Abstract
There has been much speculation regarding the functional relevance of G protein-coupled receptor heterodimers, primarily because demonstrating their existence in vivo has proven to be a considerable challenge. Here we show that the opioid agonist ligand 6′-guanidinonaltrindole (6′-GNTI) has the unique property of selectively activating only opioid receptor heterodimers but not homomers. Importantly, 6′-GNTI is an analgesic, thereby demonstrating that opioid receptor heterodimers are indeed functionally relevant in vivo. However, 6′-GNTI induces analgesia only when it is administered in the spinal cord but not in the brain, suggesting that the organization of heterodimers is tissue-specific. This study demonstrates a proof of concept for tissue-selective drug targeting based on G protein-coupled receptor heterodimerization. Importantly, targeting opioid heterodimers could provide an approach toward the design of analgesic drugs with reduced side effects.
Keywords: opioid
Many G protein-coupled receptors (GPCRs) have been shown to dimerize/oligomerize. In some cases, receptor oligomerization is essential for receptor function, i.e., for the GABAB (1), metabotropic glutamate (2), taste receptors (3), and rhodopsin (4), which has been shown to form heterodimers/oligomeric structures in native disk membranes (5). In other cases, oligomerization has been shown to play a modulatory role. In particular, dimerization of opioid receptors has been shown to alter opioid ligand properties and affect receptor trafficking in cell culture model systems (6–8) and in vivo (9). Furthermore, the phenotypes of opioid receptor knockout mice hint that receptor heterodimerization may have functional consequences (10, 11). However, determining whether opioid receptor heterodimers exist and are functionally relevant in vivo has been a true challenge.
We hypothesized that ligands that selectively targeted opioid receptor heterodimers could demonstrate the existence of heterodimers in vivo. Importantly, such a ligand could also have benefits for the treatment of pain. Many of the side effects associated with the use of opiates such as morphine are greatly reduced or eliminated when the drug is administered directly into the spinal cord (12). Thus, opiate drugs that selectively target heterodimers unique to the spinal cord could potentially produce fewer side effects. Because delta opioid peptide (DOP) receptors (DOP-Rs) and kappa opioid peptide (KOP) receptors (KOP-Rs) coexist in spinal neurons, possibly as heterodimers (13), we hypothesized that a KOP/DOP-R heterodimer could be a spinal-selective opioid target. Here we report that 6′-guanidinonaltrindole (6′-GNTI) selectively targets heterodimers and is a tissue-selective analgesic.
Materials and Methods
Receptor Constructs. The DNAs coding for the murine DOP, mu opioid peptide (MOP), KOP (14, 15), and CCR5 receptors were fused with hemagglutinin (HA) tags or FLAG tags on the amino terminus of the respective receptors. All constructs were cloned into the eukaryotic expression vector pcDNA 3.1(+) (Stratagene) and verified by DNA sequencing. Addition of epitope tags does not impair the pharmacological properties of the opioid receptors (16). Construction and functionality of the Gα-subunit Δ6-Gqi4-myr has been described (ref. 17 and references therein).
Transfections and Cells. HEK-293 cells were grown at 7% CO2 and 37°C in Dulbecco's modified Eagle's medium supplemented with 10% FCS. Clonal cell lines stably expressing the respective opioid receptors were generated by selection in drug-containing media. For transient cotransfections of Δ6-Gqi4-myr and/or DOP, KOP, or CCR5 receptors, cells were transfected with the respective amounts of cDNA by using Lipofectamine 2000 according to the manufacturer's instructions (Life Technologies, Grand Island, NY).
Immunofluorescence. Live HEK-293 cells stably expressing the respective receptors were fed monoclonal anti-HA 11 antibody (Covance, Berkeley, CA) and/or M1 FLAG antibody (Sigma) for 30 min to label receptors. Subsequently, cells were fixed with 3% formaldehyde in PBS for 20 min at room temperature and permeabilized with 0.1% Triton X-100, essentially as described in ref. 18. Receptors were labeled with subtype-selective fluorescent anti-mouse antibody directed against M1 (IgG2b) and HA (IgG1) (Molecular Probes). After staining, cells were mounted in Vectashield mounting medium (Vector Laboratories) and analyzed by using a Zeiss LSM 510 META Axioplan 2 confocal microscope.
Coimmunoprecipitation and Serial Immunoprecipitation. Cells were grown to 90% confluency in 10-cm dishes. Cells were washed twice in PBS and lysed in immunoprecipitation buffer [10 mM Tris, pH 7.4/150 mM NaCl/1 mM CaCl/25 mM KCl/0.1% Triton X-100 with protease and phosphatase inhibitors (Sigma)]. Lysate was cleared by centrifugation at 10,000 rpm (Eppendorf 5417R) for 10 min at 4°C, and cleared lysate was immunoprecipitated with 20 μl of anti-FLAG M2-conjugated Sepharose (Sigma) for 2 h at 4°C (Fig. 1b, 1st IP). Cleared lysate was then immunoprecipitated with 20 μl of HA-conjugated affinity matrix (Covance) for 2 h at 4°C (Fig. 1b, 2nd IP). All immunoprecipitates were extensively washed with immunoprecipitation buffer followed by two washes with 10 mM Tris, pH 7.5. Receptors were deglycosylated with PNGase (NEB, Beverly, MA) in 10 mM Tris, pH 7.5, for 1 h at 37°C, denatured with SDS sample buffer, and resolved by SDS/PAGE. Blots were blocked in Blotto, incubated with anti-HA antibody at a concentration of 1:500 (raw ascites, Covance) for 1 h, washed, and incubated with horseradish peroxidase-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch) at a concentration of 1:4,000 for 1 h. Blots were developed with enhanced chemiluminescence reagents (ECL, Amersham Biosciences) to detect heterodimers (Fig. 1b, 1st IP) and homomers/monomers (Fig. 1b, 2nd IP).
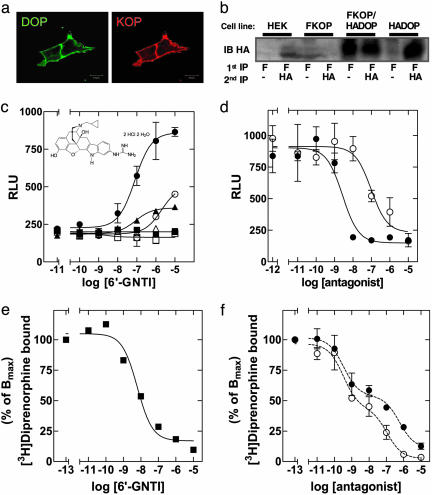
Fig. 1.
6′-GNTI specifically targets KOP/DOP heterodimers. (a) Coexpression of opioid receptor types in HEK-293 cells was visualized by immunofluorescent staining of cells stably coexpressing HA-DOP-R (DOP) and FLAG-KOP-R (KOP) with antibodies directed to the respective epitope tags. (Scale bar = 10 μm.) (b) Ratio of opioid receptor heterodimers versus homomers by serial immunoprecipitation (IP). Cells coexpressing both HA-DOP-R (HADOP) and FLAG-KOP-R (FKOP) or each individually were lysed, and the receptors were immunoprecipitated with anti-FLAG antibody. Immunoprecipitates were immunoblotted (IB) with anti-HA antibody to detect KOP/DOP heterodimers (lanes 5 and 7). After the FLAG immunoprecipitation (F), the lysates were immunoprecipitated with anti-HA antibody and immunoblotted for HA to detect the DOP homomers/monomers remaining after immunoprecipitation of the heterodimers. Compare lanes 5 (heterodimers) and 6 (homomers/monomers). (c)6′-GNTI induced Ca2+ release in HEK-293 cells expressing one or two opioid receptor types. Agonist-mediated intracellular Ca2+ release was measured in cells expressing the chimeric G protein Δ6-Gqi4-myr (200 ng for every 40,000 cells) and MOP-R (▵), DOP-R (□), or KOP-Rs (○) alone or MOP/DOP-Rs (▪), KOP/MOP-Rs (▴), or KOP/DOP-Rs (•). Intracellular Ca2+ release was measured in a Flex apparatus (Molecular Devices), where relative light units (RLU) = maximum Ca2+ peak/cell number × 1,000. Shown are representative curves carried out in duplicate (n = 4). (Inset) Structure of 6′-GNTI. (d) Effects of receptor type-selective antagonists on 6′-GNTI-induced Ca2+ release in cells expressing the KOP/DOP-R heterodimer. Cells expressing the KOP/DOP-R heterodimer were preincubated for 30 min with increasing doses of NTI (○) or NorBNI (•) and stimulated with 100 nM 6′-GNTI. Agonist-induced Ca2+ release was assessed as described in c. Data are mean ± SEM measured in duplicates. (e and f) Effect of 6′-GNTI (e) and KOP-R- and DOP-R-selective antagonists (f) on competition for [3H]diprenorphine binding to cells expressing KOP/DOP-R heterodimers. Whole-cell competition binding experiments were performed on cells stably expressing the KOP/DOP-R heterodimers. Cells were incubated with 1.5 nM [3H]diprenorphine and increasing amounts of 6′-GNTI (▪) (e), NorBNI (•)(f), or NTI (○)(f). Shown are representative curves carried out in duplicate (n = 4). Note that error bars in e are too small to be visualized.
Heterologous competition radioligand binding experiments were performed on whole HEK-293 cells stably expressing the respective receptors as described in ref. 19. The number of cells was such as to obtain 5–10% specific binding of the added radioactive ligand. Assays were performed for 60 min in 120 μl of Krebs–Ringer Hepes buffer containing 1.5 nM [3H]diprenorphine plus unlabeled ligand in concentrations ranging from 0.01 nM to 10 μM. The binding reaction was terminated by filtration over glass-fiber filters and washed in ice-cold Tris-buffered saline, pH 7.4, using a Tomtec cell harvester (Packard Instrument Company, Meriden, CT). Nonspecific binding was determined in the presence of 10 μM antagonist and amounted to ≈5–10% of total binding in the KD concentration range. Bmax, KD, and IC50 values were determined by using prism 2.1 software (GraphPad, San Diego) for one-site or two-site binding and nonlinear regression competition, respectively.
Agonist-Mediated Intracellular Ca2+ Release. Agonism was measured in HEK-293 cells stably expressing the respective receptors and the chimeric G protein Δ6-Gqi4-myr (200 ng for every 40,000 cells). One day after transfection, cells were loaded for 60 min with a Ca2+-fluorophore (Molecular Devices) and stimulated with increasing amounts of ligand as indicated in the figure legends. Intracellular Ca2+ release was measured immediately after agonist application in a Flex apparatus (Molecular Devices) for 2 min.
Analgesia Assay. Male ICR-CD1 mice (Harlan Sprague–Dawley) were injected intracerebroventricularly or intrathecally (i.t.) with increasing amounts of 6′-GNTI as indicated, and peak analgesia was measured by using the modified radiant heat tail-flick test (20). Briefly, a radiant heat source was applied to the tail, and the latency to flick away from the heat source was recorded. A positive antinociceptive response was defined as an increase in latency of at least three standard deviations above the mean of the baseline latency of the whole group. For studies with opioid receptor type-selective antagonists, antagonist was injected at the designated dose 6 min before administration of a dose of 6′-GNTI that alone produced 80% of the maximum possible effect (1 nmol per mouse). Antinociception was measured 10 min after 6′-GNTI injection. At least three groups of 10 mice were used for each drug paradigm, and each mouse was used only once. ED50 values and 95% confidence intervals were calculated by using the parallel line assay. The animal protocols used in these experiments were approved by the University of Minnesota Institutional Animal Care and use Committee.
Results and Discussion
Originally, 6′-GNTI was reported to be a KOP-R agonist based on the observation that its activity could be blocked by the KOP-R-selective antagonist nor-binaltorphimine (NorBNI) in the guinea pig ileum. However, when tested in mouse vas deferens, 6′-GNTI displayed no agonist activity (21), despite the presence of all three opioid receptor types, the MOP receptor (MOP-R), DOP-R, and KOP-R. These tissue-specific differences in 6′-GNTI activity suggested that the receptor target for 6′-GNTI existed in guinea pig ileum but not mouse vas deferens. We propose that this tissue-selective target could be an opioid receptor heterodimer.
To examine whether 6′-GNTI was a heterodimer-selective agonist on opioid receptors, we generated HEK-293 cells stably expressing epitope-tagged versions of the murine MOP-Rs, DOP-Rs or KOP-Rs alone and cell lines coexpressing MOP-Rs and DOP-Rs (MOP/DOP), KOP-Rs and MOP-Rs (KOP/MOP), or KOP-Rs and DOP-Rs (KOP/DOP) combined (Fig. 1a and data not shown). Heterodimers were readily detectable in the double stable cell lines (Fig. 1b and data not shown) and comprised at least 50% of the receptor complexes in the KOP/DOP cells (Fig. 1b). In addition, all of these cell lines expressed the chimeric Gα-subunit Δ6-Gαqi4-myr, which enabled us to assess the signaling properties of activated opioid receptors by measuring Ca2+ release from intracellular stores (17). This approach further guaranteed comparable Gα protein levels in all cell lines.
6′-GNTI was most potent (EC50 ≈ 40 nM) and efficacious (Emax ≈ 400 relative light units) at inducing Ca2+ release in the KOP/DOP cells (Table 1 and Fig. 1c, •), followed by cells expressing KOP/MOP-Rs (EC50 ≈ 100 nM) (Table 1 and Fig. 1c, ▴) and had the lowest potency on cells expressing KOP-Rs alone (EC50 ≈ 220 nM) (Table 1 and Fig. 1c, ○). In contrast, the KOP-R-selective agonist U69,593 was equally active in cells expressing KOP-Rs alone or heterodimer-expressing cells (see Table 1), suggesting that the enhanced activity of 6′-GNTI on the heterodimers did not reflect a general increase in KOP-R agonist potency in heterodimer-expressing cells. Importantly, 6′-GNTI had no agonist effect on cells expressing MOP-Rs (Fig. 1c, ▵) or DOP-Rs (Fig. 1c, □) alone or combined (MOP/DOP) (Fig. 1c, ▪). These findings were confirmed in a GTPγS-assay by using the endogenous G protein pool (data not shown). Although 6′-GNTI had no agonist effect on DOP-Rs or MOP-Rs, it bound to both of these receptors as assessed by displacement binding of the nonselective opioid radioligand [3H]diprenorphine (Table 2). Importantly, the selectivity of 6′-GNTI agonism for the KOP/DOP and KOP/MOP heterodimers did not merely reflect differences in receptor-type expression levels between cell lines, because all cell lines had matching receptor expression levels as assessed by ELISA and radioligand binding (For details, see Supporting Materials and Methods, which is published as supporting information on the PNAS web site).
Table 1. Δ6-Gαqi4myr-mediated Ca2+ release of HEK-293 cells stably expressing opioid receptors.
| Ca2+ release | 6′-GNTI | U69,593 | DPDPE | DAMGO |
|---|---|---|---|---|
| EC50, nM | ||||
| KOP | 224.4 ± 14.2 | 0.83 ± 0.07 | >1,000 | >1,000 |
| DOP | >1,000 | >1,000 | 22.1 ± 1.3 | >1,000 |
| MOP | >1,000 | >1,000 | >1,000 | 6.5 ± 2.2 |
| KOP/DOP | 39.8 ± 3.0 | 0.82 ± 2.5 | 53.1 ± 2.2 | >1,000 |
| KOP/MOP | 112.5 ± 3.1 | 0.34 ± 3.2 | >1,000 | 20.9 ± 1.8 |
| DOP/MOP | >1,000 | >1,000 | 43.2 ± 6.7 | 5.6 ± 2.6 |
| Emax, RLU | ||||
| KOP | 367.9 ± 165.7 | 518.8 ± 299.8 | — | — |
| DOP | — | — | 295.6 ± 65.4 | — |
| MOP | — | — | — | 689.4 ± 314.0 |
| KOP/DOP | 404.3 ± 140.2 | 570.7 ± 156.6 | 441.2 ± 33.0 | — |
| KOP/MOP | 272.2 ± 137.2 | 458.1 ± 206.8 | — | 536.2 ± 270.9 |
| DOP/MOP | — | — | 604.8 ± 209.1 | 531.7 ± 210.9 |
Data are mean ± SEM (n = 3-5). RLU, relative luciferase units; DAMGO, [d-Ala2-MePhe4-Gly5-ol]; —, not applicable.
Table 2. [3H]Diprenorphine displacement with 6′-GNTI and other opioid ligands.
| Binding affinity | 6′GNTI | Diprenorphine | NorBNI | NTI |
|---|---|---|---|---|
| IC50, nM | ||||
| KOP | 50.9 ± 9.8 | 1.09 ± 0.7 | 0.012 ± 0.02 | 26.6 ± 4.2 |
| DOP | 4.7 ± 1.4 | 1.9 ± 2.3 | 81.2 ± 1.4 | 0.004 ± 0.006 |
| MOP | 264.4 ± 66.7 | 0.5 ± 0.06 | 192.3 ± 37.8 | 34.6 ± 8.9 |
| IC50H, nM | ||||
| KOP/DOP | 7.7 ± 1.7 | 1.13 ± 0.2 | 0.34 ± 08 (569.8 ± 18.7)* | 0.46 ± 0.5 (112.2 ± 3.5)* |
| KOP/MOP | 34.9 ± 10.1 | 0.97 ± 0.02 | 12.0 ± 2.8 (>1,000)* | 78.9 ± 22.2 |
| DOP/MOP | 195.1 ± 48.2 | 0.99 ± 0.1 | 931.1 ± 79.5 | 37.4 ± 2.1 |
Data are mean ± SEM (n = 2-3). IC50H, high-affinity IC50.
Values in parentheses represent the low-affinity IC50 values.
Because 6′-GNTI activates the KOP/DOP and KOP/MOP heterodimers, we next examined whether the agonist effects of 6′-GNTI were blocked with KOP, DOP, and MOP receptor type-selective antagonists. In cells expressing the KOP/DOP heterodimer (Fig. 1d), both the KOP-R-selective antagonist NorBNI (Fig. 1d, •) and the DOP-R-selective antagonist naltrindole (NTI) (Fig. 1d, ○) blocked 6′-GNTI-mediated signaling. Likewise, in the KOP/MOP cells, KOP-R- and MOP-R-selective antagonists abolished 6′-GNTI-mediated signaling (data not shown). These data imply that 6′-GNTI simultaneously targets both receptor protomers in the heterodimer. In addition, 6′-GNTI was able to compete [3H]diprenorphine from the KOP/DOP heterodimer with a monophasic competition curve (Fig. 1e, ▪), suggesting that the KOP/DOP interface provides a homogeneous population of binding sites for 6′-GNTI. In contrast, [3H]diprenorphine competition binding in KOP/DOP receptor cells with NTI (Fig. 1f, ○) or NorBNI (Fig. 1f, •) resulted in biphasic competition curves. Intriguingly, affinities of NTI or NorBNI to the high- and low-affinity binding sites in the KOP/DOP-expressing cells do not match their affinities to the respective receptor protomers when expressed alone (Table 2), providing additional support for the formation of a unique ligand/receptor complex generated through heterodimer formation.
Together these data suggest that the 6′-GNTI-occupied heterodimers are a unique functional signaling unit possibly due to generation of a novel “landing pad” for 6′-GNTI and/or a conformational change generated through heterodimer formation.
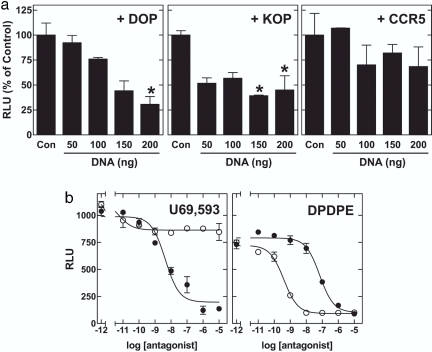
An alternative explanation for the enhanced activity of 6′-GNTI in the double receptor-expressing cells is that the mere presence of a second receptor (e.g., DOP-R) cooperatively or synergistically enhances signaling of the first receptor (e.g., KOP-R). If this cooperativity were the case, increasing the expression of DOP-Rs or KOP-Rs in the KOP/DOP cells would enhance 6′-GNTI signaling. However, increasing the expression of DOP-R (Fig. 2a Left) or KOP-R (Fig. 2a Center) in the KOP/DOP cell line decreased 6′-GNTI-mediated Ca2+ release in a gene-dose-dependent manner. These results suggest that raising the level of the DOP-Rs or KOP-Rs in the KOP/DOP cell line resulted in an unfavorable ratio between heterodimers (which are the signaling unit for 6′-GNTI) versus single receptors and/or homodimers (which bind 6′-GNTI but signal poorly). This effect was not due to nonspecific inhibition of signaling from increased expression of Gi-coupled receptors, because coexpression of the chemokine CCR5 receptor in the KOP/DOP cells (Fig. 2a Right) did not significantly alter the signaling efficiency of 6′-GNTI. Thus, synergy/cooperativity cannot explain the enhanced activity of 6′-GNTI on cells coexpressing KOP-Rs and DOP-Rs.
Fig. 2.
Mechanism of 6′-GNTI agonism. (a)6′-GNTI agonist activity on heterodimers is not due to synergy/cooperativity between two opioid receptor types. HEK-293 cells expressing the KOP/DOP-R heterodimer were transiently transfected with the chimeric G protein Δ6-Gqi4-myr (200 ng for every 40,000 cells) and increasing amounts of DOP-R (Left), KOP-R (Center), or CCR5 (Right). Cells were stimulated with 100 nM 6′-GNTI, and intracellular Ca2+ release was measured as described for Fig. 1c. Maximum stimulation in the presence of 200 ng of control pcDNA3 (Con) was set at 100%. All data sets were subjected to a one-way ANOVA analysis with Bonferroni's multiple comparison posttest. *, A significant difference from the control with P < 0.05. (b) Effects of receptor type-selective antagonists on agonist-induced Ca2+ release in cells expressing the KOP/DOP-R heterodimer. Cells expressing the KOP/DOP-R heterodimer were preincubated for 30 min with increasing doses of NTI (○) or NorBNI (•) and stimulated with 1 nM U69,593 (Left) or 50 nM DPDPE (Right). Agonist-induced Ca2+ release was assessed as described for Fig. 1c. Shown are representative curves (mean ± SEM) of at least three experiments carried out in duplicate.
Therefore, we propose that the primary functional unit for 6′-GNTI agonism is the KOP/DOP heterodimer. One can envision several mechanisms by which this unit is activated by 6′-GNTI. For example, antagonism of the DOP-R receptor enhances MOP-R activity in cells coexpressing MOP and DOP receptors (8). It thus appears that antagonism of the DOP-R facilitates agonist-mediated signaling through MOP-R in the MOP/DOP heterodimer. Because 6′-GNTI is an antagonist with high affinity for the DOP-R protomer (see Table 2), it was similarly possible that 6′-GNTI antagonism at the DOP-R facilitated 6′-GNTI signaling via the KOP-R protomer in the KOP/DOP heterodimer. However, the presence of the DOP-R-selective antagonist NTI did not enhance 6′-GNTI signaling in the DOP/KOP heterodimer (see Fig. 1d). On the contrary, both NTI and NorBNI blocked 6′-GNTI-mediated signaling via the KOP/DOP heterodimer (Fig. 1d). The same was true when the KOP/DOP heterodimer was stimulated with the KOP-R-selective agonist U69,593 (Fig. 2b Left) or the DOP-R-selective agonist [d-Pen2, d-Pen5]-enkephalin (DPDPE) (Fig. 2b Right). That is, NorBNI did not enhance DPDPE-mediated signaling on the KOP/DOP heterodimer (Fig. 2b Right, •), nor did NTI enhance U69,593-mediated signaling on the KOP/DOP heterodimer (Fig. 2b Left, ○).
In summary, neither receptor synergy nor a combination of KOP-R agonism plus DOP-R antagonism can explain the signaling profile of 6′-GNTI in the KOP/DOP cells. Together, these data support the model that 6′-GNTI engages the KOP/DOP heterodimer and generates a unique signaling entity.
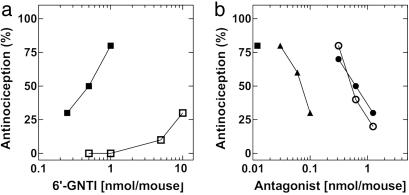
It has been suggested that KOP/DOP-R heterodimers may exist in vivo in the mouse spinal cord (22). We thus examined whether 6′-GNTI was an analgesic when administered i.t.. In fact, 6′-GNTI induced analgesia i.t. with an ED50 of 0.45 (0.31–0.63) nmol per mouse (Fig. 3a, ▪), an effect that was fully blocked by NorBNI [ED50 of 0.62 (0.617–0.627) nmol per mouse] (Fig. 3b, •), and NTI [ED50 of 0.58 (0.34–0.94) nmol per mouse] (Fig. 3b, ○). The fact that not only NorBNI but also the DOP-R-selective antagonist NTI fully blocks 6′-GNTI-mediated analgesia suggests that the in vivo target for 6′-GNTI is the KOP/DOP heterodimer rather than KOP/KOP homomers/monomers. In further support of this hypothesis, the KOP/DOP-selective bivalent antagonist KDN-21 (23) [ED50 of 0.067 (0.049–0.10) nmol per mouse] (Fig. 3b, ▴) also fully blocked 6′-GNTI-mediated analgesia with a 10-fold greater potency than either type-selective antagonist alone. Importantly, 6′-GNTI was ≈50-fold more potent i.t. (ED50 0.45, see Fig. 3a) than the KOP-R-selective agonist U50,488 [ED50 of 20.97 (18.2–24.7) nmol per mouse (23)], again suggesting that the in vivo target for 6′-GNTI was not the KOP-R alone but a KOP/DOP heterodimer. Interestingly, 6′-GNTI produced little to no analgesia when it was administered intracerebroventricularly (Fig. 3a, □). Taken together with recent studies showing spinal cord-selective activity of a bivalent antagonist selective for KOP/DOP-Rs (23), these results suggest that KOP/DOP-R heterodimers exist in the spinal cord but are not a functional analgesic unit in the brain.
Fig. 3.
6′-GNTI mediated analgesia i.t. but not intracerebroventricularly in mice. (a) Analgesia was measured by a tail-flick assay after injection of 6′-GNTI either i.t. (▪) or intracerebroventricularly (□) at the doses indicated. (b) Antagonism of 6′-GNTI-induced i.t. analgesia. NorBNI (•), NTI (○), or KDN-21 (▴) at the doses indicated was administered with 1 nmol of 6′-GNTI per mouse (a dose that produces 80% analgesia).
Together, our data suggest that opioid receptor heterodimers are indeed a distinct functional signaling unit and could provide a target for development of tissue-selective opiate analgesics with reduced side effects. It is intriguing to speculate that heterodimerization of GPCRs is more universal, incorporating GPCRs other than opioid receptors as well. If so, GPCR heterodimers could represent a large target resource for the development of therapeutics. The number of landing pads that could be generated through heterodimer formation is vastly larger than that predicted in a model of a “one ligand/one GPCR” binding pocket. In addition, it is possible that the large number of orphan GPCRs, whose ligands have yet to be identified, are, in fact, heterodimer partners of receptors whose ligands are already identified. The function of these “orphans” could be to complex with known GPCRs, thereby modulating the binding, signaling and/or trafficking of the heterodimer partner, either allosterically or through formation of a unique binding pocket. Furthermore, if other GPCR heterodimers show tissue-selective expression and/or activity (like the KOP-DOP heterodimer does), therapeutics targeting these unique signaling complexes might be expected to show reduced side effects. In conclusion, we believe that 6′-GNTI and the KOP/DOP heterodimer provide a proof-of-concept for the existence of GPCR heterodimers and may provide a framework for the development of therapeutics.
Supplementary Material
Acknowledgments
We thank Thue W. Schwartz (University of Copenhagen, Copenhagen) for providing the CCR5 cDNA, all members of the J.L.W. laboratory for engaging discussions, and Selena Bartlett and Joe Kim for critical comments on the manuscript. This work was funded by National Institute on Drug Abuse Grants R01 DA015232 (to J.L.W.) and R01 DA01533 (to P.S.P.) and funds provided by the State of California for medical research and research on alcohol and substance abuse through the University of California, San Francisco (J.L.W.).
Author contributions: M.W., P.S.P., and J.L.W. designed research; M.W., J.F., and M.M.L. performed research; M.W., R.M.J., S.K.S., E.K., and P.S.P. contributed new reagents/analytic tools; M.W., M.M.L., P.S.P., and J.L.W. analyzed data; M.W., P.S.P., and J.L.W. wrote the paper; and E.K. edited the manuscript.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GPCR, G protein-coupled receptor; DOP, delta opioid peptide; DOP-R, DOP receptor; KOP, kappa opioid peptide; KOP-R, KOP receptor; MOP, mu opioid peptide; MOP-R, MOP receptor; 6′-GNTI, 6′-guanidinonaltrindole; i.t., intrathecally; HA, hemagglutinin; NTI, naltrindole; NorBNI, nor-binaltorphimine.
See Commentary on page 8793.
References
- 1.Pin, J. P., Kniazeff, J., Binet, V., Liu, J., Maurel, D., Galvez, T., Duthey, B., Havlickova, M., Blahos, J., Prezeau, L. & Rondard, P. (2004) Biochem. Pharmacol. 68, 1565–1572. [DOI] [PubMed] [Google Scholar]
- 2.Kniazeff, J., Bessis, A. S., Maurel, D., Ansanay, H., Prezeau, L. & Pin, J. P. (2004) Nat. Struct. Mol. Biol. 11, 706–713. [DOI] [PubMed] [Google Scholar]
- 3.Nelson, G., Chandrashekar, J., Hoon, M. A., Feng, L., Zhao, G., Ryba, N. J. & Zuker, C. S. (2002) Nature 416, 199–202. [DOI] [PubMed] [Google Scholar]
- 4.Filipek, S., Krzysko, K. A., Fotiadis, D., Liang, Y., Saperstein, D. A., Engel, A. & Palczewski, K. (2004) Photochem. Photobiol. Sci. 3, 628–638. [DOI] [PubMed] [Google Scholar]
- 5.Fotiadis, D., Liang, Y., Filipek, S., Saperstein, D. A., Engel, A. & Palczewski, K. (2004) FEBS Lett. 564, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan, B. A. & Devi, L. A. (1999) Nature 399, 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George, S. R., Fan, T., Xie, Z., Tse, R., Tam, V., Varghese, G. & O'Dowd, B. F. (2000) J. Biol. Chem. 275, 26128–26135. [DOI] [PubMed] [Google Scholar]
- 8.Gomes, I., Gupta, A., Filipovska, J., Szeto, H. H., Pintar, J. E. & Devi, L. A. (2004) Proc. Natl. Acad. Sci. USA 101, 5135–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He, L., Fong, J., von Zastrow, M. & Whistler, J. L. (2002) Cell 108, 271–282. [DOI] [PubMed] [Google Scholar]
- 10.Matthes, H. W., Smadja, C., Valverde, O., Vonesch, J. L., Foutz, A. S., Boudinot, E., Denavit-Saubie, M., Severini, C., Negri, L., Roques, B. P., Maldonado, R. & Kieffer, B. L. (1998) J. Neurosci. 18, 7285–7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu, Y., King, M. A., Schuller, A. G., Nitsche, J. F., Reidl, M., Elde, R. P., Unterwald, E., Pasternak, G. W. & Pintar, J. E. (1999) Neuron 24, 243–252. [DOI] [PubMed] [Google Scholar]
- 12.Fairbanks, C. A. (2003) Adv. Drug Delivery Rev. 55, 1007–1041. [DOI] [PubMed] [Google Scholar]
- 13.Wessendorf, M. W. & Dooyema, J. (2001) Neurosci. Lett. 298, 151–154. [DOI] [PubMed] [Google Scholar]
- 14.Keith, D. E., Anton, B., Murray, S. R., Zaki, P. A., Chu, P. C., Lissin, D. V., Monteillet-Agius, G., Stewart, P. L., Evans, C. J. & von Zastrow, M. (1998) Mol. Pharmacol. 53, 377–384. [PubMed] [Google Scholar]
- 15.Chu, P., Murray, S., Lissin, D. & von Zastrow, M. (1997) J. Biol. Chem. 272, 27124–27130. [DOI] [PubMed] [Google Scholar]
- 16.Arden, J. R., Segredo, V., Wang, Z., Lameh, J. & Sadee, W. (1995) J. Neurochem. 65, 1636–1645. [DOI] [PubMed] [Google Scholar]
- 17.Kostenis, E. (2001) Trends Pharmacol. Sci. 22, 560–564. [DOI] [PubMed] [Google Scholar]
- 18.Whistler, J. L. & von Zastrow, M. (1998) Proc. Natl. Acad. Sci. USA 95, 9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn, A. K. & Whistler, J. L. (2001) Neuron 32, 829–839. [DOI] [PubMed] [Google Scholar]
- 20.Tulunay, F. C. & Takemori, A. E. (1974) J. Pharmacol. Exp. Ther. 190, 395–400. [PubMed] [Google Scholar]
- 21.Sharma, S. K., Jones, R. M., Metzger, T. G., Ferguson, D. M. & Portoghese, P. S. (2001) J. Med. Chem. 44, 2073–2079. [DOI] [PubMed] [Google Scholar]
- 22.Portoghese, P. S. & Lunzer, M. M. (2003) Eur. J. Pharmacol. 467, 233–234. [DOI] [PubMed] [Google Scholar]
- 23.Bhushan, R. G., Sharma, S. K., Xie, Z., Daniels, D. J. & Portoghese, P. S. (2004) J. Med. Chem. 47, 2969–2972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.