a particular response type would not explain the consistency that we found across conditions.
Discussion
In the present study, we found that subjects can learn from stimuli that they did not perceive. Sensitivity improvements were found for the direction of motion that was temporally paired with the targets of the RSVP task but not for motion directions presented with equal frequency but with the RSVP distractors. More importantly, in such a paradigm, we found that subjects developed a bias such that after training they saw moving dots in the paired direction when none were physically shown. This bias cannot be expected from any response bias. We concluded that it is a perceptual bias.
This perceptual bias is an important demonstration of how perceptual learning can indeed have a cost. After training, subjects not only showed increased performance for when the paired direction was displayed, but also misperceived the blank display, in which no motion stimulus was presented. Almost all theories of sensory processing assume that sensory adaptation occurs to best grasp the visual environment (40). However, the present results indicate that this is not always the case. Conditioning to one visual feature appears to be an automatic process, the result of which generalizes to other experimental contexts (for instance, conditioning in the RSVP task generalized to the tests of motion performance). Additionally, in the context of our experiment a misperception of the paired direction yields no advantage either to the RSVP task or the direction tasks. In the example of a radiologist learning to diagnose tumors, misperceptions could have very severe consequences. Thus, misperception as a result of perceptual learning could yield no advantage or be highly misleading.
Although both subject interviews and the posttest results of the contrast and detection tasks indicate that our directional stimuli were unseen by the subjects during the RSVP task, it is difficult to fully prove that no subject ever perceived these stimuli. For instance, standard experimental or internal noise can make dim stimuli visible on some trials. Even if this were the case, the rapidly changing letter stimuli with the direction of motion switching asynchronously with each letter makes it difficult for subjects to notice the pairing between target letters and the paired direction, especially with the task requirement to identify and remember the letter targets. Thus, we find it unlikely that subjects were attending differently to the paired direction than the nonpaired directions of motion. Even if subjects had seen the motion stimuli and noticed the pairing between the motion directions and the target letters, the resulting perceptual bias would still be interesting. Future work will be necessary to test whether the observed learning effects differ when a more salient motion stimulus is used in the pairing.
We believe that neurons in the MT area are likely involved in the learning observed in these studies. Neurons in the MT area have robust responses to visual motion stimuli, are very selective for particular directions of motion (41), and have been shown to be involved in visual plasticity (26). MT cells also respond to motion stimuli that are less bright (i.e., lower luminance) than those that elicit clear responses in earlier stages of the visual pathway (42). Learning also may involve higher visual areas such as the lateral intraparietal sulcus, which has strong inputs from the MT area and contains cells that are modulated strongly both by stimulus attributes and stimulus expectations (43). For instance, cells in the lateral intraparietal sulcus respond in a directionally selective manner to ambiguous motion stimuli (44) and respond in a directionally selective manner when no moving stimulus is present but when motion is inferred (45).
What is the underlying mechanism of the plasticity that results in the misperception? We suggest that presenting a stimulus that is relevant to a task can give rise to an internal reward (37) that works like an external reward in reinforcement learning (46). This reinforcement signal is likely mediated by neurotransmitters such as acetylcholine, noradrenalin, and dopamine, which are widely released from subcortical brain areas in a task-specific manner (47, 48) and have been implicated in neuronal plasticity (30, 31, 49). Specifically, we propose that when subjects detect the targets of the letter task, this internal reinforcement signal results in plasticity of neurons that are active at that time. Visual neurons (perhaps in the MT area or the lateral intraparietal sulcus), which are responsive to the weak visual signal, may thus increase their responses to the paired direction.
Furthermore, these results imply that a sensation can develop as a conditioned response. We suggest that during the experiment, the repeated presentation of the dim motion stimuli resulted in a conditioned perceptual response to see motion. At the same time, the reinforcement procedure resulted in a strengthening of signals related to the paired direction. As a result of this rather automatic processing, when tested, subjects had a conditioned visual response to perceive moving dots in the paired direction. That is, even when subjects are not aware of stimuli in the environment, learning can occur as a result of conditioning reinforced by an internal reward. This process is automatic, and learning can occur irrespective of whether it yields behavioral advantage.
Does the learning of an unattended, subliminal feature paired with a task target indicate that no attention is necessary for perceptual learning? The answer to this question depends on one's definition of attention. For instance, ideas of focused attention in which attention is oriented only to specific stimulus features at the expense of others are inconsistent with learning of unattended features. On the other hand, ideas of nonspecific attention in which the subject has an increased alertness during key points of the task do not preclude learning of subliminal features. In fact, neuromodulatory systems such as that also have been implicated in attention (50, 51).
Judging from the present findings, adult sensory plasticity can be dangerous not only because it can lead to perceptual errors, as we have shown here, but also because it can result in misjudgments of old perceptions. Research of so-called “flash-bulb” memories has shown that even memories held with strong confidence show inaccuracies in many perceptual details (52). One contribution to these inaccuracies of recall is that later experience can contaminate the old memories (53). Such misattribution of perceptual experience can lead to “false memories,” where subjects actually remember events, typically taking place in childhood, that never occurred (54, 55). Sensory learning, which updates the sensory system to better perceive the current environment, may contribute to the formation of these other errors of memory. For instance, studies of visual imagery show a role in early visual area processing (56). Thus, it is possible that plasticity in these areas may alter recalled visual imagery.
Perceptual learning is generally thought to lead to a better grasp of the environment. However, here we show that there is at least some cost to this learning. In addition to improved performance, training also led to misperceptions. Within the context of our study, these misperceptions are relatively benign, but it will be important to examine further the other possible costs of this learning. Future research should concentrate on the broader impacts of perceptual learning, beyond the specific feature improvements that have been studied up to now, to better characterize the full ramifications of this learning.
Supplementary Material
Acknowledgments
We thank Marvin Chun, Todd Herrington, and Piers Howe for comments on the manuscript, as well as John Assad, Richard Born, and Chris Pack for helpful discussions on motion processing in the macaque visual system. This work was supported by National Institutes of Health Grant R01EY015980-01, Human Frontiers Grant RGP18/2004 (to T.W.), and the Japan Society for the Promotion of Science (S.K.).
Author contributions: A.R.S. and T.W. designed research; A.R.S., J.E.N., S.R.H., S.K., and T.W. performed research; A.R.S. and S.R.H. analyzed data; and A.R.S. and T.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RSVP, rapid serial visual presentation; MT, middle temporal; RMS, root mean squared.
References
- 1.Spanis, C. W. & Squire, L. R. (1987) Behav. Neural Biol. 48, 237–245. [DOI] [PubMed] [Google Scholar]
- 2.Grossberg, S. (1980) Psychol. Rev. 87, 1–51. [DOI] [PubMed] [Google Scholar]
- 3.Marr, D. (1982) Vision: A Computational Investigation into the Human Representation and Processing of Visual Information (Freeman, San Francisco).
- 4.Wiesel, T. N. & Hubel, D. H. (1965) J. Neurophysiol. 28, 1060–1072. [DOI] [PubMed] [Google Scholar]
- 5.Hubel, D. H. & Wiesel, T. N. (1964) Naunyn Schmiedebergs Arch. Pharmacol. 248, 492–497. [DOI] [PubMed] [Google Scholar]
- 6.Dosher, B. A. & Lu, Z. L. (1998) Proc. Natl. Acad. Sci. USA 95, 13988–13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahissar, M. & Hochstein, S. (1997) Nature 387, 401–406. [DOI] [PubMed] [Google Scholar]
- 8.Chun, M. M. (2000) Trends Cognit. Sci. 4, 170–178. [DOI] [PubMed] [Google Scholar]
- 9.Fiorentini, A. & Berardi, N. (1980) Nature 287, 43–44. [DOI] [PubMed] [Google Scholar]
- 10.Poggio, T., Fahle, M. & Edelman, S. (1992) Science 256, 1018–1021. [DOI] [PubMed] [Google Scholar]
- 11.Schoups, A., Vogels, R., Qian, N. & Orban, G. (2001) Nature 412, 549–553. [DOI] [PubMed] [Google Scholar]
- 12.Sowden, P. T., Davies, I. R. & Roling, P. (2000) J. Exp. Psychol. Hum. Percept. Perform. 26, 379–390. [DOI] [PubMed] [Google Scholar]
- 13.Fahle, M., Edelman, S. & Poggio, T. (1995) Vision Res. 35, 3003–3013. [DOI] [PubMed] [Google Scholar]
- 14.Schoups, A. A., Vogels, R. & Orban, G. A. (1995) J. Physiol. (London) 483, 797–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ball, K. & Sekuler, R. (1981) J. Exp. Psychol. Hum. Percept. Perform. 7, 780–794. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe, T., Nanez, J. E. & Sasaki, Y. (2001) Nature 413, 844–848. [DOI] [PubMed] [Google Scholar]
- 17.Dill, M. & Fahle, M. (1997) Proc. R. Soc. London Ser. B 264, 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe, T., Nanez, J. E., Sr., Koyama, S., Mukai, I., Liederman, J. & Sasaki, Y. (2002) Nat. Neurosci. 5, 1003–1009. [DOI] [PubMed] [Google Scholar]
- 19.Karni, A. & Sagi, D. (1991) Proc. Natl. Acad. Sci. USA 88, 4966–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crist, R. E., Kapadia, M. K., Westheimer, G. & Gilbert, C. D. (1997) J. Neurophysiol. 78, 2889–2894. [DOI] [PubMed] [Google Scholar]
- 21.Ahissar, M. & Hochstein, S. (1993) Proc. Natl. Acad. Sci. USA 90, 5718–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, Z. (1999) Proc. Natl. Acad. Sci. USA 96, 14085–14087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, C. D., Sigman, M. & Crist, R. E. (2001) Neuron 31, 681–697. [DOI] [PubMed] [Google Scholar]
- 24.Li, W., Piech, V. & Gilbert, C. D. (2004) Nat. Neurosci. 7, 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, T. & Maunsell, J. H. (2004) J. Neurosci. 24, 1617–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zohary, E., Celebrini, S., Britten, K. H. & Newsome, W. T. (1994) Science 263, 1289–1292. [DOI] [PubMed] [Google Scholar]
- 27.Furmanski, C. S., Schluppeck, D. & Engel, S. A. (2004) Curr. Biol. 14, 573–578. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz, S., Maquet, P. & Frith, C. (2002) Proc. Natl. Acad. Sci. USA 99, 17137–17142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaina, L. M., Belliveau, J. W., des Roziers, E. B. & Zeffiro, T. A. (1998) Proc. Natl. Acad. Sci. USA 95, 12657–12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilgard, M. P. & Merzenich, M. M. (1998) Science 279, 1714–1718. [DOI] [PubMed] [Google Scholar]
- 31.Bao, S., Chan, V. T. & Merzenich, M. M. (2001) Nature 412, 79–83. [DOI] [PubMed] [Google Scholar]
- 32.Bao, S., Chang, E. F., Woods, J. & Merzenich, M. M. (2004) Nat. Neurosci. 7, 974–981. [DOI] [PubMed] [Google Scholar]
- 33.Dinse, H. R., Ragert, P., Pleger, B., Schwenkreis, P. & Tegenthoff, M. (2003) Science 301, 91–94. [DOI] [PubMed] [Google Scholar]
- 34.Kaas, J. H., Merzenich, M. M. & Killackey, H. P. (1983) Annu. Rev. Neurosci. 6, 325–356. [DOI] [PubMed] [Google Scholar]
- 35.Li, C. S., Padoa-Schioppa, C. & Bizzi, E. (2001) Neuron 30, 593–607. [DOI] [PubMed] [Google Scholar]
- 36.Pascual-Leone, A., Tarazona, F., Keenan, J., Tormos, J. M., Hamilton, R. & Catala, M. D. (1999) Neuropsychologia 37, 207–217. [DOI] [PubMed] [Google Scholar]
- 37.Seitz, A. R. & Watanabe, T. (2003) Nature 422, 36. [DOI] [PubMed] [Google Scholar]
- 38.Moulden, B., Kingdom, F. & Gatley, L. F. (1990) Perception 19, 79–101. [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Trujillo, J. & Treue, S. (2002) Neuron 35, 365–370. [DOI] [PubMed] [Google Scholar]
- 40.Gibson, E. J. (1969) Principles of Perceptual Learning and Development (Appleton-Century-Crofts, New York).
- 41.Zeki, S. M. (1974) J. Physiol. 236, 549–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sclar, G., Maunsell, J. H. & Lennie, P. (1990) Vision Res. 30, 1–10. [DOI] [PubMed] [Google Scholar]
- 43.Eskandar, E. N. & Assad, J. A. (1999) Nat. Neurosci. 2, 88–93. [DOI] [PubMed] [Google Scholar]
- 44.Williams, Z. M., Elfar, J. C., Eskandar, E. N., Toth, L. J. & Assad, J. A. (2003) Nat. Neurosci. 6, 616–623. [DOI] [PubMed] [Google Scholar]
- 45.Assad, J. A. & Maunsell, J. H. (1995) Nature 373, 518–521. [DOI] [PubMed] [Google Scholar]
- 46.Herzog, M. H. & Fahle, M. (1999) Vision Res. 39, 4232–4243. [DOI] [PubMed] [Google Scholar]
- 47.Schultz, W. (2000) Nat. Rev. Neurosci. 1, 199–207. [DOI] [PubMed] [Google Scholar]
- 48.Dalley, J. W., McGaughy, J., O'Connell, M. T., Cardinal, R. N., Levita, L. & Robbins, T. W. (2001) J. Neurosci. 21, 4908–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnsten, A. F. (1997) J. Psychopharmacol. 11, 151–162. [DOI] [PubMed] [Google Scholar]
- 50.Posner, M. I. & Petersen, S. E. (1990) Annu. Rev. Neurosci. 13, 25–42. [DOI] [PubMed] [Google Scholar]
- 51.Fan, J., McCandliss, B. D., Sommer, T., Raz, A. & Posner, M. I. (2002) J. Cognit. Neurosci. 14, 340–347. [DOI] [PubMed] [Google Scholar]
- 52.Brown, R. & Kulik, J. (1977) Cognition 5, 73–99. [Google Scholar]
- 53.Loftus, E. F. & Greene, E. (1980) Law Hum. Behav. 4, 323–334. [Google Scholar]
- 54.Loftus, E. F. & Pickrell, J. E. (1995) Psychiatr. Ann. 25, 720–725. [Google Scholar]
- 55.Mazzoni, G. A. L., Loftus, E. F., Seitz, A. & Lynn, S. J. (1999) Appl. Cognit. Psychol. 13, 125–144. [Google Scholar]
- 56.Kosslyn, S. M., Pascual-Leone, A., Felician, O., Camposano, S., Keenan, J. P., Thompson, W. L., Ganis, G., Sukel, K. E. & Alpert, N. M. (1999) Science 284, 167–170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.