Abstract
Magnetoencephalography (MEG) is an imaging technique that enables the assessment of cortical activity via direct measures of neurophysiology. It is a non-invasive and passive technique that is completely painless. MEG has gained increasing prominence in the field of pediatric neuroimaging. This dedicated review article for the pediatric population summarizes the fundamental technical and clinical aspects of MEG for the clinician. We discuss methods tailored for children to improve data quality, including child-friendly MEG facility environments and strategies to mitigate motion artifacts. We provide an in-depth overview on accurate localization of neural sources and different analysis methods, as well as data interpretation. The contemporary platforms and approaches of two quaternary pediatric referral centers are illustrated, shedding light on practical implementations in clinical settings. Finally, we describe the expanding clinical applications of MEG, including its pivotal role in presurgical evaluation of epilepsy patients, presurgical mapping of eloquent cortices (somatosensory and motor cortices, visual and auditory cortices, lateralization of language), its emerging relevance in autism spectrum disorder research and potential future clinical applications, and its utility in assessing mild traumatic brain injury. In conclusion, this review serves as a comprehensive resource of clinicians as well as researchers, offering insights into the evolving landscape of pediatric MEG. It discusses the importance of technical advancements, data acquisition strategies, and expanding clinical applications in harnessing the full potential of MEG to study neurological conditions in the pediatric population.
Keywords: Magnetoencephalography, epilepsy, neurophysiology, clinical, connectivity
Introduction
Clinical overview
Magnetoencephalography (MEG) is a non-invasive, passive, and painless imaging technique that enables cortical activity assessment via direct measures of neurophysiology. Since its introduction, MEG has undergone significant hardware and accompanying analytical software advances that improved the spatial and temporal resolution of findings generated by source reconstruction methods. 1 The contemporary MEG offers rapid multichannel acquisition of electrophysiological data from the whole cortical surface with full-head coverage.
Due to technical advances, the role of MEG in diagnosis and disease management is constantly growing, with U.S. Food and Drug Administration (FDA) approval for epilepsy1–11 and presurgical functional brain mapping.12–24 Neurologists should be aware of MEG’s expanding application for conditions including autism spectrum disorder (ASD),25–30 mild traumatic brain injury (mTBI),31–33 post-traumatic stress disorder (PTSD),34,35 extreme prematurity, 36 cerebral palsy,37–39 schizophrenia, 40 Williams and Landau-Kleffner syndromes, 41 Alzheimer’s disease,42,43 depression, 44 attention deficit hyperactivity disorder,45,46 and mapping of eloquent cortex in brain tumor patients. 47
The objective of this review is to orient the clinician to MEG’s indications, fundamental technical aspects, data acquisition and analysis, and interpretation.34,35 Pediatric clinical domains highlighted include identification of the irritative zone in epilepsy, presurgical delineation of eloquent cortices, as well as translational applications in autism, and mild traumatic brain injury (mTBI).
Technical overview
The crux of most clinical MEG applications is the ability to perform magnetic source imaging (MSI): cortical or subcortical source estimation of a signal of interest. Directional current flowing through a depolarizing neuron generates a magnetic field, detectable with MEG. 48 In contrast to all other functional imaging techniques, the data received are direct measures of post-synaptic potentials and are not reference based. With the current sub-millisecond temporal and millimeter spatial resolution, MEG can localize focal dipolar responses throughout the entire head, as opposed to subdural electroencephalography (EEG)’s regional detection. 49
To perform a MEG scan, a patient’s head is placed in a helmet that contains an array of sensors. The cortical and subcortical activities of the brain generate magnetic fields. Magnetic fields are detected with superconducting coils called superconducting quantum interference devices (SQUID).11,48 Methods such as signal space projection (SSP) 50 and synthetic gradiometer 51 are effective noise cancellation methods used during data preprocessing for removing non-cerebral magnetic noise. Importantly, MEG’s source localization can also be co-registered with structural magnetic resonance imaging (MRI) to identify specific brain areas involved in function or pathology.
Event-related field (ERF) recording is one of the more well-established methods of MEG analysis. Similar to EEG event-related potential (ERP) analysis, ERFs are measured in response to repetitive stimuli (auditory, visual, tactile, electrical, etc.). Data are then measured from onset of stimulus to neuromagnetic peaks that correlate to specific sensory or cognitive processes. The neural response(s) specific to this stimulus is represented as a waveform and termed the “evoked response.” The variability in latencies and amplitude between patients or participants can be interrogated for their physiologic relevance. 29
Neurophysiological activity can also be evaluated through cortical rhythms. To this end, graph theory and advanced computational methods can be applied to investigate the interactions of cortical and subcortical brain areas. While structural connectivity, white matter anatomical connections between different brain areas, can be measured with imaging techniques such as diffusion MRI, functional and effective connectivity require high temporal resolution, which MEG can provide. Functional connectivity is defined as statistical dependencies among remote neurophysiological events; effective connectivity refers to the effect that one neural system exerts on another, either at a synaptic or population level. 52
Functional connectivity examines brain region interconnections using time series of cerebral activity. 25 Power of induced or evoked oscillations, along with their frequencies, reflects processes like working memory, semantic processing, and sensory-motor integration.36,53 High-frequency oscillations (HFOs) (beta: 15–30 Hz, gamma: >30 Hz) are associated with event-related desynchronization (ERD) and are impacted in pathological conditions.34,54
Source estimation
Source estimation is the process of mapping the magnetic fields recorded by MEG, into a three-dimensional model of the brain. The model can be an anatomical MR image or a geometric approximation. Identifying underlying electrical currents from the induced magnetic fields does not have a unique solution since there is an infinite number of possible current dipole configurations. Transforming the sensor data into activity in specific structures of the brain requires physiologically reasonable assumptions to be made. There are several methods of source estimation, each with its own advantages and limitations.
The equivalent current dipole (ECD) method of source localization is a recursive algorithm that iterates the magnitude, strength, position, and orientation of an assumed or pre-specified number of current dipoles, until the modeled magnetic fields are a best fit to the measured magnetic fields (i.e., low fit error or high goodness-of-fit). This method is best suited for a focal source or a small number of focal sources; however, errors can result when solving for an unknown number of possibly distributed sources. 48 Localization of interictal spikes for clinical epilepsy cases are mostly performed with the ECD method.
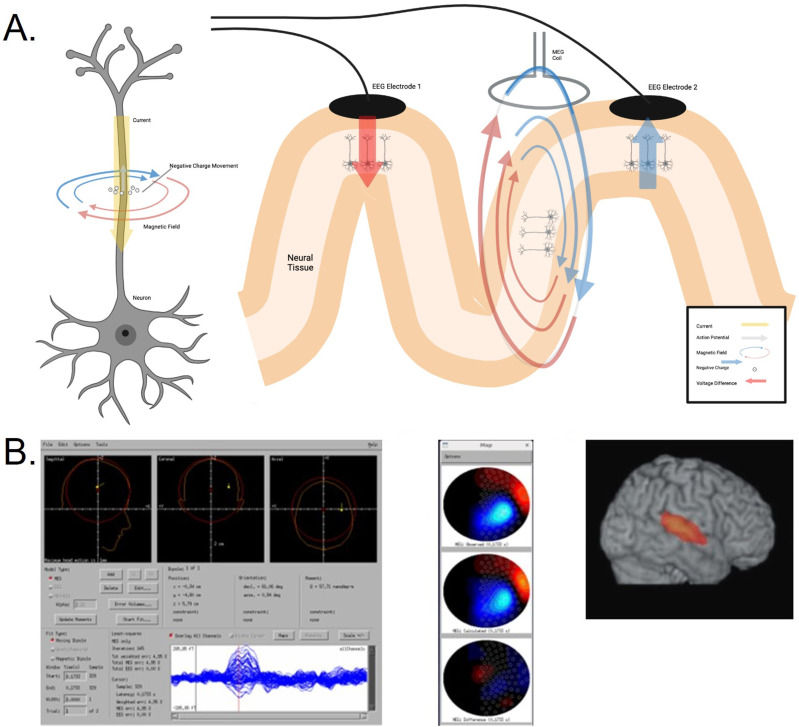
Beamforming is another method of source localization which attempts to isolate a specific focal region by using a spatial filter generated from a set of reconstructed recordings from a known source within a subject’s brain (Figure 1). This set of known values is called the leadfield. There are a number of beamforming techniques in open and closed source libraries, each having its own traits affecting accuracy. This method has been used in several recent MEG studies to improve the signal-to-noise ratio of particularly low amplitude signals of interest in the epileptic brain, such as HFOs.55–57
Figure 1.
Electrophysiological basis of MEG signals and example of dipole and beamformer source localization results. (a) Shows an action potential generated from a neuron along with a corresponding electric and magnetic field that are produced by the movement of negative charge along the outer membrane of the axon. A magnified simplified view of the measurement of electric (post-synaptic potentials) and magnetic (intracellular current) fields generated. The two EEG electrodes measure the difference in voltage from two groups of neurons along the neuronal axon, while the MEG coil measures unreferenced magnetic flux produced tangentially from a single group of neurons. This can lead to differences in field strength between MEG and EEG in both gyri and sulci, for this type of activity. Figure created using BioRender software. (b) Example of CTF’s dipole source algorithm interface. The observed waveform with overlaid sensors can be seen at the bottom, with the red vertical line indicating the selected time point. The top three views show the computed dipole location. Information regarding the exact time point, goodness-of-fit/error, and dipole coordinates are also provided. Topographic maps looking down on the head with the plot for the acquired data at the top, the reconstructed data from the forward solution in the middle, and the difference at the bottom. Example of the source localization applied using a beamforming algorithm.
Acquisition of MEG data in pediatric population
Methodology in pediatric patients
A MEG study is a non-invasive and painless procedure performed in a room shielded from external electromagnetic fields, operated by a trained technologist. A MEG session can be divided into parts with a couple of minutes break in between and depending on the study, in total can last up to an hour or more. Simultaneous EEG is occasionally used. Depending on the clinical question, the data are collected with or without stimuli, with some tasks requiring a response from the patient. The resultant data are interpreted by clinicians and scientists with MEG expertise.
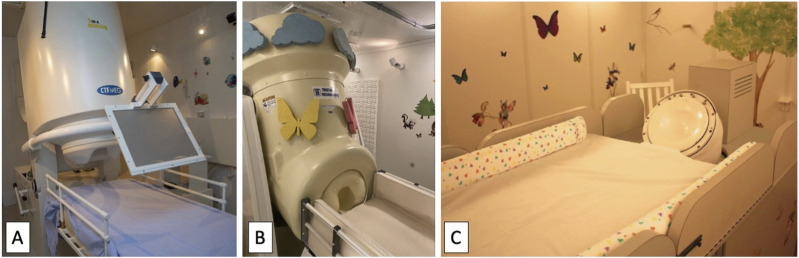
In our institutions, MEG studies have been incorporated into clinical and/or experimental protocols of patients who presented with neurological or neuropsychiatric symptoms for whom supplementation of exam, EEG, or anatomical imaging was felt to be helpful. For the studies performed at the Hospital for Sick Children (Toronto, Canada), patients and subjects were scanned supine or upright in a whole‐head 151‐channel MEG (CTF Omega, MISL, Coquitlam, Canada) and data were recorded continuously with a 600‐Hz sampling rate, DC‐100 bandpass, and the CTF Omega-specific third‐order spatial gradient noise cancellation. For those performed at the Boston Children’s Hospital (Boston, MA, USA), patients were scanned supine in the whole-head 375-channel pediatric MEG System (MagView Biomagnetometer, also known as “BabyMEG,” Tristan Technologies, San Diego, CA, USA) 58 with 1024 Hz sampling rate, at Boston Children’s Hospital, Longwood, or scanned supine in the 76-axial gradiometer pediatric MEG system 59 (“BabySQUID,” Tristan Technologies, San Diego, CA, USA) at Boston Children’s Hospital, Waltham, where MEG data were recorded continuously with a 1024 Hz sampling rate (Figure 2).
Figure 2.
Contemporary magnetoencephalography platforms. (a) Photograph of the CTF MEG system at the Hospital for Sick Children (Toronto, ON, Canada). (b) Photograph of the pediatric BabyMEG system at the Boston Children’s Hospital (Boston, MA, USA). (c) Photograph of the BabySQUID system at the Boston Children’s Hospital (Waltham, MA, USA).
The BabyMEG system, which accommodates up to 52 cm in head circumference, has better spatial resolution compared to most commercial adult systems due to its dense sensor array, small coils and short distance between each coil. 58 It also provides a better signal-to-noise ratio as there is significantly less distance between the sensors and the scalp, minimizing magnetic field weakening with distance, particularly for pediatric patients. On the other hand, adult MEG systems can accommodate head sizes that pediatric MEG systems cannot. Additionally, for children whose head circumferences approach 52 cm, collecting simultaneous MEG and EEG data may not be possible in the smaller BabyMEG system.
Data from all MEG systems are processed offline, including functional connectivity maps. Head movement is monitored to ensure <5 mm movement during runs. Monitoring the patient’s brain position relative to MEG sensors is crucial. Head Position Indicator (HPI) coils are placed at four points on the subject’s head, digitally marked for reference. Anatomical fiducial points (nasion, left and right preauricular points) are also marked digitally to locate the head inside the helmet and guide MRI co-registration (digitization process). 2 Adding more points improves co-registration accuracy but increases the time required.
Practicalities of magnetoencephalography signal capture in children
The quality of MEG data is highly sensitive to head movement. As the sensors are fixed, head movements change the relation of the sensors with neurological activity and can present a challenge for accurate localization. As some children may find staying still for the duration of the 15–45-min scan time uncomfortable or impossible, practical challenges can be solved with multiple techniques including behavioral interventions such as playing videos to keep them entertained, as well as recording during natural sleep or with anesthesia. For some young children, skipping a nap before the MEG recording is helpful for them to fall asleep easily during data acquisition. Some studies prefer to use sedation or anesthesia during MEG recordings, however, these agents can have an effect on the brain activity recorded with MEG.60–62 Also, techniques for continuous monitoring of patient head movement and compensation (e.g., signal space separation) have been developed to reduce the error generated by head movement during a MEG scan. 63 Having a child-friendly environment in the MEG facility and the shielded room, such as wall decals, age appropriate toys, and coloring books, are also helpful for creating a less intimidating atmosphere for the pediatric populations.
Clinical use of magnetoencephalography in pediatric population
Use of magnetoencephalography in epilepsy
Role of magnetoencephalography in epilepsy presurgical evaluation
Epilepsy is a disorder defined by recurrent seizures due to ictal discharges. These represent abnormal electrical discharges in the brain, and the time between seizures is thus termed the interictal period. The first-line treatment of epilepsy is anti-seizure medication, but only 60%–70% of patients achieve remission.34,64 In appropriately selected candidates, the treatment of drug-resistant epilepsy (DRE) involves surgical resection of the epileptogenic zone (EZ), defined as the area of the brain responsible for generating clinical seizures.
The preoperative workup of epilepsy surgery involves the estimation of the EZ through a wide variety of modalities, including EEG, MRI, invasive electrophysiological monitoring, subdural electrodes, intracarotid amobarbital procedure (IAP or Wada), PET, SPECT, and MEG. 1 MEG has a role in aiding the identification of the EZ and in the prediction of clinical outcomes following surgery, as further detailed below. 65
Most commonly, estimating the EZ with MEG means delineating the irritative zone, the area generating interictal epileptiform discharges (IEDs or spikes). This zone is one of the estimators of the epileptogenic zone. Delineating the irritative zone with MEG involves dipole source modeling of the magnetic field deflections caused by IEDs. The field measured by the MEG is compared with a simulated field modeled by a computer to determine the location of a point source dipole capable of generating the discharge. This information is then overlaid on an MRI of the patient’s brain, to estimate the structural location of IEDs’ generation—the irritative zone (Figure 3).
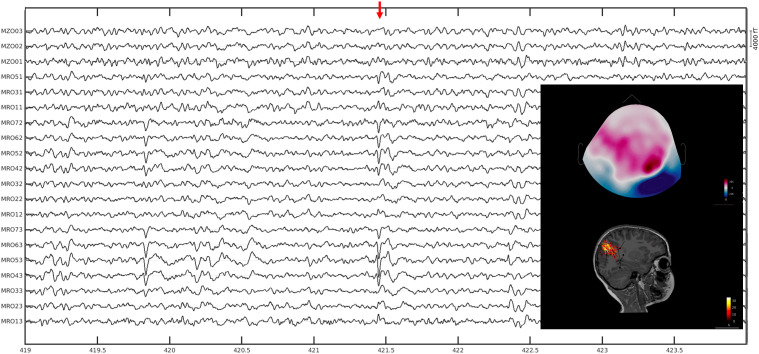
Figure 3.
Identifying the irritative zone (Example 1). A pediatric patient (younger than 10 years of age) with intractable epilepsy. Spontaneous data were collected with the whole-head pediatric MEG system at Boston Children’s Hospital while the patient was awake. Data were bandpass filtered between 4 and 70 Hz with a notch filter at 60 Hz, bad channels and segments of the data with motion artifacts were removed from the analysis. Data was analyzed with the Brainstorm software. T1-weighted MRI data of the patient were processed through the Freesurfer software and registered to the patient’s brain for source modeling to be performed using the Boundary Element Method. Equivalent current dipoles were thresholded at 70%. No ictal activity was observed in the data. Most of the interictal spikes had a tight cluster of dipoles localized in the right parietal lobe where the center of the cluster surrounds the intraparietal sulcus extending both to the superior and inferior parietal lobule. The anterior aspect of the intraparietal sulcus had a suspected focal cortical dysplasia. There were also scattered dipoles localized to the right frontal lobe, primarily in the parasagittal region.
While due to the time limit of MEG sessions (not to exceed 1–2 h as per IFCN-endorsed practical guidelines) and head movement control challenges, most studies investigating MEG in epilepsy presurgical planning are focused on characterizing interictal discharges, 66 in certain settings MEG can be also used to investigate ictal discharges.6–8 Studies with adult epilepsy patients have shown that MEG has shown success in discovering interictal discharges and employment of successful dipole source modeling providing additional useful information for presurgical evaluation.3–5 Furthermore, in another study of 121 children with temporal lobe and temporal lobe plus epilepsy, MSI localization data showed significant predictive value. 67 Further demonstrating the importance of multi-modal evaluation, three recent studies on pediatric patients demonstrated and quantified the presurgical accuracy of both MSI and electric source imaging (ESI) in localizing IEDs, highlighting the relative superiority of MSI, the complementary role of the two techniques, and the benefit of applying complementary clustering analysis.55,66,68
Studies that successfully recorded ictal MEG discharges have small sample sizes. Among these, there is a general agreement that ictal MEG is superior to interictal MEG in terms of sensitivity and localization.6–8 Finally, an increasing number of recent studies have been reporting the potential of MEG in detecting and localizing epileptic HFOs, which are relatively novel interictal epilepsy biomarkers. 49 The recent studies of Tamilia and colleagues55,69 demonstrated that MEG can detect and localize interictal ripples in children with DRE, consistently with the ripple-zone defined by intracranial EEG, and is more likely to predict seizure freedom in children when ripple-zones are resected. Ripples overlapping on spikes and ripples at onset of a propagating ripple activity are more often linked to the EZ. 69 Migliorelli and colleagues also demonstrated in their cohort of nine patients (8 children) that interictal ripples on MEG can help localize the EZ and correlated with HFOs on intracranial EEG. 70
Non-surgical uses of magnetoencephalography in epilepsy
While the predominant focus of MEG has been in presurgical evaluation, research has also shown possible burgeoning roles in non-surgical epilepsy patients in the understanding of underlying mechanisms and prognostication. For example, connectivity studies in childhood absence epilepsy show that strength of HFOs in MEG reflects the severity of absence seizures 71 and ictal HFOs can be used to localize seizure onset zones such as those at the thalami, cingulate gyri, and anterior cerebellar regions.72,73
For childhood epilepsy with centrotemporal spikes (CECTS), a recent study by Kai Niu and colleagues correlated improved cognitive outcomes of patients who showed normalization of connectivity networks after 1 year of anti-seizure medication. 74 Another study of 28 pediatric patients with CECTS compared to 14 control subjects suggested that abnormal network topology might explain underlying cognitive impairments. 75
In summary, both ictal and interictal MEG measurements, as well as connectivity measurements, have been shown to be helpful in presurgical evaluation of epilepsy patients, both in terms of localization of the epileptogenic zone and predicting post-surgical seizure freedom (Figure 4). Though ictal MEG discharges tend to be more accurate, they are more difficult to obtain due to limited MEG session length and difficulty in minimizing head movements. Moreover, MEG should be used in conjunction with other imaging modalities such as EEG, MRI, PET, and/or SPECT for a more comprehensive assessment.
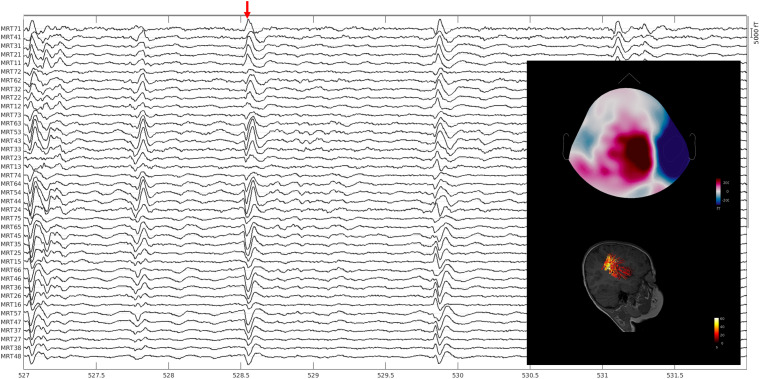
Figure 4.
Identifying the irritative zone (Example 2). A pediatric patient (younger than 5 years of age) with tuberous sclerosis complex, developmental delay, infantile spasms, refractory complex partial seizures, and status epilepticus. Simultaneous spontaneous data during natural sleep were collected using the whole-head pediatric MEG system at Boston Children’s Hospital. Data were bandpass filtered between 4 and 60 Hz with a notch filter at 60 Hz, bad channels and data segments were removed from the analysis. The data was analyzed with the Brainstorm software. Patient’s T1-weighted MRI data was processed with the Freesurfer software and co-registered to the patient’s brain for source modeling to be performed by the Boundary Element Method. Equivalent current dipoles were thresholded at 85%. No ictal activity was observed. Interictal activity was observed over the right hemisphere where dipole localization involved the inferior right precentral gyrus, adjacent anterior inferior right parietal lobe, posterior right insula, and posterior aspect of the right superior temporal gyrus. There is a tuber in the precentral gyrus just superior to the dipole cluster in the right inferior precentral gyrus.
Use of magnetoencephalography for mapping of eloquent cortices
Prior to neurosurgical procedures, neurosurgical landmarks, such as somatosensory, motor, visual, and auditory cortices, must be identified to prevent inadvertent loss of brain function (Figure 5). Currently, the gold standard for identification of these eloquent cortices is invasive cortical stimulation and the IAP for language lateralization.34,35 These invasive procedures have inherent risks of morbidity. 35 The use of MEG for mapping eloquent cortices has been extensively studied in literature, including with pediatric epilepsy patients.76,77 The American Clinical Magnetoencephalography Society (ACMEGS) has released two position statements and a clinical practice guideline to support the use of MEG for presurgical mapping.35,78
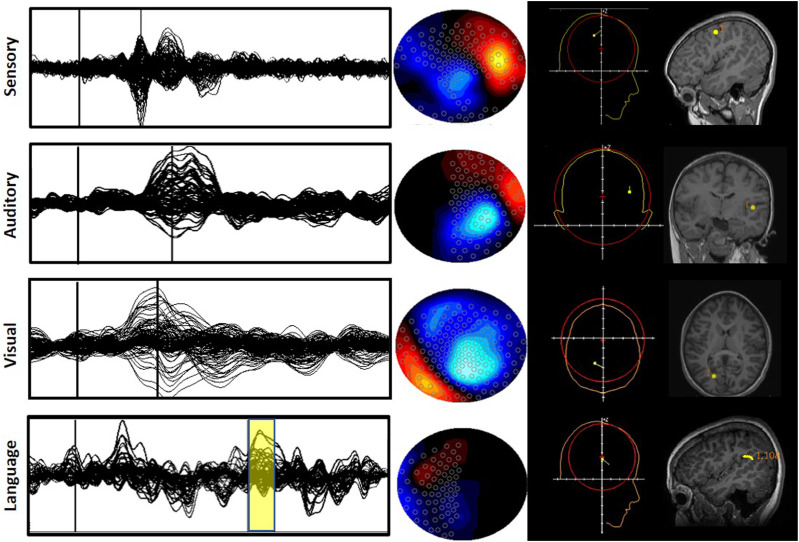
Figure 5.
Mapping of eloquent cortices. Example of typical averaged waveforms with overlaid sensors (first column) for somatosensory, auditory, visual, and language paradigms. The thin vertical lines (for sensory, auditory, and visual) indicate the time point of interest for the dipole fit, and the yellow box (for language) indicates the time points for a moving dipole fit. The middle column shows topographic plots at the selected time points. The projection by convention is looking down on the head with the nose at the top and the ears at the sides. The last column shows the dipole location obtained from the source reconstruction algorithm, and then overlaid onto individual MRIs after co-registration with fiducials.
Somatosensory and motor cortex
The mapping of the somatosensory cortex is often required prior to tumor resection surgeries, because tumors have been known to shift the position and shape of the central sulcus. 12 MEG has been validated against fMRI and invasive techniques for this purpose.12,35 Electrical or tactile stimulations of the median nerve or the fingers, respectively, generate a somatosensory evoked field (SEF) from the primary somatosensory cortex.12,35 The mapping of both primary and secondary somatosensory cortices was shown to be reliable in patients under anesthesia and in coma.12,35 MEG has also been used to localize the somatosensory cortex in typically developing children and pediatric epilepsy patients.79,80
MEG is superior to other imaging modalities in identifying the motor cortex due to its greater temporal resolution. 12 All movements (active, passive, or imagined) can be utilized to map the motor cortex and its associated areas. 35 Two methods have been suggested to map the motor cortex, either via repeated motor movements to produce an average evoked field or by using EMG (electromyography) to detect movement onset for correlation with MEG signals.81,82 A study by Gaetz and colleagues successfully localized the hand region primary motor cortex, as a result of right and left index finger movements, in pediatric patients undergoing presurgical evaluation. 83
Visual and auditory cortices
The localization of the visual cortex is difficult due to the crossing of the visual pathway at the optic chiasm resulting in simultaneous activation of multiple occipital areas within the brain, which may result in signal cancellations. 12 However, it has been established that visual evoked fields (VEFs) in each brain hemisphere can be stimulated with a hemifield reversing checkerboard pattern and localized with the ECD approach 12,35; this mapping has been validated against fMRI. 15 VEF analysis is part of clinical practice during the presurgical evaluation of patients with epilepsy or brain tumors, especially if posterior brain regions are involved. Future clinical use of VEFs includes early identification of disorders such as multiple sclerosis, Parkinson’s disease, and stroke. 84
The localization of the auditory cortex and cortices responsible for language function are also being explored with MEG. Cheour and colleagues were able to use MEG successfully to study auditory discrimination in infants. 85 Moreover, peripheral and central lesions of auditory pathways can affect auditory evoked fields, localized to Heschl’s gyri and the supratemporal auditory cortices. 86 Language pathways can be re-routed in patients suffering from long-term epileptic activity, tumors, or stroke 86 ; therefore, mapping prior to surgery is important to prevent deficits. Some studies have attempted to localize the frontal cortical area responsible for expressive language, 87 while others focused on localizing the receptive area within the temporal lobe or tempo-parietal junction.23,24 However, the protocols are not standardized, and more experimental research remains to be performed on this topic. Thus, auditory cortex mapping is currently not frequently used in a clinical setting alone, but more often applied in conjunction with the lateralization of language. 35
Lateralization of language
Clinical MEG studies of language mapping have focused on the lateralization of language function: determining the dominant language hemisphere and the intrahemispheric localization of language function prior to surgery. Language mapping is particularly important for epilepsy patients because they show more variability in hemispheric dominance compared to neurologically typical individuals.88,89 The standard procedure for determining language dominance has been the Wada test for years 90 ; however, this test is invasive, and it does work for intrahemispheric localization of language. 91 For now, fMRI might be the non-invasive test of choice in the lateralization of language, due to its greater standardization of technology and protocol.34,92 However, MEG is a promising technique yielding relatively high concordance rates with both IAP and fMRI. For example, a study of 85 surgical candidates (ages: 8–56 years) for drug-resistant epilepsy (DRE) showed 87% concordance between MEG and IAP. 16
MEG is used to assess the localization of receptive and expressive language function in epilepsy patients through a variety of tasks (for a review see 91 ). One of the most commonly used receptive language mapping tasks is passive listening tasks where patients listen to words, tones, or vowels. Such tasks are the easiest for pediatric populations since they do not require any active participation from the patient. Other tasks may require patients to actively respond via button presses such as semantic decision tasks where the patient decides if a given word is abstract (“freedom”) or concrete (e.g., “table”) 93 or word recognition tasks that require patients to indicate when a previously presented target word is among a list of words presented 16 but these active tasks are not very commonly used for pediatric populations. According to a review study, word recognition task helps with the intrahemispheric localization of language and has a high concordance rate between MEG and IAP and has been used in both children and adult populations. 91
For the mapping of expressive language, tasks such as picture naming, word generation, phonemic fluency, and verb generation have been used. 91 One of the most commonly used tasks is the verb generation task where participants are asked to generate a verb associated with a given written noun or picture of an object (e.g., “BALL”—“throw”/“catch”). 94 This method had high concordance rates with other methods, such as cortical stimulation mapping and fMRI, for localization of language in 89% of the pediatric epilepsy patients. 94 Another expressive language task, especially easier for younger populations, is the verbal fluency task where patients are asked to produce words that start with a letter they are given. 91
New evidence suggests that atypical language lateralization may be common in pediatric patients with a variety of neurological disorders.20,21,30,95 Thus, such patients require a multi-modal approach to ensure the most accurate lateralization of language prior to surgery. This can be especially true when neurovascular decoupling compromises the utility of fMRI alone, such as arteriovenous malformations or vasogenic edema.
In summary, the mapping of eloquent cortices is an important step before neurosurgical procedures. A growing body of literature has supported the use of MEG in the identification of somatosensory, motor, visual, and auditory cortices as well as in the lateralization of language. ACMEGS recommends the routine clinical use of non-invasive MEG/MSI in the presurgical evaluation of patients with brain lesions and epilepsy. 35
Use of magnetoencephalography in autism spectrum disorder
ASD is a neurodevelopmental condition characterized by challenges in social-emotional reciprocity and the presence of restricted or repetitive behaviors or interests. 96 Though it is a clinical diagnosis, attempts have been made to study the atypical neuronal processing using fMRI,97,98 EEG,99,100 and MEG.25–29,101 MEG ASD studies have been done in both adults and children.26,28 As MEG is quieter than MRI and provokes less claustrophobia, it confers an advantage in children with ASD.
A recent systematic review summarized studies prior to 2015 that utilized MEG to investigate brain function in ASD. 25 Out of 38 studies that met inclusion criteria, 24 focused on responses to visual, auditory, or somatosensory stimuli. The rest investigated cognitive tasks or spontaneous activity. Studies using auditory stimuli (Figure 6) generally showed a significant difference between the evoked potentials and spontaneous activity between children with ASD and neurotypical controls.86,95,102,103 However, the review noted great variability in the specific differences discovered among the studies, which the authors attributed to differences in stimulation used, heterogeneity of the participants, and heterogeneity of methods. 26 MEG research to date has been conducted at the group level, future studies are needed to examine whether MEG can predict ASD presence, type, and severity at the individual level, especially helping identify individuals with auditory and language impairments who might need intervention.104–107
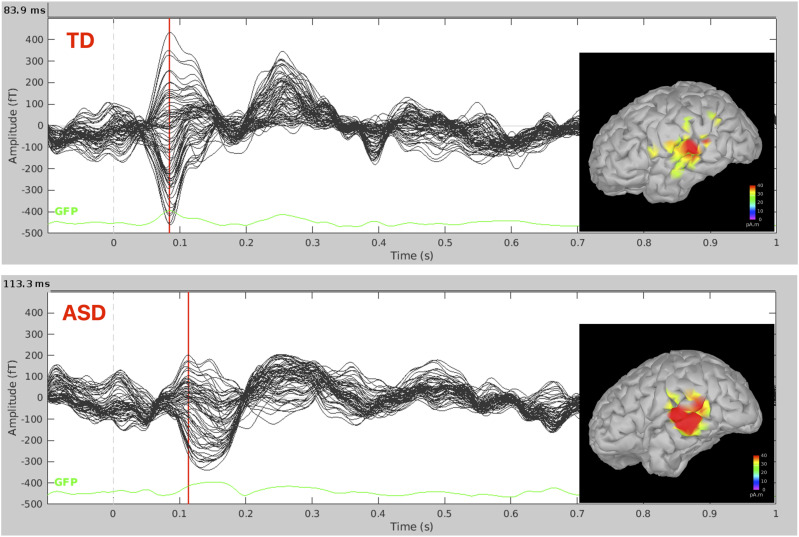
Figure 6.
Auditory evoked fields. Auditory evoked fields generated by the auditory presentation of beep sounds (∼220 epochs) from the left hemispheres of a typically developing (TD) child (aged between 7 and 12 years old) and a child with an autism spectrum disorder (ASD) (aged between 7 and 12 years old). Data were collected with the BabySQUID system, at Boston Children’s Hospital, Waltham. BabySQUID (Tristan Technologies, Inc., San Diego, CA, USA) is a pediatric MEG system (143) (BCH, Waltham), with a partial head coverage sensor array (coverage area ∼265 cm2) consisting of 76-axial gradiometers (10 mm pickup coil diameter, 30 mm baseline, and 12–14 mm coil center-to-coil center spacing, gap of 7–10 mm between each coil and scalp). During the recording, children lay on the BabySQUID bed, resting their head on the helmet. Minimum norm estimate (in pico ampere meter) solutions overlaid on the participant’s own cortical surface for the TD participant and on an age matched template brain for the child with ASD at the peak of M100 which was delayed in the child with ASD compared to the TD child as shown in previous studies [103].
Studies suggest atypical development of the primary auditory cortex in ASD and have implications for clinical markers for the diagnosis of ASD. Latencies in M50, M100, and mismatch field (MMF), and auditory event-related fields104,107 have shown significant findings in ASD, including both language impairment and overall level of support needed.106,108 One study also showed that frontal and temporal residual M100 latencies were found to be longer in children with ASD with auditory sensitivity. 109 M100 latencies, in another study, were additionally associated with the level of support needed. 106 Berman and colleagues showed that the latency of M100 response to be related to ASD level of support needed and the MMF delay to language impairment. 95 Matsuzaki and colleagues showed that latency delays in ASD are associated with poorer language ability persisting into adulthood. 102 Roberts et al. (2019) showed M50 and M100 auditory responses of children with ASD with significant language disability had significantly delayed latencies compared to controls. 103
MEG has also been used to study functional connectivity in children with ASD supporting the fMRI and immunocytochemistry derived hypothesis of long-range under-connectivity and local over-connectivity. A MEG study conducted during awake state when children were watching a video, found reduced long-range functional connectivity in the theta band in children with ASD compared to TD children between the left-anterior and right-posterior brain regions, where the reduction was correlated with clinical severity in the right-handed children with ASD. 26 Another MEG study also found decreased connectivity in the theta frequency in children with ASD compared to TD controls in a memory task. 110 A MEG study of face response found reduced functional connectivity between the fusiform face area and the inferior frontal gyrus in children with ASD that correlated with more severe symptomatology. 111
There is a high comorbidity between epileptic activity and ASD; however, it is not known if epileptiform activity, in the absence of seizures, contributes to ASD symptoms. 112 A study reported that MEG successfully identified abnormal discharges in 82% of children with ASD in contrast to EEG which identified in only 68% 27 (for a review on EEG studies of epilepsy and ASD both in children and adults please see 112 ). MEG studies have shown subclinical epileptiform activity especially in the perisylvian regions in children with ASD.27,113
Face processing in children with ASD has also been studied using MEG. A study found altered functional connectivity, increased alpha band phase synchronization, and happy face processing in ASD compared to controls. 114 Abnormal reduction in the local and long-range functional connectivity in children with ASD for inverted faces has been shown in a MEG study. 115 Another study showed that adolescents with ASD had atypical neural activity in insula, anterior and posterior cingulate, and temporal and orbitofrontal regions for angry and happy face processing. 116
In summary, MEG has been used to study a variety of neuronal processes in children with ASD. It has been used to understand the neurobiology of ASD and how it functions as compared to neurotypical individuals. MEG can add information in the characterization of neural processing in ASD in the hopes of future clinical applications in diagnosis, stratification, and treatment response.
Use of magnetoencephalography in mild traumatic brain injury
Traumatic brain injury (TBI) is a major public health problem among children and adolescents. 117 70%–90% of pediatric TBIs are classified as mild traumatic brain injury (mTBI), commonly referred to as a concussion.117–119 mTBI is responsible for a substantial number of deaths, hospitalizations, and emergency department visits during childhood. 120
mTBI occurs when the head experiences acceleration and deceleration forces from a direct impact or rotational forces that affect the brain. 121 Injury symptoms spontaneously resolve in around 70%–80% of cases within 3 months.122,123 However, at least 20% of injured individuals continue to experience chronic physical, cognitive, and emotional impairments, known as persistent post-concussive symptoms.122–124 These chronic effects of mTBI in childhood and adolescence have been associated with reduced quality of life, difficulties in school, and poorer long-term psychological and behavioral outcomes.125,126
Traditional neuroimaging methods such as computed tomography (CT) and MRI are commonly used to clinically assess mTBI. CT scan excludes hemorrhage and skull fracture, but isn’t reliable for detecting mTBI and visualizing certain brain structures. Structural MRI improves visualization but may lack sensitivity for detecting white matter microstructure changes. 127 MEG can be more sensitive to brain injury as it can detect cellular activity not detected by macroscopic structural neuroimaging. Previous adult mTBI studies have shown that resting state MEG can detect brain abnormalities that go along with persistent behavioral changes when traditional imaging methods fail to do so.128,129 Those abnormalities include a pathological increase in slow-wave oscillations in the delta and theta frequency bands in frontal and temporal regions in symptomatic adults after mTBI,130–133 which are evidence of neurological damage. 134
More recent studies also assessed the pediatric population with mTBI using MEG, revealing abnormalities in the electromagnetic signals from the children and adolescents with mTBI. Those abnormalities included pathological slow-wave oscillations,124,135,136 similar to adult mTBI studies, but also showed dysregulated alpha activity124,135 in pediatric patients with chronic mTBI. Huang et al. discovered increased low frequency activity in bilateral temporal lobes and hypoactivity in prefrontal cortices and precuneus areas in alpha bands using rs-MEG. They also observed hyperactivity in the inferior temporal lobe, parahippocampus, and insula. Additionally, the study found hyper- and hypoactivity in frontal, temporal, occipital, and cerebellar regions in beta and gamma bands, 135 suggesting abnormal communication patterns in both remote and local networks. 137 While the slow-wave results are comparable to what has been found in adult mTBI studies, the outcomes of other frequency bands may be distinct to pediatric mTBI.
In the alpha band, reduced alpha peak power also suggests damage to white matter tracts, consistent with axonal injury.138,139 Thus, the hypoactivity findings may indicate a deficiency in thalamocortical interactions, which could cause a decline in functional suppression within the prefrontal cortices and precuneus cortical regions of children with mTBI. 140 On the other hand, the hyperactivity in the alpha bands observed in the inferior temporal lobe, parahippocampus, and insula may be associated with increased functional inhibition in these regions. This inhibition may lead to deficits in memory function and sensory information integration.
To better assess the post-concussive symptoms and their recovery, recently Huang et al. found that MEG data combined with machine learning algorithms can accurately distinguish between children with mTBI and the control group, with a sensitivity of 83.7% and a specificity of 85.7%. The researchers also found that the MEG data can be used to track the recovery of children with mTBI over time, as the differences between the two groups became smaller as the children with mTBI recovered. 141
In summary, mTBI affects neural communication in the brain across different frequencies, with long-lasting consequences for children and adolescents. These effects are not limited to specific regions and persist for approximately 1 year after the injury. MEG can be a valuable tool for objectively assessing and monitoring pediatric mTBI, leading to better diagnosis and treatment for children.
Conclusions
MEG is a non-invasive technique that enables study of the brain’s neural activity in real time. Within our institutions, the use of MEG has contributed to identifying epileptogenic foci and eloquent cortices, as well as to further our understanding of the neuronal processes underlying ASD and mTBI. These processes represent only a few of the many uses of MEG to delineate neurological pathologies. As technology advances, we will likely see more routine clinical uses of MEG, either alone or in combination with other brain imaging techniques.
Supplemental Material
Supplemental Material for Magnetoencephalography for the pediatric population, indications, acquisition and interpretation for the clinician by Adam A. Dmytriw, Aristides Hadjinicolaou, Georgios Ntolkeras, Eleonora Tamilia, Matthew Pesce, Laura F. Berto, P. Ellen Grant, Elizabeth Pang, and Banu Ahtam in The Neuroradiology Journal.
Author contributorship
A.A.D., MD MPH MSc: conception and design of the study, literature review, and drafting a significant portion of the manuscript and figures. A.H., MD: literature review and drafting a significant portion of the manuscript and figures. G.N., MD: literature review, drafting a significant portion of the manuscript and figures, and acquisition and analysis of data. E.T., PhD: literature review, drafting a significant portion of the manuscript and figures, and acquisition and analysis of data. M.P., BS: literature review, drafting a significant portion of the manuscript and figures, and acquisition and analysis of data. L.F. Be.: literature review and drafting a significant portion of the manuscript and figures. P.E.G., MD MSc: literature review and drafting a significant portion of the manuscript and figures. E.P., PhD: conception and design of the study and acquisition and analysis of data. B.A., DPhil MSc: literature review, drafting a significant portion of the manuscript and figures, and acquisition and analysis of data.
Acknowledgments
We would like to express our sincere appreciation to Yoshio Okada, PhD, Christos Papadelis, PhD, Phillip Pearl, MD, Elizabeth Holland, BA, and Timothy Bardouille, PhD.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
Ethical statement
Ethical approval
Data presented in this review paper have been collected at Hospital for Sick Children (Toronto, Canada) and Boston Children’s Hospital (Boston, MA, USA). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the SickKids Research Ethics Board (REB# 1000053377 and REB# 1000049940) and the Boston Children’s Hospital Institutional Review Board (IRB-P00000247 and IRB-P00023088).
Consent to participate
An informed written consent was obtained from all parents of children from whom data were collected.
ORCID iDs
Adam A. Dmytriw https://orcid.org/0000-0003-0131-5699
Banu Ahtam https://orcid.org/0000-0003-2036-092X
References
- 1.Stefan H, Rampp S, Knowlton RC. Magnetoencephalography adds to the surgical evaluation process. Epilepsy Behav 2011; 20: 172–177. [DOI] [PubMed] [Google Scholar]
- 2.Ray A, Bowyer SM. Clinical applications of magnetoencephalography in epilepsy. Ann Indian Acad Neurol 2010; 13: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefan H, Hummel C, Scheler G, et al. Magnetic brain source imaging of focal epileptic activity: a synopsis of 455 cases. Brain 2003; 126: 2396–2405. [DOI] [PubMed] [Google Scholar]
- 4.Englot DJ, Nagarajan SS, Imber BS, et al. Epileptogenic zone localization using magnetoencephalography predicts seizure freedom in epilepsy surgery. Epilepsia 2015; 56: 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murakami H, Wang ZI, Marashly A, et al. Correlating magnetoencephalography to stereo-electroencephalography in patients undergoing epilepsy surgery. Brain 2016; 139: 2935–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliashiv DS, Elsas SM, Squires K, et al. Ictal magnetic source imaging as a localizing tool in partial epilepsy. Neurology 2002; 59: 1600–1610. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara H, Greiner HM, Hemasilpin N, et al. Ictal MEG onset source localization compared to intracranial EEG and outcome: improved epilepsy presurgical evaluation in pediatrics. Epilepsy Res 2012; 99: 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medvedovsky M, Taulu S, Gaily E, et al. Sensitivity and specificity of seizure-onset zone estimation by ictal magnetoencephalography. Epilepsia 2012; 53: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino G, Hedrich T, Chowdhury R, et al. Source localization of the seizure onset zone from ictal EEG/MEG data. Hum Brain Mapp 2016; 37: 2528–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duez L, Beniczky S, Tankisi H, et al. Added diagnostic value of magnetoencephalography (MEG) in patients suspected for epilepsy, where previous, extensive EEG workup was unrevealing. Clin Neurophysiol 2016; 127: 3301–3305. [DOI] [PubMed] [Google Scholar]
- 11.Papadelis C, Harini C, Ahtam B, et al. Current and emerging potential for magnetoencephalography in pediatric epilepsy. J Pediatr Epilepsy 2013; 02: 073–085. [Google Scholar]
- 12.Stufflebeam SM. Clinical magnetoencephalography for neurosurgery. Neurosurg Clin N Am 2011; 22: 153–167, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firsching R, Bondar I, Heinze HJ, et al. Practicability of magnetoencephalography-guided neuronavigation. Neurosurg Rev 2002; 25: 73–78. [DOI] [PubMed] [Google Scholar]
- 14.Cheyne D, Bostan AC, Gaetz W, et al. Event-related beamforming: a robust method for presurgical functional mapping using MEG. Clin Neurophysiol 2007; 118: 1691–1704. [DOI] [PubMed] [Google Scholar]
- 15.Moradi F, Liu LC, Cheng K, et al. Consistent and precise localization of brain activity in human primary visual cortex by MEG and fMRI. Neuroimage 2003; 18: 595–609. [DOI] [PubMed] [Google Scholar]
- 16.Papanicolaou AC, Simos PG, Castillo EM, et al. Magnetocephalography: a noninvasive alternative to the Wada procedure. J Neurosurg 2004; 100: 867–876. [DOI] [PubMed] [Google Scholar]
- 17.Knake S, Halgren E, Shiraishi H, et al. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res 2006; 69: 80–86. [DOI] [PubMed] [Google Scholar]
- 18.Raghavan M, Li Z, Carlson C, et al. MEG language lateralization in partial epilepsy using dSPM of auditory event-related fields. Epilepsy Behav 2017; 73: 247–255. [DOI] [PubMed] [Google Scholar]
- 19.Breier JI, Castillo EM, Simos PG, et al. Atypical language representation in patients with chronic seizure disorder and achievement deficits with magnetoencephalography. Epilepsia 2005; 46: 540–548. [DOI] [PubMed] [Google Scholar]
- 20.Pataraia E, Simos PG, Castillo EM, et al. Reorganization of language-specific cortex in patients with lesions or mesial temporal epilepsy. Neurology 2004; 63: 1825–1832. [DOI] [PubMed] [Google Scholar]
- 21.Kadis DS, Iida K, Kerr EN, et al. Intrahemispheric reorganization of language in children with medically intractable epilepsy of the left hemisphere. J Int Neuropsychol Soc 2007; 13: 505–516. [DOI] [PubMed] [Google Scholar]
- 22.Herdman AT, Pang EW, Ressel V, et al. Task-related modulation of early cortical responses during language production: an event-related synthetic aperture magnetometry study. Cereb Cortex 2007; 17: 2536–2543. [DOI] [PubMed] [Google Scholar]
- 23.Rezaie R, Narayana S, Schiller K, et al. Assessment of hemispheric dominance for receptive language in pediatric patients under sedation using magnetoencephalography. Front Hum Neurosci 2014; 8: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Poppel M, Wheless JW, Clarke DF, et al. Passive language mapping with magnetoencephalography in pediatric patients with epilepsy. J Neurosurg Pediatr 2012; 10: 96–102. [DOI] [PubMed] [Google Scholar]
- 25.O’Reilly C, Lewis JD, Elsabbagh M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS One 2017; 12: e0175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kikuchi M, Yoshimura Y, Hiraishi H, et al. Reduced long-range functional connectivity in young children with autism spectrum disorder. Soc Cogn Affect Neurosci 2015; 10: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewine JD, Andrews R, Chez M, et al. Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics 1999; 104: 405–418. [DOI] [PubMed] [Google Scholar]
- 28.Bailey AJ, Braeutigam S, Jousmäki V, et al. Abnormal activation of face processing systems at early and intermediate latency in individuals with autism spectrum disorder: a magnetoencephalographic study. Eur J Neurosci 2005; 21: 2575–2585. [DOI] [PubMed] [Google Scholar]
- 29.Sun L, Grutzner C, Bolte S, et al. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal-posterior cortices. J Neurosci 2012; 32: 9563–9573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahtam B, Braeutigam S, Bailey A. Semantic processing in autism spectrum disorders is associated with the timing of language acquisition: a magnetoencephalographic study. Front Hum Neurosci 2020; 14: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Costa L, Robertson A, Bethune A, et al. Delayed and disorganised brain activation detected with magnetoencephalography after mild traumatic brain injury. J Neurol Neurosurg Psychiatry 2015; 86: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pang EW, Dunkley BT, Doesburg SM, et al. Reduced brain connectivity and mental flexibility in mild traumatic brain injury. Ann Clin Transl Neurol 2016; 3: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakorin VA, Doesburg SM, da Costa L, et al. Detecting mild traumatic brain injury using resting state magnetoencephalographic connectivity. PLoS Comput Biol 2016; 12: e1004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braeutigam S. Magnetoencephalography: fundamentals and established and emerging clinical applications in radiology. ISRN Radiology 2013; 2013: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagić AI, Bowyer SM, Kirsch HE, et al. American clinical MEG society (ACMEGS) position statement #2: the value of magnetoencephalography (MEG)/magnetic source imaging (MSI) in noninvasive presurgical mapping of eloquent cortices of patients preparing for surgical interventions. J Clin Neurophysiol 2017; 34: 189–195. [DOI] [PubMed] [Google Scholar]
- 36.Ye AX, AuCoin-Power M, Taylor MJ, et al. Disconnected neuromagnetic networks in children born very preterm: disconnected MEG networks in preterm children. Neuroimage: Clinical 2015; 9: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VerMaas JR, Gehringer JE, Wilson TW, et al. Children with cerebral palsy display altered neural oscillations within the visual MT/V5 cortices. Neuroimage Clin 2019; 23: 101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurz MJ, Proskovec AL, Gehringer JE, et al. Children with cerebral palsy have altered oscillatory activity in the motor and visual cortices during a knee motor task. Neuroimage: Clinical 2017; 15: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadelis C, Ahtam B, Nazarova M, et al. Cortical somatosensory reorganization in children with spastic cerebral palsy: a multimodal neuroimaging study. Front Hum Neurosci 2014; 8: 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dima D, Frangou S, Burge L, et al. Abnormal intrinsic and extrinsic connectivity within the magnetic mismatch negativity brain network in schizophrenia: a preliminary study. Schizophr Res 2012; 135: 23–27. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura M, Watanabe S, Inagaki M, et al. Electrophysiological study of face inversion effects in Williams syndrome. Brain Dev 2013; 35: 323–330. [DOI] [PubMed] [Google Scholar]
- 42.de Haan W, van der Flier WM, Koene T, et al. Disrupted modular brain dynamics reflect cognitive dysfunction in Alzheimer’s disease. Neuroimage 2012; 59: 3085–3093. [DOI] [PubMed] [Google Scholar]
- 43.Cheng C-H, Wang P-N, Hsu W-Y, et al. Inadequate inhibition of redundant auditory inputs in Alzheimer’s disease: an MEG study. Biol Psychol 2012; 89: 365–373. [DOI] [PubMed] [Google Scholar]
- 44.Takei Y, Kumano S, Hattori S, et al. Preattentive dysfunction in major depression: a magnetoencephalography study using auditory mismatch negativity. Psychophysiology 2009; 46: 52–61. [DOI] [PubMed] [Google Scholar]
- 45.Dockstader C, Gaetz W, Cheyne D, et al. Abnormal neural reactivity to unpredictable sensory events in attention-deficit/hyperactivity disorder. Biol Psychiatr 2009; 66: 376–383. [DOI] [PubMed] [Google Scholar]
- 46.Helenius P, Laasonen M, Hokkanen L, et al. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia 2011; 49: 1889–1896. [DOI] [PubMed] [Google Scholar]
- 47.Dockstader C, Wang F, Bouffet E, et al. Gamma deficits as a neural signature of cognitive impairment in children treated for brain tumors. J Neurosci 2014; 34: 8813–8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hämäläinen M, Hari R, Ilmoniemi RJ, et al. Magnetoencephalography—theory, instrumentation, and applications to noninvasive studies of the working human brain. Rev Mod Phys 1993; 65: 413–497. [Google Scholar]
- 49.Tamilia E, Madsen JR, Grant PE, et al. Current and emerging potential of magnetoencephalography in the detection and localization of high-frequency oscillations in epilepsy. Front Neurol 2017; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uusitalo MA, Ilmoniemi RJ. Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 1997; 35: 135–140. [DOI] [PubMed] [Google Scholar]
- 51.Sun L, Hämäläinen MS, Okada Y. Noise cancellation for a whole-head magnetometer-based MEG system in hospital environment. Biomedical Physics & Engineering Express 2018; 4: 055014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp 1994; 2: 56–78. [Google Scholar]
- 53.Dunkley BT, Sedge PA, Doesburg SM, et al. Theta, mental flexibility, and post-traumatic stress disorder: connecting in the parietal cortex. PLoS One 2015; 10: e0123541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dockstader C, Gaetz W, Cheyne D, et al. MEG event-related desynchronization and synchronization deficits during basic somatosensory processing in individuals with ADHD. Behav Brain Funct 2008; 4: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tamilia E, Matarrese MAG, Ntolkeras G, et al. Noninvasive mapping of ripple onset predicts outcome in epilepsy surgery. Ann Neurol 2021; 89: 911–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Klink N, Hillebrand A, Zijlmans M. Identification of epileptic high frequency oscillations in the time domain by using MEG beamformer-based virtual sensors. Clin Neurophysiol 2016; 127: 197–208. [DOI] [PubMed] [Google Scholar]
- 57.Velmurugan J, Nagarajan SS, Mariyappa N, et al. Magnetoencephalography imaging of high frequency oscillations strengthens presurgical localization and outcome prediction. Brain 2019; 142: 3514–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada Y, Hämäläinen M, Pratt K, et al. BabyMEG: a whole-head pediatric magnetoencephalography system for human brain development research. Rev Sci Instrum 2016; 87: 094301. [DOI] [PubMed] [Google Scholar]
- 59.Okada Y, Pratt K, Atwood C, et al. BabySQUID: a mobile, high-resolution multichannel magnetoencephalography system for neonatal brain assessment. Rev Sci Instrum 2006; 77: 024301. [Google Scholar]
- 60.König MW, Mahmoud MA, Fujiwara H, et al. Influence of anesthetic management on quality of magnetoencephalography scan data in pediatric patients: a case series. Paediatr Anaesth 2009; 19: 507–512. [DOI] [PubMed] [Google Scholar]
- 61.Kirchberger K, Schmitt H, Hummel C, et al. Clonidine- and methohexital-induced epileptiform discharges detected by magnetoencephalography (MEG) in patients with localization-related epilepsies. Epilepsia 1998; 39: 1104–1112. [DOI] [PubMed] [Google Scholar]
- 62.Szmuk P, Kee S, Pivalizza EG, et al. Anaesthesia for magnetoencephalography in children with intractable seizures. Paediatr Anaesth 2003; 13: 811–817. [DOI] [PubMed] [Google Scholar]
- 63.Larson E, Taulu S. The importance of properly compensating for head movements during MEG acquisition across different age groups. Brain Topogr 2017; 30: 172–181. [DOI] [PubMed] [Google Scholar]
- 64.Marson A, Burnside G, Appleton R, et al. The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 2021; 397: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gofshteyn JS, Le T, Kessler S, et al. Synthetic aperture magnetometry and excess kurtosis mapping of Magnetoencephalography (MEG) is predictive of epilepsy surgical outcome in a large pediatric cohort. Epilepsy Res 2019; 155: 106151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ntolkeras G, Tamilia E, AlHilani M, et al. Presurgical accuracy of dipole clustering in MRI-negative pediatric patients with epilepsy: validation against intracranial EEG and resection. Clin Neurophysiol 2022; 141: 126–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martire DJ, Wong S, Workewych A, et al. Temporal-plus epilepsy in children: a connectomic analysis in magnetoencephalography. Epilepsia 2020; 61: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 68.Tamilia E, AlHilani M, Tanaka N, et al. Assessing the localization accuracy and clinical utility of electric and magnetic source imaging in children with epilepsy. Clin Neurophysiol 2019; 130: 491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tamilia E, Dirodi M, Alhilani M, et al. Scalp ripples as prognostic biomarkers of epileptogenicity in pediatric surgery. Ann Clin Transl Neurol 2020; 7: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Migliorelli C, Alonso JF, Romero S, et al. Automated detection of epileptic ripples in MEG using beamformer-based virtual sensors. J Neural Eng 2017; 14: 046013. [DOI] [PubMed] [Google Scholar]
- 71.Tang L, Xiang J, Huang S, et al. Neuromagnetic high-frequency oscillations correlate with seizure severity in absence epilepsy. Clin Neurophysiol 2016; 127: 1120–1129. [DOI] [PubMed] [Google Scholar]
- 72.Youssofzadeh V, Agler W, Tenney JR, et al. Whole-brain MEG connectivity-based analyses reveals critical hubs in childhood absence epilepsy. Epilepsy Res 2018; 145: 102–109. [DOI] [PubMed] [Google Scholar]
- 73.Miao A, Xiang J, Tang L, et al. Using ictal high-frequency oscillations (80--500 Hz) to localize seizure onset zones in childhood absence epilepsy: a MEG study. Neurosci Lett 2014; 566: 21–26. [DOI] [PubMed] [Google Scholar]
- 74.Niu K, Li Y, Zhang T, et al. Impact of antiepileptic drugs on cognition and neuromagnetic activity in childhood epilepsy with centrotemporal spikes: a magnetoencephalography study. Front Hum Neurosci 2021; 15: 720596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P, Li Y, Sun Y, et al. Altered functional connectivity in newly diagnosed benign epilepsy with unilateral or bilateral centrotemporal spikes: a multi-frequency MEG study. Epilepsy Behav 2021; 124: 108276. [DOI] [PubMed] [Google Scholar]
- 76.Bowyer SM, Pang EW, Huang M, et al. Presurgical functional mapping with magnetoencephalography. Neuroimaging Clin N Am 2020; 30: 159–174. [DOI] [PubMed] [Google Scholar]
- 77.Collinge S, Prendergast G, Mayers ST, et al. Pre-surgical mapping of eloquent cortex for paediatric epilepsy surgery candidates: evidence from a review of advanced functional neuroimaging. Seizure 2017; 52: 136–146. [DOI] [PubMed] [Google Scholar]
- 78.Bagic A, Funke ME, Ebersole J, et al. American Clinical MEG Society (ACMEGS) position statement: the value of magnetoencephalography (MEG)/magnetic source imaging (MSI) in noninvasive presurgical evaluation of patients with medically intractable localization-related epilepsy. J Clin Neurophysiol 2009; 26: 290–293. [DOI] [PubMed] [Google Scholar]
- 79.Gaetz W, Otsubo H, Pang EW. Magnetoencephalography for clinical pediatrics: the effect of head positioning on measurement of somatosensory-evoked fields. Clin Neurophysiol 2008; 119: 1923–1933. [DOI] [PubMed] [Google Scholar]
- 80.Bast T, Wright T, Boor R, et al. Combined EEG and MEG analysis of early somatosensory evoked activity in children and adolescents with focal epilepsies. Clin Neurophysiol 2007; 118: 1721–1735. [DOI] [PubMed] [Google Scholar]
- 81.Hallett M, DelRosso LM, Elble R, et al. Evaluation of movement and brain activity. Clin Neurophysiol 2021; 132: 2608–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shitara H, Shinozaki T, Takagishi K, et al. Movement and afferent representations in human motor areas: a simultaneous neuroimaging and transcranial magnetic/peripheral nerve-stimulation study. Front Hum Neurosci 2013; 7: 554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gaetz W, Cheyne D, Rutka JT, et al. Presurgical localization of primary motor cortex in pediatric patients with brain lesions by the use of spatially filtered magnetoencephalography. Neurosurgery 2009; 64: 177–185, discussion ons 186. [DOI] [PubMed] [Google Scholar]
- 84.Zillgitt A, Barkley GL, Bowyer SM. Visual mapping with magnetoencephalography: an update on the current state of clinical research and practice with considerations for clinical practice guidelines. J Clin Neurophysiol 2020; 37: 585–591. [DOI] [PubMed] [Google Scholar]
- 85.Cheour M, Imada T, Taulu S, et al. Magnetoencephalography is feasible for infant assessment of auditory discrimination. Exp Neurol 2004; 190(Suppl 1): 44–51. [DOI] [PubMed] [Google Scholar]
- 86.Shvarts V, Mäkelä JP. Auditory mapping with MEG: an update on the current state of clinical research and practice with considerations for clinical practice guidelines. J Clin Neurophysiol 2020; 37: 574–584. [DOI] [PubMed] [Google Scholar]
- 87.Pang EW, Wang F, Malone M, et al. Localization of Broca’s area using verb generation tasks in the MEG: validation against fMRI. Neurosci Lett 2011; 490: 215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hamberger MJ, Cole J. Language organization and reorganization in epilepsy. Neuropsychol Rev 2011; 21: 240–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berl MM, Balsamo LM, Xu B, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology 2005; 65: 1604–1611. [DOI] [PubMed] [Google Scholar]
- 90.Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J Neurosurg 2007; 106: 1117–1133. [DOI] [PubMed] [Google Scholar]
- 91.Pirmoradi M, Béland R, Nguyen DK, et al. Language tasks used for the presurgical assessment of epileptic patients with MEG. Epileptic Disord 2010; 12: 97–108. [DOI] [PubMed] [Google Scholar]
- 92.Rodin D, Bar-Yosef O, Smith ML, et al. Language dominance in children with epilepsy: concordance of fMRI with intracarotid amytal testing and cortical stimulation. Epilepsy Behav 2013; 29: 7–12. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka N, Liu H, Reinsberger C, et al. Language lateralization represented by spatiotemporal mapping of magnetoencephalography. AJNR Am J Neuroradiol 2013; 34: 558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Foley E, Cross JH, Thai NJ, et al. MEG assessment of expressive language in children evaluated for epilepsy surgery. Brain Topogr 2019; 32: 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Berman JI, Edgar JC, Blaskey L, et al. Multimodal diffusion-MRI and MEG assessment of auditory and language system development in autism spectrum disorder. Front Neuroanat; 10. DOI: 10.3389/fnana.2016.00030, Epub ahead of print 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed., text rev.). American Psychiatric Association, 2022. [Google Scholar]
- 97.Abrams DA, Lynch CJ, Cheng KM, et al. Underconnectivity between voice-selective cortex and reward circuitry in children with autism. Proc Natl Acad Sci U S A 2013; 110: 12060–12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci 2012; 14: 319–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bosl WJ, Tager-Flusberg H, Nelson CA. EEG analytics for early detection of autism spectrum disorder: a data-driven approach. Sci Rep 2018; 8: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milovanovic M, Grujicic R. Electroencephalography in assessment of autism spectrum disorders: a review. Front Psychiatry 2021; 12: 686021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roberts TPL, Kuschner ES, Edgar JC. Biomarkers for autism spectrum disorder: opportunities for magnetoencephalography (MEG). J Neurodev Disord 2021; 13: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsuzaki J, Ku M, Dipiero M, et al. Delayed auditory evoked responses in autism spectrum disorder across the life span. Dev Neurosci 2019; 41: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts TPL, Matsuzaki J, Blaskey L, et al. Delayed M50/M100 evoked response component latency in minimally verbal/nonverbal children who have autism spectrum disorder. Mol Autism 2019; 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edgar JC, Fisk CLI, Berman JI, et al. Auditory encoding abnormalities in children with autism spectrum disorder suggest delayed development of auditory cortex. Mol Autism 2015; 6: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roberts TPL, Bloy L, Ku M, et al. A multimodal study of the contributions of conduction velocity to the auditory evoked neuromagnetic response: anomalies in autism spectrum disorder. Autism Res 2020; 13: 1730–1745. [DOI] [PubMed] [Google Scholar]
- 106.Green HL, Edgar JC, Matsuzaki J, et al. Magnetoencephalography research in pediatric autism spectrum disorder. Neuroimaging Clin N Am 2020; 30: 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roberts TPL, Schmidt GL, Egeth M, et al. Electrophysiological signatures: magnetoencephalographic studies of the neural correlates of language impairment in autism spectrum disorders. Int J Psychophysiol 2008; 68: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Edgar JC, Khan SY, Blaskey L, et al. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in autism spectrum disorders. J Autism Dev Disord 2015; 45: 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Matsuzaki J, Kagitani-Shimono K, Sugata H, et al. Delayed mismatch field latencies in autism spectrum disorder with abnormal auditory sensitivity: a magnetoencephalographic study. Front Hum Neurosci 2017; 11: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato J, Safar K, Vogan VM, et al. Functional connectivity changes during working memory in autism spectrum disorder: a two-year longitudinal MEG study. Neuroimage Clin 2023; 37: 103364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khan S, Michmizos K, Tommerdahl M, et al. Somatosensory cortex functional connectivity abnormalities in autism show opposite trends, depending on direction and spatial scale. Brain 2015; 138: 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boutros NN, Lajiness-O’Neill R, Zillgitt A, et al. EEG changes associated with autistic spectrum disorders. Neuropsychiatric Electrophysiology 2015; 1: 1–20. [Google Scholar]
- 113.Muñoz-Yunta JA, Ortiz T, Palau-Baduell M, et al. Magnetoencephalographic pattern of epileptiform activity in children with early-onset autism spectrum disorders. Clin Neurophysiol 2008; 119: 626–634. [DOI] [PubMed] [Google Scholar]
- 114.Safar K, Wong SM, Leung RC, et al. Increased functional connectivity during emotional face processing in children with autism spectrum disorder. Front Hum Neurosci 2018; 12: 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mamashli F, Kozhemiako N, Khan S, et al. Children with autism spectrum disorder show altered functional connectivity and abnormal maturation trajectories in response to inverted faces. Autism Res 2021; 14: 1101–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Leung RC, Pang EW, Cassel D, et al. Early neural activation during facial affect processing in adolescents with Autism Spectrum Disorder. Neuroimage Clin 2015; 7: 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lumba-Brown A, Yeates KO, Sarmiento K, et al. Diagnosis and management of mild traumatic brain injury in children: a systematic review. JAMA Pediatr 2018; 172: e182847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lefevre-Dognin C, Cogné M, Perdrieau V, et al. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie 2021; 67: 218–221. [DOI] [PubMed] [Google Scholar]
- 119.Gardner RC, Yaffe K. Epidemiology of mild traumatic brain injury and neurodegenerative disease. Mol Cell Neurosci 2015; 66: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taylor CA, Bell JM, Breiding MJ, et al. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ 2017; 66: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 1995; 45: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 122.Haarbauer-Krupa J, Pugh MJ, Prager EM, et al. Epidemiology of chronic effects of traumatic brain injury. J Neurotrauma 2021; 38: 3235–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Konrad C, Geburek AJ, Rist F, et al. Long-term cognitive and emotional consequences of mild traumatic brain injury. Psychol Med 2011; 41: 1197–1211. [DOI] [PubMed] [Google Scholar]
- 124.Safar K, Zhang J, Emami Z, et al. Mild traumatic brain injury is associated with dysregulated neural network functioning in children and adolescents. Brain Commun 2021; 3: fcab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moran LM, Taylor HG, Rusin J, et al. Quality of life in pediatric mild traumatic brain injury and its relationship to postconcussive symptoms. J Pediatr Psychol 2012; 37: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yeates KO. Mild traumatic brain injury and postconcussive symptoms in children and adolescents. J Int Neuropsychol Soc 2010; 16: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuh EL, Hawryluk GWJ, Manley GT. Imaging concussion: a review. Neurosurgery 2014; 75(Suppl 4): S50–63. [DOI] [PubMed] [Google Scholar]
- 128.Huang M-X, Nichols S, Baker DG, et al. Single-subject-based whole-brain MEG slow-wave imaging approach for detecting abnormality in patients with mild traumatic brain injury. Neuroimage Clin 2014; 5: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang M-X, Nichols S, Robb A, et al. An automatic MEG low-frequency source imaging approach for detecting injuries in mild and moderate TBI patients with blast and non-blast causes. Neuroimage 2012; 61: 1067–1082. [DOI] [PubMed] [Google Scholar]
- 130.Dunkley BT, Da Costa L, Bethune A, et al. Low-frequency connectivity is associated with mild traumatic brain injury. Neuroimage Clin 2015; 7: 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang M-X, Theilmann RJ, Robb A, et al. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J Neurotrauma 2009; 26: 1213–1226. [DOI] [PubMed] [Google Scholar]
- 132.Kaltiainen H, Helle L, Liljeström M, et al. Theta-band oscillations as an indicator of mild traumatic brain injury. Brain Topogr 2018; 31: 1037–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robb SA, Nichols S, Drake A, et al. Magnetoencephalography slow-wave detection in patients with mild traumatic brain injury and ongoing symptoms correlated with long-term neuropsychological outcome. J Neurotrauma 2015; 32: 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gloor P, Ball G, Schaul N. Brain lesions that produce delta waves in the EEG. Neurology 1977; 27: 326–333. [DOI] [PubMed] [Google Scholar]
- 135.Huang M-X, Robb Swan A, Angeles Quinto A, et al. Resting-state magnetoencephalography source imaging pilot study in children with mild traumatic brain injury. J Neurotrauma 2020; 37: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Davenport EM, Urban JE, Vaughan C, et al. MEG measured delta waves increase in adolescents after concussion. Brain Behav 2022; 12: e2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kopell N, Ermentrout GB, Whittington MA, et al. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 2000; 97: 1867–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Valdés-Hernández PA, Ojeda-González A, Martínez-Montes E, et al. White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. Neuroimage 2010; 49: 2328–2339. [DOI] [PubMed] [Google Scholar]
- 139.Nunez PL, Srinivasan R. A theoretical basis for standing and traveling brain waves measured with human EEG with implications for an integrated consciousness. Clin Neurophysiol 2006; 117: 2424–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fries P. Rhythms for cognition: communication through coherence. Neuron 2015; 88: 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang M-X, Angeles-Quinto A, Robb-Swan A, et al. Assessing pediatric mild traumatic brain injury and its recovery using resting-state magnetoencephalography source magnitude imaging and machine learning. J Neurotrauma April 2023. DOI: 10.1089/neu.2022.0220, Epub ahead of print 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Magnetoencephalography for the pediatric population, indications, acquisition and interpretation for the clinician by Adam A. Dmytriw, Aristides Hadjinicolaou, Georgios Ntolkeras, Eleonora Tamilia, Matthew Pesce, Laura F. Berto, P. Ellen Grant, Elizabeth Pang, and Banu Ahtam in The Neuroradiology Journal.