Abstract
There is limited research on the effect of dietary quality on hepatocellular carcinoma (HCC) risk in populations with relatively high risk of HCC. Using data from Singapore Chinese Health Study, a prospective cohort study, of 63 257 Chinese aged 45 to 74, we assessed four diet-quality index (DQI) scores: the Alternative Health Eating Index-2010 (AHEI-2010), Alternate Mediterranean Diet (aMED), Dietary Approaches to Stop Hypertension (DASH) and Heathy Diet Indicator (HDI). We identified 561 incident HCC cases among the cohort participants after a mean of 17.6 years of follow-up. Cox proportional hazard regression model was used to estimate hazard ratio (HR) and 95% confidence interval (CI) for HCC in relation to these DQI scores. Unconditional logistic regression method was used to evaluate the associations between DQIs and HCC risk among a subset of individuals who tested negative for hepatitis B surface antigen (HBsAg). High scores of AHEI-2010, aMED and DASH, representing higher dietary quality, were associated with lower risk of HCC (all Ptrend < .05). Compared with the lowest quartile, HRs (95% CIs) of HCC for the highest quartile of AHEI-2010, aMED and DASH were 0.69 (0.53–0.89), 0.70 (0.52–0.95) and 0.67 (0.51–0.87), respectively. No significant association between HDI and HCC risk was observed. Among HBsAg-negative individuals, similar inverse associations were observed, and the strongest inverse association was for aMED (HRQ4vsQ1 = 0.46, 95% CI: 0.23–0.94, Ptrend = .10). These findings support the notion that adherence to a healthier diet may lower the risk of HCC, suggesting that dietary modification may be an effective approach for primary prevention of HCC.
Keywords: diet-quality index (DQI) scores, hepatocellular carcinoma, risk factor
1 |. INTRODUCTION
In 2018, liver cancer was ranked sixth most common cancer and fourth leading cause of cancer death worldwide.1 More than 90% of primary liver cancer cases are hepatocellular carcinoma (HCC).2 The established major risk factors for HCC are chronic infection with hepatitis B virus (HBV), hepatitis C virus and alcohol abuse, of which approximately two-thirds of HCC cases are attributable to viral hepatitis.3 In the United States, the incidence rate of liver cancer has been increasing by 3% to 4% per year since the mid-1970’s and was three times higher in the 2012 to 2016 period than in the 1975 to 1979 period.4 The rising incidence of liver cancer is believed to be attributable to increasing prevalence of nonalcoholic fatty liver disease (NALFD), which is closely associated with obesity and diabetes. With the implementation of effective universal hepatitis B vaccination and available curative therapy for hepatitis C, it is expected that the impact of NAFLD on HCC development continues increasing.
Evaluating the role of diet in the development of health outcomes is a challenging task due to the synergistic interaction of different nutrients and compounds within individual foods as different foods that are consumed together.5 In 2012, the National Cancer Institute launched the Dietary Patterns Methods Project (DPMP) with the aim to strengthen research evidence on dietary indices, dietary patterns as well as health for the Dietary Guidelines for Americans.6 In DPMP, four diet-quality indices (DQIs) were developed to capture full aspects of diet, the complexity of foods, nutrients and beverages consumed and their associations with cancers and cardiovascular diseases. These four DQIs, based on comprehensive literature review, included: (a) the Healthy Eating Index-2010 (HEI-2010); (b) the Alternative Health Eating Index-2010 (AHEI-2010); (c) the Alternate Mediterranean Diet (aMED) and (d) the Dietary Approaches to Stop Hypertension (DASH).5
Few prospective cohort studies have examined the role of dietary patterns, using these DQIs in the development of HCC. To date, only four prospective cohorts, all conducted in the United States,7–9 have evaluated the association between these DQIs and risk of primary liver cancer or specifically HCC and reported that AHEI-2010, aMED and DASH were inversely associated with the risk of HCC.
People living in Asia have different dietary habits from those in the United States, such as lower consumption of alcohol, soy foods and rice, and lower consumption of dairy products and red meat.10,11 To our knowledge, no prospective study has been conducted in an Asian population that examines the association between measures of dietary quality and HCC risk. We, therefore, evaluated the association between the DQIs and the risk of developing HCC, using data from the Singapore Chinese Health Study (SCHS), a population-based prospective cohort of more than 60 000 individuals with up to 25 years of follow-up.
2 |. METHODS
2.1 |. Study population
Data for the current analysis were obtained from the SCHS, which was described in details elsewhere.12 Briefly, the SCHS is an on-going population-based prospective cohort study that recruited 63 257 Chinese men and women, aged 45 to 74 from two main dialect groups of Chinese in Singapore (ie, Hokkiens and Cantonese) who resided in the government-built housing estates between April 1993 and December 1998. The Hokkiens and Cantonese, who accounted for more than two-thirds of Chinese in Singapore, were originated from the Fujian and Guangdong provinces in Southern China.
At baseline, participants were interviewed at their homes by trained interviewers, using a structured questionnaire to collect information on demographics, body weight and height, lifetime use of tobacco, current physical activity, menstrual/reproductive history (women only), occupational exposure, medical history and family history of cancer. During 1994 to 1999 recruitment period, blood and urine samples were collected from a 3% random sample of cohort participants. Between July 1999 and December 2003, all surviving cohort participants were recontacted to provide blood and urine samples. Participants were asked by phone interview to provide update information on alcohol use, tobacco smoking, medical history, current physical activity and body weight. They were also asked to donate a blood and if declined, a mouthwash sample was collected instead and urine samples for research use. Of all the subjects that we recontacted successfully, 28 346 subjects (approximately 57%) consented to donating blood for research.
2.2 |. Dietary assessment
Dietary assessment in SCHS was performed using a semi-quantitative food frequency questionnaires (FFQs) that was validated against a series of 24-hour dietary recall (24-HDR) interviews13 and selected biomarker studies on random subsets of cohort participants (n = 810).14,15 The FFQ contained 165 food items and food groups commonly consumed in Singapore. Study participants were asked how frequently (in eight categories: ranging from “never or hardly ever” to “two or more times a day”) they consumed the food or food group and followed by a question on the amount of food consumed, using photographs to choose from three portion sizes (ie, small, medium, and large). Average daily intake of approximately 100 nutrients and nonnutrient compounds was calculated for each study participant using the Singapore Food Composition Database.13 Between April 1994 and March 1997, the FFQ was validated against two 24-HDRs, one on a weekday and the other on a weekend that was approximately 2-month apart, among a random sample of 810 participants of the SCHS. The correlation coefficients between the FFQ and 24-HDR for the majority of calorie-adjusted nutrients ranged from 0.24 to 0.79.13
2.3 |. DQI scores
Four DQI scores were created using the dietary information from the SCHS FFQ in the current analysis. These DQIs were AHEI-2010, aMED, DASH and Heathy Diet Indicator (HDI). Previously, we identified two distinct Chinese dietary patterns in the SCHS, including the vegetable-fruit-soy (VFS) pattern, which was characterized by vegetables, fruit, and soy food and the meat-dim-sum (MDS) pattern, which was rich in meat and refined starchy foods.16 AHEI-2010 was chosen in our analysis because the VFS pattern and the aHEI-201 having similar associations with disease prevention and common beneficial good groups. aMED was chosen based on Mediterranean diet whereas HDI reflects the World Health Organization (WHO) dietary guidelines. DASH, with primary aim to lower blood pressure, was chosen based on its diet of high in fruits and vegetables, moderate in low-fat dairy products and low in animal protein but with substantial amount of plant protein from legumes and nuts.17 The higher the scores were the better the adherence to dietary guidelines.
These four DQIs were calculated based on the food groups and dietary nutrients derived from the Singapore Food Composition Database.18 We presented the dietary components and standards for scoring in Table S1. In the calculation of these DQIs, daily consumption of foods in grams was converted to standard serving equivalents. For example, we defined the serving size as follows: 67 g (0.5 cup of local vegetables) to represent one serving of vegetables,19,20 16 g to represent one serving of whole grains (ie, one slice of whole-wheat bread, 0.5 cup of oatmeal),21 28 g to represent one serving of nuts or one tablespoon (or 16 g) to represent one serving of peanut butter,19 90 g to represent one serving of fish,20 and 10 g to represent one serving of alcohol.20 For the representation of healthy dietary pattern components, we excluded potatoes and preserved vegetables from total vegetables, preserved or dried fruit from total fruit, and sweetened soy products and sweetened bean soup from legumes.
2.3.1 |. AHEI-2010 score
The AHEI-2010 was originally developed to examine the role of foods and nutrients on chronic disease risk22 and has 11 components.19 In our analysis, we included nine components: (a) vegetables, (b) fruit, (c) whole grains, (d) legumes, (e) long chain (n-3) fat, (f) polyunsaturated fatty acids (PUFAs), (g) red/processed meat, (h) sugar-sweetened beverage and fruit juice and (i) sodium after excluding alcohol (an independent risk factor for liver cancer23) and trans fat (unavailable in the study population). Each component was assigned a score of 0 to 10 according to the level of consumption and healthy/unhealthy status of the component. The summed score of AHEI-2010 ranged from 0 to 90. (Supplementary Table S1).
2.3.2 |. aMED score
The aMED was originally developed to examine the association between dietary habits of Mediterranean populations and the risk of chronic diseases.24,25 It originally included nine items.26 In our analysis, we included eight components: (a) vegetables, (b) fruit and nuts, (c) cereals, (d) dairy, (e) legumes, (f) fish, (g) the ratio of monounsaturated fatty acid (MUFA) over saturated fatty acids (SFAs) and (h) meat and meat products after excluding alcohol (an independent risk factor23). Each component was assigned a score of 0 or 1 according to the consumption level (below or above the study population specific median) and healthy/unhealthy status of the component. The summed score of aMED ranged from 0 to 8 (Supplementary Table S1).
2.3.3 |. DASH score
The DASH was originally developed for hypertension management and included eight components.27 In our analysis, we included eight components as outlined as: (a) vegetables, (b) fruit, (c) whole grains, (d) nuts and legumes, (e) low-fat dairy, (f) red and processed meat, (g) sweetened beverages and (h) sodium. Each component was assigned a score of 1 to 5 according to its quintiles of consumption and healthy/unhealthy status of the component. The summed score of DASH ranged from 5 to 40 (Table S1).
2.3.4 |. HDI score
The HDI score was originally developed following the 1990 WHO Dietary Guidelines to reflect an optimal diet to prevent chronic disease.28,29 In the current analysis, we used an updated version of the HDI, following the 2003 WHO Dietary Guidelines,30 which included seven components: (a) percentages of energy intake from SFAs, (b) PUFAs (g/d), (c) monosaccharide and disaccharide, and protein (mg/d), (d) cholesterol (mg/d), (e) fruit and vegetable combined (g/d), (f) total dietary fiber (g/d) and (g) nonstarch polysaccharides (g/d). Each component was assigned a score of 0 to 10 according to the decile of consumption and healthy/unhealthy status of the component. The summed score of HDI ranged from 0 to 70 (Supplementary Table S1).
2.4 |. Assessment of other covariates
Body mass index (BMI) was calculated as the weight in kilograms divided by height in meters squared. Smoking habits included in the current analysis were smoking status (ie, never, current and former smokers).31 For physical activity, we used a continuous scale of eight, that is, never, 0.5 to 1, 2 to 3, 4 to 6, 7 to 10, 11 to 20, 21 to 30 and 31 hours or more per week, for each of three physical activity categories: (a) strenuous sports (ie, jogging, bicycling on hills, tennis, squash, swimming laps, or aerobics); (b) vigorous work (ie, moving heavy furniture, loading or unloading trucks, shoveling, or equivalent manual labor) and (c) moderate activities (ie, brisk walking, bowling, bicycling on level ground, Tai Chi and Chi Kung).32
2.5 |. Ascertainment of HCC case
Incident HCC cases and deaths were identified by annual linkage analysis of all surviving cohort participants with the national databases of the Singapore Cancer Registry and the Singapore Birth and Death Registry, respectively. The International Classification of Diseases—Oncology, second Edition Codes C22.0 were used to determine HCC cases.33 To date, only 56 (<0.1%) of the entire cohort participants were known to be lost to follow-up due to migration out of Singapore, the ascertainment of incident cancer cases and deaths among the cohort participants had been virtually complete. As of December 2015, after excluding 1936 participants with a history of cancer at baseline, and with an average 17.7 years of follow-up, the current analysis identified 561 incident case of HCC in this cohort.
2.6 |. Case-control study of HCC
We also constructed a case-control study of HCC within SCHS, which was derived from 28 346 participants who provided baseline blood samples. All 220 incident HCC cases diagnosed before December 31, 2015 who donated a baseline blood sample were eligible for the subanalysis. For each case, we randomly selected two to three control subjects. Eligible were individuals who donated a baseline blood sample and were alive and free of cancer at the date of diagnosis of the index case. Controls were individually matched to the index case by age at enrollment (±2 years old), gender, dialect groups (ie, Hokkien, Cantonese), and date of baseline blood collection (± 6 months).
Serum samples of all subjects of the case-control study of HCC were tested for serological markers of HBV exposure using standard assays as described previously.20,32,33 Laboratory personnel were blind on the case/control status of the test samples. Briefly, we tested the presence of HBsAg on the first 302 samples using commercialized test kit (AUSRIA, Abbott, Laboratories, North Chicago, Illinois). Negative samples were further tested for the presence of anti-HBc and anti-HBs, using standardized test kit (Corab and Ausab, Abbott Laboratories, North Chicago, Illinois). For the late 360 samples, only was HBsAg status determined using the same assay.
2.7 |. Statistical analysis
Means and SD were calculated for continuous variables while counts and proportions were computed for categorical variables. The t test and χ2 test were used to compare the distributions of continuous and categorical variables, respectively, between cases and noncases as well as across quartiles of the each high-quality index. Person-years at risk for each participant was calculated from the date of blood draw to the date of HCC diagnosis, death, migration out of Singapore, or December 31, 2015, whichever occurred first.
The Cox proportional hazard regression method was used to determine the association between DQIs and the risk of HCC. Hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) of HCC with higher levels of DQIs (in quartile or continuous scale per SD) were derived from the Cox proportional hazard regression models with additional potential confounders, which were age, gender, dialect group (Hokkien or Cantonese), level of education (no formal education, primary school, secondary or higher education), year of enrollment (1993–1995 and 1996–1998), BMI (<25 vs ≥25 kg/m2), smoking status (ever vs never), alcohol drinking (nondrinkers, 1–7 drinks/week and >7 drinks/week), diabetes (yes vs no) and total energy intake (Kcal/d). Liner trend for HCC risk with DQIs was tested based on ordinal values of their quartiles. The proportional hazard assumption was assessed using Schoenfeld residuals test and graph for residuals over time and was confirmed by an interaction test between predictors and follow-up time. No violation was found.
We further performed stratified analysis by BMI level (<25 or ≥25 kg/m2) and history of Type 2 diabetes (no or yes). A sensitivity analysis was conducted by excluding HCC cases and person-years observed within the first 2 years postenrollment. Furthermore, we performed an analysis of the association between individual food and nutrient components of the four DQI scores and HCC risk to better understand the differences in results of these scores.
We used conditional logistic regression method in analysis for the entire data set of the nested case-control study to evaluate the association between a DQI score and the risk of HCC with additional adjustment for HBsAg serological status. In the stratified analysis by HBsAg status, unconditional logistic regression method was used with the inclusion of all matching factors since the matched pairs were broken. All statistical analyses were conducted using the computing software SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). All P values were two sided and .05 was used as a threshold of statistical significance.
3 |. RESULTS
After a mean (SD) follow-up duration of 17.6 (5.3) years for 61 321 participants, we identified 561 incident HCC cases. The median (inter-quartile range) age at cancer diagnosis was 71 years (65, 76).
Cases and noncases did not differ significantly in distributions by level of education, physical activity, and consumption of fruits, fiber, fish and total calories. Compared to noncases, cases were older and had a higher proportion of men, persons with a Hokkien dialect, heavy smokers, heavy drinkers and those with a history of Type 2 diabetes. Cases also had a higher BMI and consumed more red meat but less vegetable (all P < .05) (Table 1).
TABLE 1.
Distributions of baseline characteristics among study participants, the Singapore Chinese Healthy Study, 1993–2016
| Characteristics | Cases (n = 561) | Noncases (n = 60 760) | P value |
|---|---|---|---|
|
| |||
| Median age (IQR), years | 60 (53, 65) | 55 (49, 62) | <.0001 |
| Gender (%)a | |||
| Male | 405 (72.2) | 26 888 (44.2) | <.0001 |
| Female | 156 (27.8) | 33 872 (55.7) | |
| Dialecta | |||
| Cantonese | 213 (38.0) | 28 112 (46.3) | <.0001 |
| Hokkien | 348 (62.0) | 32 648 (53.7) | |
| Level of education (%)a | |||
| No formal education | 144 (25.7) | 16 517 (27.2) | 0.12 |
| Primary school | 273 (48.7) | 26 951 (44.4) | |
| Secondary school or higher | 144 (25.7) | 17 292 (28.5) | |
| Mean body mass index (±SD)b, Kg/m2 | 24.0 (±3.6) | 23.1 (±3.3) | <.0001 |
| Smoking status (%) | |||
| Never smoker | 281 (50.1) | 42 302 (69.6) | <.0001 |
| Former smoker | 99 (17.6) | 6582 (10.8) | |
| Current smoker | 181 (32.3) | 11 876 (19.5) | |
| Alcohol consumption (%)b | |||
| Nondrinkers | 427 (76.1) | 49 223 (81.0) | <.0001 |
| 1–7 drinks/week | 81 (14.4) | 8691 (14.3) | |
| ≥7 drinks/week | 53 (9.4) | 2846 (4.7) | |
| Diabetes (%)b | |||
| No | 476 (84.8) | 55 376 (91.1) | <.0001 |
| Yes | 85 (15.2) | 5384 (8.9) | |
| Any weekly physical activityc (%) | |||
| No | 507 (90.4) | 55 305 (91.0) | .59 |
| Yes | 54 (9.6) | 5455 (9.0) | |
| Mean total energy intake (±SD), kcal | 1590.1 (±594.8) | 1556.3 (±565.9) | .16 |
| Red meat (g/d) (±SD) | 32.7 (±25.2) | 30.6 (±24.3) | .04 |
| Vegetables (g/d) (±SD) | 101.1 (±55.6) | 110.7 (±63.6) | .0003 |
| Fruits (g/d) (±SD) | 189.0 (±156.3) | 202.6 (±169.5) | .06 |
| Fiber (g/d) (±SD) | 12.3 (±5.7) | 12.7 (5.8) | .17 |
| Fish (g/d) (±SD) | 56.7 (31.3) | 55.2 (31.6) | .70 |
Note: Values are means ± SDs for continuous variables and numbers (percentages) for categorical variables unless specified.
Abbreviation: IQR, interquartile range.
Variables reported at baseline.
Variables reported at the follow-up 1.
Physical activity represents amount of strenuous physical activity and/or vigorous work.
The correlation coefficients were .65 between AHEI-2010 and aMED, .76 between AHEI-2010 and DASH, .31 between AHEI-2010 and HDI, .59 between aMED and DASH, .32 between aMED and HDI and .33 between DASH and HDI (all P values <.0001). Across the four DQI scores, there were high proportions of Cantonese, participants with higher education level, never smokers, nondrinkers and persons with a history of diabetes in the highest quartile of the DASH and HDI scores; more persons with a history of diabetes in the highest quartile of the AHEI-2010 score; a higher proportion of physically active participants in the highest quartile of aMED and HDI scores, compared with the lowest quartile of their respective DQIs. Of all four DQIs, participants in the highest quartile reported lower consumption of red meat, and higher intake of vegetables, fruits and fiber. However, the intake of fish was higher in the highest quartile of AHEI-2010 and aMED, but lower for HDI, and similar for DASH as compared with the lowest quartile of respective DQIs (Table 2).
TABLE 2.
Distributions of baseline characteristics among study participants by highest vs lowest quartile of the diet quality indices in the Singapore Chinese Health Study, 1993–2015
| AHEI-2010 (n, %) |
aMED (n, %) |
DASH (n, %) |
HDI (n, %) |
|||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 15 330) | Q4 (n = 15 330) | Q1 (n = 12 915) | Q4 (n = 11 960) | Q1 (n = 17 112) | Q4 (n = 13 231) | Q1 (n = 15 330) | Q4 (n = 15 330) | |
|
| ||||||||
| Age (mean ± SD) | 56.3 (8.1) | 56.1 (7.8) | 57.6 (8.2) | 55.1 (7.6) | 55.8 (7.9) | 56.8 (7.9) | 56.5 (8.0) | 56.0 (7.8) |
| Gender (%)a | ||||||||
| Male | 8498 (55.4) | 5681 (37.1) | 6073 (47.0) | 5116 (42.8) | 9489 (55.4) | 4443 (33.6) | 6626 (43.2) | 7644 (49.9) |
| Female | 6832 (44.6) | 9649 (62.9) | 6842 (53.0) | 6844 (57.2) | 7623 (44.6) | 8788 (66.4) | 8704 (56.8) | 7686 (50.1) |
| Dialecta | ||||||||
| Cantonese | 6526 (42.6) | 7778 (50.7) | 4977 (38.5) | 6525 (54.6) | 7105 (41.5) | 6894 (52.1) | 6097 (39.8) | 8129 (53.0) |
| Hokkien | 8804 (57.4) | 7552 (49.3) | 7938 (61.5) | 5435 (45.4) | 10 007 (58.5) | 6337 (47.9) | 9233 (60.2) | 7201 (47.0) |
| Level of education (%)a | ||||||||
| No formal education | 4548 (29.6) | 3439 (22.4) | 4708 (36.5) | 2097(17.5) | 4889 (28.6) | 3159 (23.9) | 4951 (31.6) | 3221 (21.0) |
| Primary school | 7123 (46.5) | 6430 (41.9) | 5829 (45.1) | 5004 (41.8) | 8044 (47.0) | 5513 (41.7) | 6709 (43.8) | 6830 (44.5) |
| Secondary school or higher | 3849 (25.1) | 5461 (35.6) | 2378 (18.4) | 4859 (40.6) | 4179 (24.4) | 4559 (34.5) | 3770 (24.6) | 5279 (34.4) |
| Mean body mass index (±SD)b, Kg/m2 | 23.0 ± 3.3 | 23.2 ± 3.2 | 23.0 ± 3.3 | 23.1 ± 3.3 | 23.2 ± 3.3 | 23.0 ± 3.2 | 23.2 ± 3.4 | 23.1 ± 3.2 |
| Smoking status (%) | ||||||||
| Never smoker | 8936 (58.3) | 12 109 (79.0) | 7952 (61.6) | 9187 (76.8) | 9848 (57.5) | 10 713 (81.0) | 9754 (63.6) | 11 095 (72.4) |
| Former smoker | 1873 (12.2) | 1540 (10.0) | 1372 (10.6) | 1325(11.1) | 2036(11.9) | 1280 (9.7) | 1539 (10.0) | 1971 (12.9) |
| Current smoker | 4521 (29.5) | 1681 (11.0) | 3591 (27.8) | 1448 (12.1) | 5228 (30.5) | 1238 (9.4) | 4037 (26.3) | 2264 (14.8) |
| Alcohol consumption (%)b | ||||||||
| Nondrinkers | 11 620 (75.8) | 12 995 (84.8) | 10 521 (81.5) | 9514 (79.5) | 12 779 (74.7) | 11 528 (87.1) | 11 906 (77.7) | 12 567 (82.0) |
| 1–7 drinks/week | 2460 (16.0) | 1938 (12.6) | 1556 (12.4) | 2001 (167) | 2913 (17.0) | 1456 (11.0) | 2164 (14.1) | 2353 (15.3) |
| ≥7 drinks/week | 1250 (8.2) | 357 (2.6) | 798 (6.2) | 445 (3.7) | 1420 (8.3) | 247 (1.9) | 1260 (8.2) | 410 (2.7) |
| Diabetes (%)b | ||||||||
| No | 14 196 (92.6) | 13 767 (89.8) | 11 834 (91.6) | 10 865 (90.8) | 15 870 (92.7) | 11 818 (89.3) | 13 889 (90.6) | 14 105 (92.0) |
| Yes | 1134 (7.4) | 1563 (10.2) | 1081 (8.4) | 1095 (9.2) | 1242 (7.3) | 1413 (10.7) | 1441 (9.4) | 1225 (8.0) |
| Any weekly physical activitya (%) | ||||||||
| No | 13 507 (88.1) | 14 089 (91.9) | 11 800 (91.4) | 10 673 (89.2) | 15 191 (88.8) | 12 217 (92.3) | 14 094 (91.9) | 13 748 (89.7) |
| Yes | 1623 (11.9) | 1241 (8.1) | 1115 (8.6) | 1287 (10.8) | 1921(11.2) | 1014 (7.7) | 1236 (8.1) | 1582 (10.3) |
| Mean total energy intake (±SD), Kcal | 1563.5 ± 604.2 | 1632.0 ± 543.3 | 1284.3 ± 441.8 | 1860.7 ± 613.9 | 1545.3 ± 565.6 | 1580.2 ± 513.0 | 1474 ± 665.2 | 1739.5 ± 507.3 |
| Red meat (g/d) (±SD) | 22.0 ± 12.8 | 15.2 ± 9.1 | 20.5 ± 11.7 | 17.3 ± 10.8 | 24.8 ± 11.7 | 12.3 ± 19.4 | 23.9 ± 13.3 | 14.5 ± 8.2 |
| Vegetables (g/d) (±SD) | 81.7 ± 44.7 | 150.6 ± 78.2 | 67.6 ± 31.4 | 161.1 ±71.5 | 85.0 ± 46.2 | 142.0 ± 72.4 | 100.1 ± 63.4 | 129.4 ± 63.6 |
| Fruits (g/d) (±SD) | 137.6 ± 131.6 | 301.2 ± 196.6 | 98.8 ± 92.9 | 321.3 ± 189.9 | 130.5 ± 124.7 | 290.1 ± 182.9 | 133.9 ± 145.3 | 290.6 ± 179.1 |
| Fiber (g/d) (±SD) | 9.9 ± 4.5 | 16.8 ± 6.4 | 8.0 ± 3.2 | 18.1 ± 6.2 | 9.7 ± 4.2 | 16.6 ± 6.0 | 10.2 ± 5.6 | 16.0 ± 5.4 |
| Fish (g/d) (±) | 28.0 ± 15.6 | 41.1 ± 17.9 | 30.9 ± 16.5 | 40.5 ± 16.9 | 36.0 ± 17.5 | 35.0 ± 18.2 | 43.4 ± 20.1 | 28.0 ± 12.3 |
Note: Values are means ± SDs for continuous variables unless otherwise specified; percentages are based on nonmissing data.
Abbreviations: AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean diet; DASH, Dietary Approaches to Stop Hypertension; DQI, Diet Quality Index; HDI, Healthy Diet Indicator; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; Q1, lowest quartile or first quartile; Q4, highest quartile or forth quartile; SFA, saturated fatty acid; SSB, sugar sweetened beverages.
Variables reported at baseline.
Variables reported at the follow-up 1.
The associations between each of the four DQIs and HCC risk are shown in Table 3. Higher scores for AHEI-2010, aMED, DASH and HDI were associated with lower risk of HCC (all Ptrend < .05); the corresponding HRs and 95% CIs for the highest quartile compared to the lowest quartile were 0.69 (0.53–0.89), 0.70 (0.52–0.95), 0.67 (0.51–0.87) and 0.85 (0.55–1.09), respectively (Table 3).
TABLE 3.
Associations between the diet quality indices and risk of hepatocellular carcinoma in the Singapore Chinese Health Study
| DQI | Person-year | Cases | HR (95% CI)a |
|---|---|---|---|
|
| |||
| AHEI-2010 | |||
| Quartile 1 (17.0–42.1) (lowest) | 261 019 | 168 | 1.00 |
| Quartile 2 (42.1–46.7) | 269 137 | 144 | 0.91 (0.73–1.14) |
| Quartile 3 (46.7–51.5) | 272 975 | 149 | 0.99 (0.79–1.24) |
| Quartile 4 (51.5–75.9) (highest) | 280 358 | 100 | 0.69 (0.53–0.89) |
| Ptrend | 0.02 | ||
| Continuous scale (per SD increase) | 0.90 (0.82–0.98) | ||
| aMED | |||
| Quartile 1 (0–2.0) (lowest) | 217 893 | 144 | 1.00 |
| Quartile 2 (3.0) | 208 942 | 109 | 0.85 (0.66–1.09) |
| Quartile 3 (4.0–5.0) | 435 987 | 226 | 0.91 (0.73–1.13) |
| Quartile 4 (6.0–8.0) (highest) | 220 668 | 82 | 0.70 (0.52–0.95) |
| Ptrend | 0.06 | ||
| Continuous scale (per SD increase) | 0.90 (0.82–0.99) | ||
| DASH | |||
| Quartile 1 (8.0–21.0) (lowest) | 296 200 | 192 | 1.00 |
| Quartile 2 (22.0–23.0) | 192 590 | 108 | 0.91 (0.72–1.16) |
| Quartile 3 (24.0–27.0) | 356 057 | 177 | 0.86 (0.70–1.06) |
| Quartile 4 (28.0–39.0) (highest) | 238 642 | 84 | 0.67 (0.51–0.87) |
| Ptrend | 0.004 | ||
| Continuous scale (per SD increase) | 0.86 (0.79–0.94) | ||
| HDI | |||
| Quartile 1 (14.2–45.2) (lowest) | 263 182 | 150 | 1.00 |
| Quartile 2 (45.2–50.2) | 270 457 | 159 | 1.08 (0.85–1.33) |
| Quartile 3 (50.2–55.1) | 272 064 | 121 | 0.79 (0.62–1.00) |
| Quartile 4 (55.1–70.0) (highest) | 277 786 | 131 | 0.85 (0.66–1.09) |
| Ptrend | 0.04 | ||
| Continuous scale (per SD increase) | 0.92 (0.84–1.00) | ||
Note: Bold values indicate P < .05.
Abbreviations: AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean diet; BMI, body mass index; DASH, Dietary Approaches to Stop Hypertension; DQI, diet-quality index; HDI, Healthy Diet Indicator.
Models adjusted for age, sex, dialect, year of enrollment, education level, smoking status, coffee drinking status, alcohol drinking status, total energy intake, BMI, and diabetes status.
In the case-control analysis, we did not find a significant inverse association between the AHEI-2010, DASH and HDI indexes and HCC risk (Table 4). We, however, found an inverse association between aMED and HCC incidence among HBsAg-negative subjects of whom the risk of HCC decreased by more than 50% for those with the highest related to the lowest quartiles (HRQ4vsQ1 = 0.46, 95% CI: 0.23–0.94; Ptrend = .10). The analysis for HBsAg-positive individuals was not informative due to the small sample size (Table 4).
TABLE 4.
Associations between the high-diet quality indices and risk of hepatocellular carcinoma in subset of participants with known hepatitis B surface antigen (HBsAg) serology status in the Singapore Chinese Health Studya
| Q1 | Q2 | Q3 | Q4 | P trend | |
|---|---|---|---|---|---|
|
| |||||
| AHEI-2010 | |||||
| All subjectsb | |||||
| Cases/controls | 54/148 | 39/140 | 60/125 | 46/134 | |
| OR (95% CI) | 1.00 | 0.72 (0.42–1.23) | 1.29 (0.79–2.12) | 0.77 (0.45–1.31) | 0.83 |
| HBsAg-negativec | |||||
| Cases/controls | 37/141 | 25/134 | 43/118 | 24/130 | |
| OR (95% CI) | 1.00 | 0.68 (0.38–1.22) | 1.31 (0.78–2.22) | 0.62 (0.34–1.12) | 0.47 |
| aMED | |||||
| All subjectsb | |||||
| Cases/controls | 44/160 | 35/119 | 86/211 | 34/112 | |
| OR (95% CI) | 1.00 | 0.67 (0.38–1.19) | 0.91 (0.55–1.50) | 0.48 (0.25–0.91) | 0.11 |
| HBsAg-negativec | |||||
| Cases/controls | 31/101 | 25/112 | 56/205 | 17/105 | |
| OR (95% CI) | 1.00 | 0.77 (0.42–1.40) | 0.90 (0.53–1.52) | 0.46 (0.23–0.94) | 0.10 |
| All subjects | |||||
| DASH | |||||
| All subjectsb | |||||
| Cases/controls | 59/156 | 35/85 | 64/170 | 41/136 | |
| OR (95% CI) | 1.00 | 1.04 (0.59–1.81) | 0.89 (0.55–1.42) | 0.70 (0.41–1.20) | 0.19 |
| HBsAg-negativec | |||||
| Cases/controls | 40/149 | 26/79 | 38/164 | 25/131 | |
| OR (95% CI) | 1.00 | 1.18 (0.65–2.12) | 0.80 (0.48–1.33) | 0.61 (0.34–1.10) | 0.07 |
| HDI | |||||
| All subjectsb | |||||
| Cases/controls | 58/128 | 50/111 | 36/141 | 55/167 | |
| OR (95% CI) | 1.00 | 1.02 (0.61–1.70) | 0.58 (0.34–1.00) | 0.73 (0.44–1.21) | 0.09 |
| HBsAg-negativec | |||||
| Cases/controls | 35/121 | 33/106 | 24/136 | 37/160 | |
| OR (95% CI) | 1.00 | 1.02 (0.59–1.78) | 0.57 (0.32–1.03) | 0.76 (0.44–1.31) | 0.14 |
Note: Bold values indicate P < .05.
Abbreviations: AHEI-2010, Alternative Healthy Eating Index-2010; aMED, alternate Mediterranean diet; CI, confidence interval; DASH, Dietary Approaches to Stop Hypertension; DQI, Diet Quality Index; HDI, Healthy Diet Indicator.
Models adjusted for age, sex, dialect, year of enrollment, education level, smoking status, coffee drinking status, alcohol drinking status, total energy intake, BMI, diabetes status.
Conditional logistic regression model was performed.
Unconditional logistic regression model was performed.
In the stratified analysis by BMI level, we found statistically significant inverse associations for AHEI-2010, aMED and DASH with HCC risk in participants with BMI < 25 kg/m2 or those without a history of Type 2 diabetes (Table S2). In addition, highest quartile of DASH index was associated with significantly lower risk of HCC in participants with a history of diabetes and the highest quartile of HDI with lower risk of HCC in participants without a history of diabetes (both Ptrend < .05). We did not find a statistically significant association for the DQIs with HCC risk in other subgroups. There was only significant interaction between HDI and history of diabetes in the association with HCC risk (Pinteraction = .03) (Table S2).
We conducted sensitivity analysis after excluding HCC cases and person-years within the first 2 years of observation and found that the results remained almost the same as those based on the whole dataset (Table S3). Compared with the lowest quartiles, HRs (95% CIs) of HCC for the highest quartiles of AHE-2010, aMED, DASH and HDI were 0.72 (0.55–0.93), 0.70 (0.51–0.95), 0.69 (0.52–0.91) and 0.79 (0.61–1.02), respectively.
We also evaluated food groups and nutrients that are components of the four DQIs in relation to HCC risk. After adjusting for potential confounders, higher intakes of legumes and long-chain n-3 PUFAs were associated with a lower risk of HCC while consumptions of MDS and PUFAs were associated with increased risk of HCC (Figures 1 and 2).
FIGURE 1.
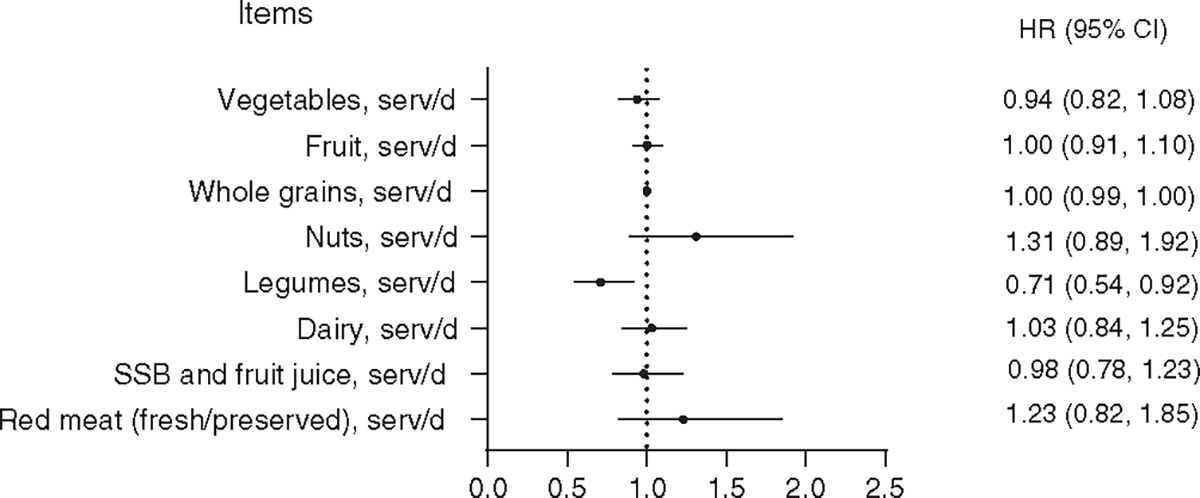
Association between food groups of diet-quality index scores and hepatocellular carcinoma in the Singapore Chinese Health Study. Models adjusted for age, sex, dialect, year of enrollment, education level, smoking status, coffee drinking status, alcohol drinking status, total energy intake, BMI, diabetes status, vegetables, fruits, whole grains, nuts, legumes, dairy, SSB, red meat and fish. Fish was removed from the figure as the HR and 95% CI = 1.00 (1.00–1.00). CI, confidence interval; SSB, sugar-sweetened beverage
FIGURE 2.
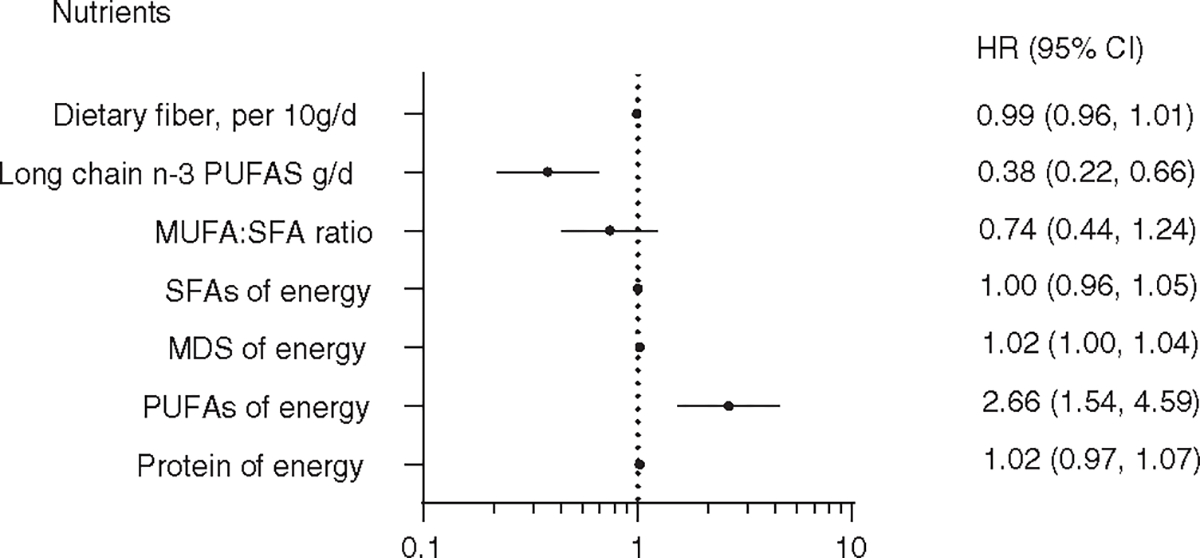
Association between nutrient components of diet-quality index scores and hepatocellular carcinoma in the Singapore Chinese Health Study. Models adjusted for age, sex, dialect, year of enrollment, education level, smoking status, coffee drinking status, alcohol drinking status, total energy intake, BMI, diabetes status, fiber, long-chain n-3 PUFAs, MUFA:SFA ratio, SFA, MDS, PUFAs, protein, sodium, and cholesterol. Sodium and cholesterol were removed from the Figure as the HRs and 95% CIs = 1.00 (1.00–1.00). PUFAs, polyunsaturated fatty acids, MDS, monosaccharide and disaccharide; MUFAs, monounsaturated fatty acids, SFA, short-chain fatty acid,
4 |. DISCUSSION
In the current analysis, we found higher scores of four DQIs (ie, AHEI-2010, aMED, DASH and HDI), reflecting higher quality dietary patterns, all were associated with a 15% to 33% lower risk of developing HCC. The protective effect of DQIs against the development of HCC was confirmed among a subset of individuals with negative HBsAg, which ruled out the possibility of confounding effect by chronic infection with HBV.
To our knowledge, this is the first prospective cohort study that used DQI scores derived in an Asian population whose dietary habits were distinct from Americans. Our results on the association between higher scores of AHEI-2010 and lower risk of HCC are consistent with findings among participants of Health Professional Follow-up Study (HPFS) and Nurses’ Health Study (NHS),8 the AARP9 and Multiethnic Cohort Study (MEC).7 Our study showed a 31% lower HCC risk for those who were most adherent to AHEI-2010. A similar reduction (by 28%-39%) in HCC risk was observed in diverse populations (ie, Caucasian, African Americans, Hispanic and Asian Americans) with different dietary habits in the United States.7–9
Similarly, the reduction in HCC risk was by 30% for those who had highest score of aMED in our study as compared with a 25% to 39% risk reduction among participants of MEC7 and AARP.9 In addition to prospective cohort studies, a case-control study of two Mediterranean countries (ie, Greece and Italy) involving 518 HCC cases and 772 controls34 reported a statistically significant 50% lower risk of HCC associated with higher traditional MED score (≥5) compared with the lowest score (0–3). For DASH score, we found that the HCC risk was 33% lower for the highest score compared with the lowest score, which is also in line with results in MEC7 HPFS and NHS cohorts8 (a 25%-48% risk reduction). The risk reduction for HCC using HDI score was 15% for the highest score in comparison with the lowest score, but this score was not examined the other prospective cohort studies.7–9 These consistent results from diverse populations suggest that diet plays an important role in the risk of HCC development, especially for nonviral-related HCC.
The four evaluated dietary quality scores (ie, AHEI-2010, aMED, DASH and HDI) were moderately to highly correlated to each other. These dietary quality scores focused on food groups, comprising higher intakes of vegetables, fruits, whole grains, nuts and legumes and lower intakes of red meat, sodium and sugar sweetened beverages and fruit juices (ie, AHEI-2010 and DASH). In contrast, the HDI score was based on WHO recommendation with a focus on nutrients including fatty acids, cholesterol, monosaccharide and disaccharide and protein. The differences in compositions of food groups and dietary nutrients between DQIs might explain their different associations with HCC risk. The associations of these DQIs (ie, AHEI-2010, aMED, DASH and HDI) with HCC risk were comparable to one another, suggesting that these DQIs reflected similar dietary patterns in our study population. These DQIs were used following dietary patterns for Singaporean Chinese who reside in Singapore and are well-represented healthy dietary patterns for reducing risk of HCC in this population.
Results from prior studies also suggested that quality of diet contributes to the development of obesity, insulin resistance,35 Type 2 diabetes and systemic hepatic inflammation36,37 all of which are underlying causes for NAFLD,38 a spectrum of liver diseases from simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis and liver cirrhosis, the underlying condition for HCC and vice versa.39,40 Recently, Godos et al41 outlined the possible molecular mechanisms of dietary components in aMED that might provide protective effects in NAFLD, such as the consumption of fish, nuts and olive oil are associated with higher intakes of MUFAs, consequently leading to lower liver inflammation, lipogenesis, oxidative stress or steatosis. Whole grain is thought to reduce liver inflammation and increase insulin sensitivity.42 The other possible mechanism that higher levels of vitamins E and D, deriving from cooking oils and fish/supplemental foods, help reduce levels of liver inflammation and steatosis and increase glucose and lipid metabolism, respectively.43
In the analysis of individual food groups and nutrients in relation to HCC risk, we observed a significant inverse association between legumes and HCC risk, but null association for nut consumption. In the AHEI-2010 and DASH scores, legumes were combined with nuts, but in the aMED score, legumes was an independent component of the score. This suggests that using the combined category of “nuts and legumes” in some of the DQI scores may not be optimal. In Chinese population, consumption of legumes in the form of soy products is often much higher than nut intakes. This finding is consistent with results from the Shanghai Women’s Health Study and Shanghai Men’s Health Study in which Zhang et al44 showed that legumes and legume products were associated with a lower risk of liver cancer, but not nuts. We also found that long-chain n-3 PUFAs was inversely associated with HCC risk while total PUFAs was associated with increased risk of HCC. It is well known that PUFAs is divided into two groups: n-3 PUFAs and n-6 PUFAs, depending on the position of the first double bond from the methyl end of the carbon chain.45 Experimental studies have shown that n-3 PUFAs have anti-inflammatory effects via different mechanisms, including activation of the anti-inflammatory transcription factor NR1C3 (ie, peroxisome proliferator-activated receptor γ) and binding to the G-protein-coupled receptor GPR120, disruption of lipid raft or alteration of cell membrane phospholipid fatty acid composition.46 However, the amount of n-3 PUFAs intake is much less than n-6 PUFAs intakes. In our cohort, the intakes of n-3 PUFAs and n-6 PUFAs of the total PUFAs are 10% and 90% (0.5 vs 4.5 g/d), respectively. Thus, the positive association between PUFAs intakes and HCC risk found in our analysis is driven by the n-6 PUFAs, which is consistent with our prior report47 (ie, n-6 PUFAs increased HCC risk). The metabolism of n-6 PUFAs is found to increase the levels of pro-inflammatory products, including prostaglandin E2, TNF-α, IL-6, plasminogen activator inhibitor-1, indirect C-reactive protein or thromboxane,48,49 which have been involved in the progression from advanced fibrosis in NASH, to cirrhosis and finally HCC.50,51
Strengths of our study included a prospective study design and large sample size; comprehensive evaluation of diet and potential confounding factors; complete and long-term follow-up, comprehensive ascertainment of cancer incidence and deaths by via the linkage with Singapore national cancer registry and death registry; our ability to evaluate the diet-quality score in an Asian population; and finally, the availability of HBsAg testing results that allowed us to evaluate the association between DQI scores and HCC without the confounding effect of HBV infection. Limitations of our study included the use of the baseline assessment of the diet using an FFQ, which was inherent to measurement error due to the change in dietary habit over time. However, the study of reproducibility and validity of the SCHS FFQ13 showed reasonable correlations between of most groups/items and nutrients with those in 24-HDR in our cohort. Furthermore, because of the prospective study design, changes in the diet after baseline administration of FFQ would probably result in nondifferential misclassification, leading to an underestimation of the true association.52 Our AHEI-2010 did not include trans fat, but our prior study53 showed that the plasma trans fat concentration in our cohort participants was very low, suggesting that this omission had limited impact on the AHEI-2010. In addition, because of low intake of dairy products in our cohort participants, total dairy products were used instead of the low-fat dairy products in the calculation of DASH score. Although we carefully adjusted for important confounding factors, including demographic and lifestyle factors in our analyses, we could not rule out the possibility of residual confounding due to imperfectly measured or unknown confounders that cannot be excluded.
In summary, our current analysis of a population-based prospective cohort study, for the first time in an Asian population, revealed that higher adherence to dietary recommendations, reflecting in the AHEI-2010, aMED and DASH, was significantly associated with lower risk of HCC incidence. Our findings support adherence to high-quality diet with a focus on different healthy plant-based foods, including vegetables, fruits, and nuts/legumes and lower consumption of sodium and higher intake of PUFAs for lowering the risk of HCC incidence that can be recommended to Chinese and broader to East Asian populations.
Supplementary Material
What’s new?
Can improving the daily diet of high-risk populations lower their risk of hepatocellular carcinoma (HCC)? In this large, prospective Asian study, the authors found that the answer is yes. Three different diet-quality index (DQI) scores were associated with a significant drop in HCC risk, by as much as 30%. These included the AHEI-2010, aMED, and DASH. These findings suggest that public-health programs emphasizing dietary modification may offer an effective strategy for the prevention of HCC.
ACKNOWLEDGMENTS
We acknowledge the founding and longstanding principal investigator of the Singapore Chinese Health Study, Mimi C. Yu. We thank Siew-Hong Low of the National University of Singapore for supervising the fieldwork of the Singapore Chinese Health Study. We also thank the Singapore Cancer Registry for the identification of incident cancer cases among participants of the Singapore Chinese Health Study. The Singapore Chinese Health Study was supported by the National Institutes of Health (NIH) of the United States (grants # R01 CA144034 and UM1 CA182876). Hung N. Luu is partially supported by the University of Pittsburgh Medical Center Hillman Cancer Center start-up grant.
Abbreviations:
- AHEI-2010
Alternative Health Eating Index-2010
- aMED
Alternate Mediterranean diet
- BMI
body mass index
- CI
confidence interval
- DASH
Dietary Approaches to Stop Hypertension
- DPMP
Dietary Patterns Methods Project
- DQI
diet-quality index
- FFQ
food frequency questionnaire
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HDI
Heathy Diet Indicator
- HPFS
Health Professional Follow-up Study
- HR
hazard ratio
- MDS
meat-dim-sum
- MEC
Multiethnic Cohort Study
- MUFAs
monounsaturated fatty acids
- NAFLD
nonalcoholic fatty liver disease
- NHS
Nurses’ Health Study
- PUFAs
polyunsaturated fatty acids
- SCHS
Singapore Chinese Health Study
- SFAs
saturated fatty acids
- VFS
vegetable-fruit-soy
- WHO
World Health Organization
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ETHICS STATEMENT
All study participants provided written informed consent. The SCHS has been approved by the Institutional Review Boards of the National University of Singapore and the University of Pittsburgh.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Deidentified data relevant to the report can be shared and are available upon request through the University of Pittsburgh for researchers who meet the criteria for access to confidential data. Data are accessible to the corresponding author and are available from the University of Pittsburgh Institutional Data Access/Ethics Committee with the following contact information: 3500 Fifth Avenue, Hieber Building Main Office, Suite 106 Pittsburgh, PA 15213. Main Phone: (412) 383–1480. Main Fax: (412) 383–1508. askirb@pitt.edu.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/archive/csr/1975_2015/; based on November 2017 SEER data submission, posted to the SEER web site, April 2018. 2018 [Google Scholar]
- 5.Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liese AD, Krebs-Smith SM, Subar AF, et al. The dietary patterns Methods project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogumil D, Park S-Y, Le Marchand L, et al. High-quality diets are associated with reduced risk of hepatocellular carcinoma and chronic liver disease: the multiethnic cohort. Hepatol Commun. 2019;3:437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma Y, Yang W, Simon TG, et al. Dietary patterns and risk of hepatocellular carcinoma among U.S. men and women. Hepatology. 2018;70:677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W-Q, Park Y, McGlynn KA, et al. Index-based dietary patterns and risk of incident hepatocellular carcinoma and mortality from chronic liver disease in a prospective study. Hepatology. 2014;60:588–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Zhang X, Xiang Y-B, et al. Adherence to dietary guidelines and mortality: a report from prospective cohort studies of 134,000 Chinese adults in urban Shanghai. Am J Clin Nutr. 2014;100:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurotani K, Akter S, Kashino I, et al. Quality of diet and mortality among Japanese men and women: Japan public health center based prospective study. BMJ. 2016;352:i1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J-M, Stram DO, Arakawa K, Lee H-P, Yu MC. Dietary cryptoxanthin and reduced risk of lung cancer: the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2003;12:890–898. [PubMed] [Google Scholar]
- 13.Hankin JH, Stram DO, Arakawa K, et al. Singapore Chinese Health Study: development, validation, and calibration of the quantitative food frequency questionnaire. Nutr Cancer. 2001;39:187–195. [DOI] [PubMed] [Google Scholar]
- 14.Seow A, Shi CY, Franke AA, Hankin JH, Lee HP, Yu MC. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–140. [PubMed] [Google Scholar]
- 15.Seow A, Shi CY, Chung FL, et al. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: relationship with dietary total ITC and glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–781. [PubMed] [Google Scholar]
- 16.Butler LM, Wu AH, Wang R, Koh W-P, Yuan J-M, Yu MC. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. 2010;91:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 18.Neelakantan N, Koh W-P, Yuan J-M, van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J Nutr. 2018;148:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Health Promotion Board. My Healthy Plate Fact Sheet [Internet]. 2010. https://www.ntu.edu.sg/Students/Undergraduate/StudentServices/HealthAndCounselling/Documents/HPB_MyHealthyPlate_FactSheet_FA(hires).pdf
- 21.The Whole Grains Council. Oldways Whole Grains Council 101: what counts as a serving? [Internet]. 2017. https://wholegrainscouncil.org/whole-grains-101/how-much-enough/what-counts-serving [Google Scholar]
- 22.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. [DOI] [PubMed] [Google Scholar]
- 23.Koh W-P, Robien K, Wang R, Govindarajan S, Yuan J-M, Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. Br J Cancer. 2011;105:1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trichopoulou A, Kouris-Blazos A, Wahlqvist ML, et al. Diet and overall survival in elderly people. BMJ. 1995;311:1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 26.Fung TT, Hu FB, McCullough ML, Newby PK, Willett WC, Holmes MD. Diet quality is associated with the risk of estrogen receptor-negative breast cancer in postmenopausal women. J Nutr. 2006;136:466–472. [DOI] [PubMed] [Google Scholar]
- 27.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization (WHO). Diet, nutrition, and the prevention of chronic diseases. Report of a WHO Study Group. WHO Technical Report Series. 1990 [PubMed] [Google Scholar]
- 29.Huijbregts P, Feskens E, Räsänen L, et al. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ. 1997;315:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jankovic N, Geelen A, Streppel MT, et al. WHO guidelines for a healthy diet and mortality from cardiovascular disease in European and American elderly: the CHANCES project. Am J Clin Nutr. 2015;102:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh W-P, Yuan J-M, Sun C-L, Lee H-P, Yu MC. Middle-aged and older Chinese men and women in Singapore who smoke have less healthy diets and lifestyles than nonsmokers. J Nutr. 2005;135:2473–2477. [DOI] [PubMed] [Google Scholar]
- 32.Eaglehouse YL, Koh W-P, Wang R, Aizhen J, Yuan J-M, Butler LM. Physical activity, sedentary time, and risk of colorectal cancer: the Singapore Chinese Health Study. Eur J Cancer Prev. 2017;26:469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkin DM, Whelan S, Ferlay J, Teppo L, Thomas D, eds. Cancer Incidence in Five Continents. Vol. 8: Cancer Incidence in Five Continents. Vol 8. Lyon: IARC Press; 2002:781. [Google Scholar]
- 34.Turati F, Trichopoulos D, Polesel J, et al. Mediterranean diet and hepatocellular carcinoma. J Hepatol. 2014;60:606–611. [DOI] [PubMed] [Google Scholar]
- 35.Adachi Y, Nojima M, Mori M, et al. Insulin-like growth factor-related components and the risk of liver cancer in a nested case-control study. Tumour Biol. 2016;37:15125–15132. [DOI] [PubMed] [Google Scholar]
- 36.Ohishi W, Cologne JB, Fujiwara S, et al. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134:154–163. [DOI] [PubMed] [Google Scholar]
- 37.Nakagawa H, Maeda S, Yoshida H, et al. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer. 2009;125:2264–2269. [DOI] [PubMed] [Google Scholar]
- 38.Mirmiran P, Amirhamidi Z, Ejtahed H-S, Bahadoran Z, Azizi F. Relationship between diet and non-alcoholic fatty liver disease: a review article. Iran J Public Health. 2017;46:1007–1017. [PMC free article] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD), European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. [DOI] [PubMed] [Google Scholar]
- 40.Burt AD, Lackner C, Tiniakos DG. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis. 2015;35:207–220. [DOI] [PubMed] [Google Scholar]
- 41.Godos J, Federico A, Dallio M, Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int J Food Sci Nutr. 2017;68:18–27. [DOI] [PubMed] [Google Scholar]
- 42.Ross AB, Godin J-P, Minehira K, Kirwan JP. Increasing whole grain intake as part of prevention and treatment of nonalcoholic fatty liver disease. Int J Endocrinol. 2013;585876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Xiang Y-B, Li H-L, et al. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women’s and men’s health studies. Cancer Sci. 2013;104:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trombetta A, Maggiora M, Martinasso G, Cotogni P, Canuto RA, Muzio G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem Biol Interact. 2007;165:239–250. [DOI] [PubMed] [Google Scholar]
- 46.Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koh W-P, Dan YY, Goh GB-B, Jin A, Wang R, Yuan J-M. Dietary fatty acids and risk of hepatocellular carcinoma in the Singapore Chinese health study. Liver Int. 2016;36:893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monteiro J, Leslie M, Moghadasian MH, Arendt BM, Allard JP, Ma DWL. The role of n - 6 and n - 3 polyunsaturated fatty acids in the manifestation of the metabolic syndrome in cardiovascular disease and non-alcoholic fatty liver disease. Food Funct. 2014;5:426–435. [DOI] [PubMed] [Google Scholar]
- 49.Calder PC. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot Essent Fatty Acids. 2006;75:197–202. [DOI] [PubMed] [Google Scholar]
- 50.Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13:631–647. [DOI] [PubMed] [Google Scholar]
- 51.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: growing evidence of an epidemic? Hepatol. 2012;42:1–14. [DOI] [PubMed] [Google Scholar]
- 52.Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst. 2011;103:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neelakantan N, Naidoo N, Koh W-P, Yuan J-M, van Dam RM. The alternative healthy eating index is associated with a lower risk of fatal and nonfatal acute myocardial infarction in a Chinese adult population. J Nutr. 2016;146:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data relevant to the report can be shared and are available upon request through the University of Pittsburgh for researchers who meet the criteria for access to confidential data. Data are accessible to the corresponding author and are available from the University of Pittsburgh Institutional Data Access/Ethics Committee with the following contact information: 3500 Fifth Avenue, Hieber Building Main Office, Suite 106 Pittsburgh, PA 15213. Main Phone: (412) 383–1480. Main Fax: (412) 383–1508. askirb@pitt.edu.


