Abstract
Abstract
Objectives
Optimising postoperative pain following knee replacement is important for patients, healthcare professionals and healthcare funders. Adductor canal blocks (ACB) are widely used but there is uncertainty about their efficacy when combined with local infiltration analgesia (LIA) compared with either LIA or ACB alone.
Design
A systematic review and meta-analyses of randomised controlled. The primary outcome was pain over the first 72 hours. Secondary outcomes included morphine use, range of movement, distance walked, length of hospital stay, health economic outcomes and reported adverse events.
Data sources
MEDLINE, Embase, EB Health - KSR Evidence, Cochrane Central Register of Controlled Trials, CINAHL, International HTA database, ClinicalTrials.gov and the International Clinical Trials Registry Platform (WHO) were searched up to June 2023.
Eligibility criteria
Randomised controlled trials involving patients undergoing primary total knee replacement comparing LIA combined with ACB to either LIA or ACB alone.
Data extraction and synthesis
All eligible studies were data extracted independently by two reviewers. Studies were pooled for each outcome at each timepoint in a random effects meta-analysis.
Results
We identified 13 completed studies including 1154 participants. 12 studies compared LIA vs combination and 5 compared ACB vs combination. We identified that participants receiving the combination had lower pain scores at rest at 24 hours compared with LIA alone (SMD 0.42, 95% CI 0.20 to 0.64) or ACB alone (SMD 0.63, 95% CI 0.42 to 0.83). Pain on movement at 24 hours was also lower for patients with combination vs LIA alone (SMD 0.37, 95% CI 0.01 to 0.73) or ACB alone (SMD 0.81, 95% CI 0.35 to 1.26). We also identified that patients on combination used less morphine than on LIA alone (MD 1.06, 95% CI −0.09 to 2.20) or ACB alone (MD 5.94, 95% CI −2.41 to 14.29). The same was seen with range of motion at 24 hours with combination having a larger improvement than LIA alone (MD −5.19, 95% CI −5.55 to −4.83) or ACB alone (MD −3.80, 95% CI −4.37 to −3.23). These findings were consistent across all time points; however, there were no studies deemed to be at a low risk of bias.
Conclusions
Further well-designed and conducted randomised controlled trials are needed to confirm if a combination of LIA and ACB is superior to either option alone for patients undergoing primary total knee arthroplasty.
PROSPERO registration number
CRD42023436895.
Keywords: Systematic Review, Meta-Analysis, Knee, Pain management, Adult surgery
Strengths and limitations of this study.
We used a comprehensive search strategy to identify all possible eligible studies.
All title and abstracts and full-text articles were screened independently by two researchers to ensure no potentially relevant studies were missed.
Contacts for trial registries and abstracts were contacted to obtain as much data from available trials as possible for analysis.
The methodological quality of the included trials was assessed using the most up to date tools.
One limitation is that we excluded non-English publications as we would not be able to confirm their eligibility.
Introduction
Joint replacement is the most common elective surgical procedure conducted within the National Health Service, with around 100 000 primary knee replacements implanted each year.1 2 While highly clinically and cost effective in the long term, during the acute postoperative period, over half of patients report severe pain associated with the surgery despite multimodal approaches.3 Optimising postoperative pain is not only important for patients but also for healthcare professionals and healthcare payers. Postoperative pain is associated with increased morbidity and mortality and is a major determinant of length of stay which is a significant component of cost of this procedure.4,6
The optimum analgesic strategy would be one that delivered a long-lasting sensory, but not motor block, without systemic side effects. Local infiltration analgesia (LIA) at the surgical site represents one such strategy but the clinical benefit is time-limited with patients reporting rebound pain, often within 24 hours of surgery. While novel sustained release local anaesthetics have been developed to try to prolong the duration of analgesia, clinical trials have yet to find these clinically or cost effective and as such attention has turned to regional anaesthesia.7 8
While a range of peripheral nerve blocks have been used in knee replacement, many result in motor blockade. This is problematic as early mobilisation is beneficial for the knee to avoid stiffness and avoid systemic complications associated with immobility. Furthermore, independence and ability to self-care have been identified through qualitative work as important early in recovery from the patient perspective.9
The adductor canal block (ACB), where local anaesthetic is infiltrated into the adductor canal, provides predominantly a sensory block via its action on the saphenous nerve and posterior branch of the obturator nerve. Compared with femoral nerve block, ACB provides similar levels of analgesia but with better preservation of quadriceps strength and mobilisation.10 LIA and ACB are both commonly used in clinical practice, both independently and in combination, and there is uncertainty as to the optimum analgesic strategy.
The aim of this systematic review is to establish the efficacy of LIA combined with ACB compared with either LIA or ACB alone on postoperative pain following total knee replacement. The clinical relevance of this is that it will inform the optimum analgesic strategy for this patient group.
Methods
A systematic review and meta-analysis of the published literature was conducted and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 guidelines. The review was prospectively registered with PROSPERO (CRD42023436895).
Eligibility criteria
Studies were included in this review if they met the following criteria:
Study design: randomised controlled trials
Participants: over the age of 18, undergoing a primary total knee replacement (TKR), irrespective of any comorbidities. Patients given general anaesthetic, spinal or epidural anaesthesia were all included. Studies involving patients undergoing unicompartmental, revision or simultaneous bilateral knee replacements were excluded.
Intervention: combined LIA and ACB. Trials will be included regardless of the agent or concentration used in both the LIA and ACB. Trials assessing ACB given postoperatively after the patient had left theatre were excluded.
Comparator: the control was LIA and/or ACB alone.
Search methods for identification of studies
An information specialist (HF) designed a preliminary search for Ovid Medline, in consultation with the review team. This search strategy was then translated for use on the following bibliographic databases using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource. The search strategies were designed to identify RCTs on specified types of analgesia or anaesthesia for patients with total knee replacement. No restrictions on date or language were applied to the searches. The full search strategies are available in the Figshare repository supplementary materials.12
The following databases and trial registries were searched on 13 June 2023:
MEDLINE(R) ALL (Ovid) (1946 to 12 June 2023);
Embase (Ovid) (1974 to 12 June 2023);
EB Health - KSR Evidence (Ovid) (2015 to 2023 week 24);
Cochrane Central Register of Controlled Trials (Wiley): 2023, Issue 6 in the Cochrane Library;
CINAHL Complete (EBSCO) (Inception - Current);
International HTA database (https://database.inahta.org/search/advanced) (Inception - Current);
ClinicalTrials.gov (US NIH), (all available years);
International Clinical Trials Registry Platform (WHO), (all available years).
We contacted the authors of identified studies for information on unpublished or ongoing trials, or to request additional trial data.
Data collection and analysis
The results of the databases were deduplicated in EndNote 2013 then imported into Covidence14 for screening and data extraction.
Titles and abstracts were initially screened and then full texts were obtained for all remaining records, at both stages, records were screened independently for eligibility by two reviewers with any disagreements resolved by discussion. Data extraction and quality assessment were completed independently by two reviewers using a study-specific extraction template in Covidence with any disagreements resolved by discussion. The study quality was assessed using the risk of bias 2 tool.15 16
The following data were extracted: author(s), year, country, setting, funding source, participant characteristics (including eligibility criteria and demographic data), and the number of participant withdrawals and dropouts. Study design data was also extracted including number of trial arms, unit of randomisation and timing of follow-ups.
We also extracted outcome data for:
Pain (based on a Visual Analogue or Numeric Rating Scale) both at rest and on movement at 12, 24, 48 and 72 hours
Morphine use at 12, 24, 48 and 72 hours
Range of movement at 12, 24, 48 and 72 hours
Distance walked
Length of hospital stay
Any health economic outcome
Any reported adverse events
Where necessary, study authors were emailed for further data or clarification.
Statistical methods
Key study and participant characteristics, and study quality are summarised narratively and presented in tables or figures.
Studies were pooled for each outcome at each timepoint in a random effects meta-analysis using the method of DerSimonian and Laird.17 Variability due to between-study heterogeneity was quantified using the I² statistics and the Cochrane guidelines for interpretation.18 Results for each outcome are presented as mean differences (length of stay, morphine consumption, distance walked and range of movement) or standardised mean differences (SMD) (pain at rest and pain on movement) with the associated 95% CIs. All quantitative analyses were undertaken in Stata (v17 or later).
Public and patient involvement
Patients and the public were not involved in the design, or conduct, or reporting or dissemination of this research.
Results
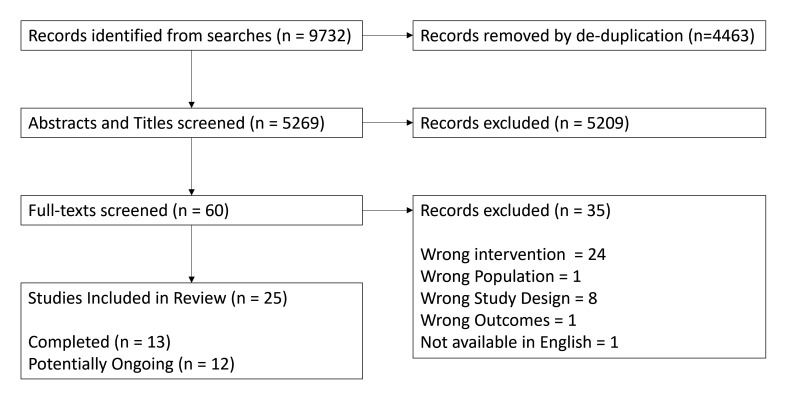
We identified 9732 records from databases, following de-duplication, we screened the titles and abstracts of 5269 records. We then assessed 60 full-text records of which 25 were eligible, records excluded and the reasons for exclusion are available in the Figshare repository supplementary materials.19 The screening process is summarised in figure 1. Of the 25 included studies, there were 13 completed20,32 studies and 12 which are ongoing.33,44
Figure 1. Flowchart of screening process.
A summary of the study characteristics is included in table 1. Of the 13 completed studies, 12 compared LIA alone vs combination, 5 compared ACB alone vs combination. Four of the studies included arms for both LIA alone and ACB alone.
Table 1. Characteristics of included studies.
| Study details | Arms included (#) | Alone | Combined | Characteristics | Other analgesics used |
| Goytizolo et al23 2019USA | LIA alone (56) vs combination (55) | Deep: bupivacaine (30 mL, 0.5%) with epinephrine, morphine (1 mL, 8 mg/mL), methyl-prednisolone (1 mL, 40 mg/mL), cefazolin (500 mg, 10 mL), normal saline solution (22 mL) Superficial: bupivacaine (20 mL, 0.25%) | Ultrasound-guided ACB: bupivacaine (15 mL, 0.25%), preservative-free dexamethasone (2 mg)LIA: as described | Age: 65 (10) vs 67 (7)ASA: I - 3 vs 1; II - 51 vs 51; III - 2 vs 3Female/male: 33/23 vs 34/21 | Preoperative: oral meloxicam and extended-release oxycodone (10 mg). Spinal anaesthetic. Ondansetron (4 mg) and famotidine (20 mg) |
| Luo et al25 2022China | LIA alone (30) vs combination (30) | LIA: ropivacaine (200 mg), morphine (10 mg), betamethasone (5 mg), diluted normal saline (60 mL). Pre-implantation and post-implantation LIA performed around all relevant structures | Ultrasound-guided ACB: after general anaesthesia before skin incision ropivacaine (0.5%) or normal saline (20 mL). LIA as described | Age: 65.3 (4.86) vs 65.4 (5.22)ASA: I - 0 vs 0; II - 25 vs 24; III - 5 vs 6Female/male: 22/8 vs 23/7 | Parecoxib 20 mg two times a day immediately after the operation until discharge, oxycodone or morphine when immediate pain >4 on a 0–10 VAS was reported |
| Grosso et al24 2018USA | LIA alone (54) vs ACB alone (55) vs combination (54) | LIA: bupivacaine (50 mL, 0.25%). A standard protocol was followed. Tissues and subcutaneous tissues. Ultrasound-guided ACB: performed in the preoperative block area by the anaesthesia team prior to surgery using bupivacaine (15 mL, 0.5%) | Combined as described | Age: 73 vs 69 vs 71ASA: NRFemale/male: 39/34 vs 33/36 vs 32/19 | Preoperative: acetaminophen, oxycodone, celecoxib and gabapentin, 1 hour priorPostoperative: included acetaminophen, ketorolac then celecoxib (3 months), gabapentin (10 days), oral opioids (as needed) and intravenous hydromorphone |
| Zhou et al32 2018China | LIA alone (20) vs ACB alone (20) vs combination (20) | LIA: ropivacaine (100 mL, 2 mg/mL) with epinephrine (0.5 mL, 1 mg/mL) in two 50 mL syringes. Ultrasound-guided ACB: ropivacaine (30 mL, 0.375%), epinephrine (5 µg/mL), 21-gauge needle | Combined as described | Age: 69 (7.1) vs 67.1 (10.2) vs 66.4 (5.8)ASA: not reportedFemale/male: 16/4 vs 14/6 vs 13/7 | All subjects received a standardised general anaesthesia |
| Rajkumar et al22 2021India | LIA alone (50) vs ACB alone (50) vs combination (50) | LIA: administered by a single operating surgeon for all the patients. Ropivacaine (30 mL, 0.5%) in normal saline (20 mL) was injected around periarticular structures Ultrasound-guided ACB: after skin closure, an anaesthetist ropivacaine (30 mL, 0.2%), dexamethasone (8 mg). | Combined as described | Age: 62.24 (5.76) vs 65.2 (6.16) vs 64.88 (7.58)ASA: not reportedFemale/male: 38/12 vs 34/16 vs 36/14 | All the patients received the same pre-emptive analgesia |
| Nicolino et al29 2020Argentina | LIA alone (36) vs combination (34) | LIA: posterior capsule (30 mL), medial femoral (10 mL), lateral periosteum on each side (10 mL) and tibial periosteum (10 mL). Ropivacaine (20 mL, 0.75%), of morphine (8 mg, 1 mg/mL in 0.3 mL epinephrine), prednisolone (40 mg), saline solution (30 mL). A 16G abbocath was used. Sham ACB: internal saphenous nerve was infiltrated with saline (5 mL). | Ultrasound-guided ACB: regional SNB with ropivacaine (5 mL, 0.75%) and lidocaine (5 mL,2%) was administered by the anaesthetist.LIA: as described | Age: 72.58 (8.41) vs 71.97 (6.86)ASA: not reportedFemale/male: 27/9 vs 23/11 | Epidural anaesthesia of bupivacaine (15–20 mg, 5%), combined with midazolam sedation |
| Atchabahian et al20 2019USA | LIA alone (28) vs combination (23) | LIA: bupivacaine, ketorolac, epinephrine and morphine, as well as liposomal bupivacaine | ACB: mid-thigh level with bupivacaine (30 mL, 0.25%) with 1:200 000 epinephrine and LIA performed as described | Age: 64.0 (7.4) vs 63.5 (8.1)ASA: I - 1 vs 0; II - 16 vs 14; III - 11 vs 9Female/male: 18/10 vs 19/4 | Spinal anaesthetic and postoperative pain management were standardised |
| Rauf49 2013Ireland | LIA alone (10) vs combination (10) | No description provided | No description provided | Age: NRASA: NRFemale/male: NR | Spinal anaesthesia+/−sedation |
| Wang et al31 2019China | LIA alone (48) vs combination (48) | LIA: 20 mL cocktail (0.2% ropivacaine and 2.0 mg/mL of epinephrine). Infiltration of the collateral ligaments before implantation. After, infiltration of quadriceps and retinacular tissues with 20 mL, fat and subcutaneous tissues with 40 mL. Sham ACB: isotonic saline (20 mL) | Ultrasound-guided ACB: A 22-gauge, 100 mm needle was used to inject 20 mL of 0.2% ropivacaine and 2.0 mg/mL of epinephrine | Age: 64.0 (6.7) vs 64.8 (7.0)ASA: I - 1 vs 2; II - 30 vs 29; III - 14 vs 14Female/male: 33/12 vs 30/15 | Preoperative: loxoprofen (60 mg) two times a day Postoperative: loxoprofen (60 mg), prolonged-release oxycodone hydrochloride tablets (10 mg) were administered two times a day to control postoperative pain |
| Tziona et al28 2018Greece | ACB alone (20) vs combination (20) | Ultrasound-guided ACB: ropivacaine (25 mL, 0.375%) and dexamethasone (8 mg) before start of the surgery. Sham LIA: normal saline (0.9%,120 mL) | ACB: As describedLIA: ropivacaine (250 mg) and epinephrine (0.5 mg) in normal saline (0.9%),120-mL solution of ropivacaine (0.2%) | Age: 71.5 (8.5) vs 72.0 (7.2)ASA: I - 3 vs 2; II - 14 vs 14; III - 16 vs 15Female/male: 16/4 vs 15/5 | Preoperative: pregabalin (75 mg) per os 1 hour before surgery |
| Sawhney et al27 2016Canada | LIA alone (54) vs ACB (51) alone vs combination (54) | Ultrasound-guided ACB: under conscious sedation with fentanyl 50 µg and midazolam 1 mg. Subcutaneous injection of lidocaine (2 mL,1%) to anaesthetise the AC site. Ropivacaine (30 mL,0.5%) was injected into the canal. Sham ACB: the procedure stopped after they received lidocaine. LIA: a blinded 110 mL solution bag was prepared by the pharmacy and delivered to the operating room - ropivacaine (300 mg), morphine (10 mg) and ketorolac (30 mg) or saline (110 mL, 0.9%) | Combined as described | Age: 67.6 (9.4) vs 66.4 (9.6) vs 68.3 (9.7)ASA: NRFemale/male: 36/18 vs 31/20 vs 33/21 | Premedication: acetaminophen (1000 mg), celecoxib (200 mg), gabapentin (300 mg) |
| Biswas et al21 2018Canada | LIA alone (65) vs combination (69) | LIA: ropivacaine (150 mL, 0.2%), ketorolac (30 mg), epinephrine (0.6 mg). Half in the posterior capsule and posterior soft tissues of the knee under direct vision after osteotomy but before insertion of the implants. Half of the solution was administered into the anterior soft tissues after placement of the implants and before skin closure.Sham ACB: 30 mL of placebo solution (0.9% NaCl) with method as described for ACB | Ultrasound-guided ACB: A mid-ACB was used to block the saphenous and the nerve to vastus medialis. Lidocaine (1–2 mL, 2%) skin infiltration. The study solution was injected deep to the vastoadductor membrane (ropivacaine, 30 mL, 0.5% with 1:400 000 epinephrine) | Age: 64.2 (8.1) vs 64.2 (8.2)ASA: NRFemale/male: 41/21 vs 37/31 | Preoperative: immediately preoperatively sedation was achieved with intravenous midazolam (1–2 mg) and intravenous fentanyl (25–50 µg) as needed.Postoperative: acetaminophen (3–4 g daily up to 5 days), celecoxib (100–200 mg two times a day up to 5 days), or an NSAID drug, and hydromorphone (1–2 mg), or oxycodone (5–10 mg) every 2 hours as needed. Intravenous patient-controlled analgesia with hydromorphone or morphine was prescribed as a ‘rescue’ modality if oral analgesics failed to achieve an NRS of <5 |
| Nader et al26 2016USA | LIA alone (20) vs combination (20) | LIA: 100 mL of ropivacaine (200 mg), morphine (4 mg), ketorolac (30 mg), clonidine (100 µg) and epinephrine (0.5 mg) as part of the multimodal analgesia regimenSham ACB: normal saline (10 mL) | Ultrasound-guided ACB: bupivacaine (10 mL, 0.25%) with epinephrine 1:300 000LIA: as described | Age: 67 (59–72) vs 68ASA: NRFemale/male: 13/7 vs 15/5 | Preoperative: oxycodone (10 mg), celecoxib (400 mg). Spinal anaesthesia: bupivacaine (2.5 mL, 0.5%). Postoperative: ketorolac (15 mg every 12 hours for 24 hours), hydrocodone (10 mg), acetaminophen (325 mg 4–6 hourly) as needed to maintain a pain NRS of ≤4. Oxycodone (10 mg) every 12 hours as needed for NRS >4Breakthrough pain: intravenous hydromorphone (0.5 mg) or tramadol (50 mg) |
ACBadductor canal blocksLIAlocal infiltration analgesiaNRnot reported
Risk of bias
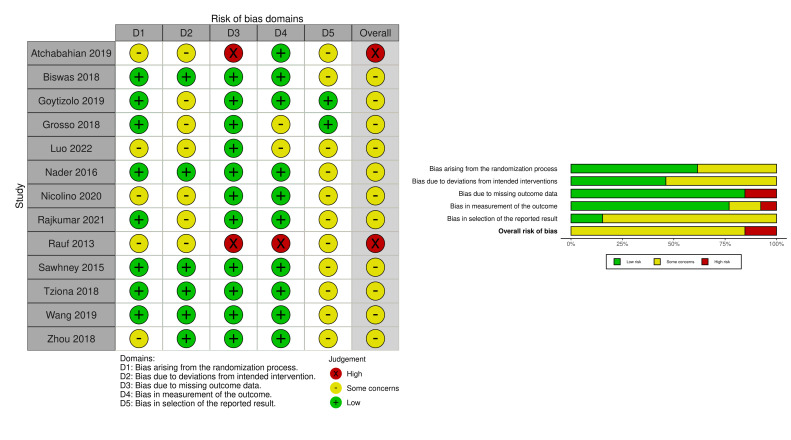
The risk of bias was assessed for all 13 completed studies (figure 2). Two studies were deemed to be at a high risk of bias20 30 mainly due to missing outcome data and the measurement of the outcome. The remaining 11 were deemed to have some concerns. There were no studies judged to be of low risk of bias. Within each category assessed, over 50% of studies were deemed to be of low risk of bias arising from the randomisation process, missing outcome data and measurement of the outcome. There was more concern regarding bias arising from deviations from the intended interventions and in the selection of the reported results. Overall, all studies were considered to have some concerns or be at a high risk of bias.
Figure 2. Risk of bias summary.
Pain
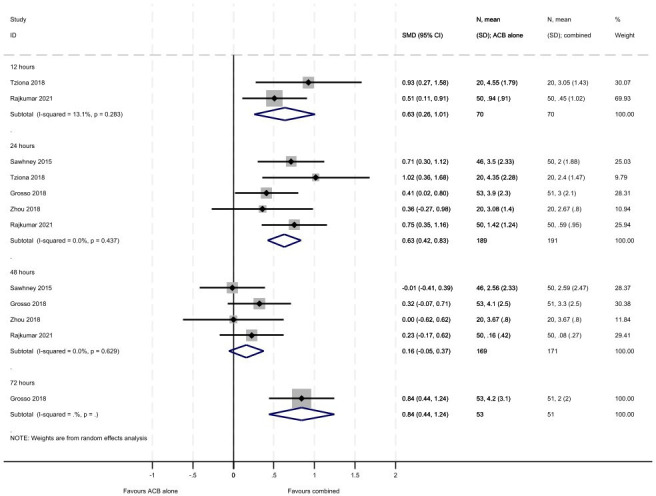
Across the papers reporting pain as an outcome, both Visual Analogue Scales and Numerical Rating Scales were used and these were also of different lengths. From online supplemental tables 1 and 2 and figures3 4, there are consistent results across all time points (12, 24, 48 and 72 hours) for the studies reporting pain at rest. The combined treatment group showed greater reductions in pain at rest compared with LIA alone and ACB alone; although non-statistically significant differences were seen at 48 hours. For the comparison with LIA alone, greater reductions in pain at rest were seen within the first 12 hours (SMD 0.80, 95% CI 0.11 to 1.48, 5 studies including 450 participants) compared with later time points although all favoured combined treatment (24 hours: SMD 0.42, 95% CI 0.20 to 0.64, 11 studies including 859 participants; 48 hours: SMD 0.22, 95% CI −0.03 to 0.47, 10 studies including 804 participants; 72 hours: SMD 0.35, 95% CI 0.02 to 0.69, 3 studies including 252 participants). There were moderate to considerate levels of heterogeneity across all analyses for pain. For the comparison with ACB alone, similar reductions in pain at rest were seen within the first 12 hours (SMD 0.63, 95% CI 0.26 to 1.01, 2 studies including 140 participants) and 24 hours (SMD 0.63, 95% CI 0.42 to 0.83, 5 studies including 380 participants) compared with 48 hours (SMD 0.16, 95% CI −0.05 to 0.37, 4 studies including 340 participants). There was a single study assessing pain at rest at 72 hours which highlighted a similar pattern of reductions in the combined treatment group (SMD 0.84, 95% CI 0.44 to 1.24, including 104 participants).
Figure 3. Meta-analysis of pain at rest (LIA vs combined). LIA, local infiltration analgesia.
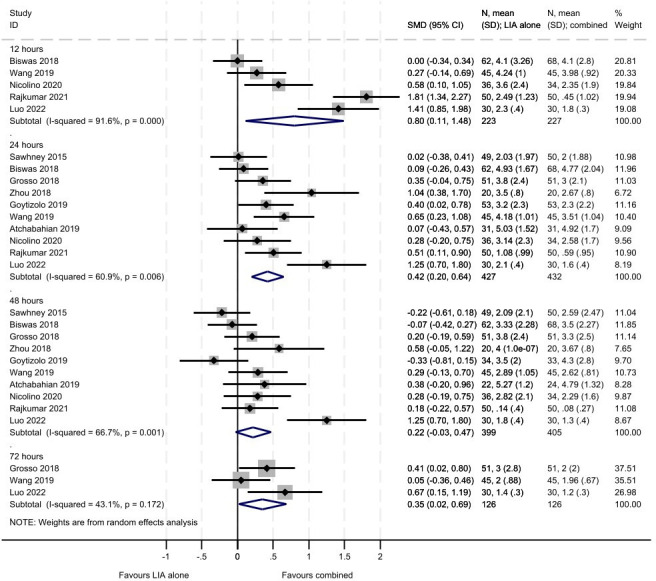
Figure 4. Meta-analysis of pain at rest (ACB vs combined). ACB, adductor canal blocks.
A similar pattern to pain at rest was seen for studies reporting pain on movement in the LIA alone compared with combined treatment comparison across all time points, with the largest reductions in pain on movement observed at 12 hours (SMD 0.94, 95% CI 0.23 to 1.65, 4 studies including 380 participants) compared with later time points (24 hours: SMD 0.37, 95% CI 0.01 to 0.73, 8 studies including 626 participants; 48 hours: SMD 0.54, 95% CI 0.12 to 0.96, 7 studies including 585 participants; 72 hours: SMD 0.35, 95% CI −0.23 to 0.92, 2 studies including 150 participants; online supplemental tables 1 and 2 and online supplemental figures 1 and 2). The combined treatment showed greater reductions in pain on movement compared with LIA alone; although the differences were not statistically significant at 72 hours. For the studies comparing ACB to combined treatment, larger reductions in pain on movement were seen in the combined treatment group at all time points assessed (12 hours: SMD 0.76, 95% CI 0.42 to 1.10, 2 studies including 140 participants; 24 hours: SMD 0.81, 95% CI 0.35 to 1.26, 4 studies including 276 participants; 48 hours: SMD 0.66, 95% CI 0.40 to 0.93, 3 studies including 236 participants).
Morphine usage
There were consistent findings across all time points (online supplemental table 1) which favoured combined treatment rather than LIA alone but none of the differences were statistically significant (12 hours: MD 2.96, 95% CI −3.88 to 9.80, 3 studies with 363 participants; 24 hours: MD 1.06,95% CI −0.09 to 2.20, 8 studies with 681 participants; 48 hours: MD 0.19, 95% CI −0.52 to 0.90, 5 studies with 489 participants; 72 hours: MD 2.44, 95% CI −2.39 to 7.28, 3 studies with 252 participants). The same pattern was seen in the comparison with ACB alone, demonstrating less morphine use in the combined treatment group (12 hours: MD 2.41, 95% CI −5.22 to 10.04, 2 studies including 144 participants; 24 hours: MD 5.94, 95% CI −2.41 to 14.29, 3 studies including 240 participants; 48 hours: MD 8.11, 95% CI −7.80 to 24.02, 2 studies including 200 participants; 72 hours: MD 8.70, 95% CI 2.03 to 15.37, 1 study including 104 participants).
Distance walked
For the LIA alone comparison, there were single studies reporting the distance walked at 12 (130 participants) and 72 hours (90 participants), two studies at 48 hours (189 participants) and three studies at 24 hours (319 participants). For the ACB alone comparison, there were single studies reporting distance walked at 24 (96 participants) and 48 hours (96 participants). The findings indicate little evidence of a difference in the distance walked over time between the LIA alone and combined treatment groups but greater distance walked in the combined treatment group compared with ACB alone (online supplemental tables 1 and 2).
Range of motion
From online supplemental tables 1 and 2, we can see that at 24 and 48 hours, the estimates favour the combined treatment compared with the LIA alone (24 hours: MD −5.19, 95% CI −5.55 to −4.83, 4 studies with 380 participants; 48 hours: MD −4.13, 95% CI −6.31 to −1.95, 3 studies with 250 participants). These findings were driven by a single study Rajkumar, 2021 and the pooled results were statistically significant. The later time points (72 hours and 1, 6 and 12 months) for this comparison were assessed in either one or two studies which found little evidence of a difference in the range of movement between groups. Range of movement was assessed in single studies for each time point (24 and 48 hours and 1 month) in the ACB alone compared with combined treatment. Results at 1 month did not find a statistically significant difference but the results were significantly different at 24 and 48 hours; however, all favoured combined treatment (24 hours: MD −3.80, 95% CI −4.37 to −3.23, 96 participants; 48 hours: MD −1.80, 95% CI −2.21 to −1.39, 100 participants; 1 month: MD −5.00, 95% CI −10.21 to 0.21, 104 participants).
Length of hospital stay
Data on average length of stay (in days) were reported in six studies comparing LIA alone to combined treatment and three studies comparing ACB alone to combined treatment. The pooled estimate demonstrated no difference in the length of stay for LIA alone in comparison to combined treatment but a shorter length of stay for combined treatment compared with ACB alone (MD 0.35, 95% CI 0.11 to 0.59; online supplemental tables 1 and 2.
Adverse events
Adverse events were reported in 9 of the 13 studies. Nausea and vomiting were the most commonly reported adverse events (8/9 trials). Two trials did not report figures but noted there was no difference between the groups. Two trials reported drowsiness or dizziness. Events related to wound healing (swelling, delayed healing, infection etc) were reported in two trials. Urinary retention was reported in two trials. Pruritus was reported in four trials. Venous thrombotic events were reported in one trial. Insufficient details were reported to provide modality-specific events across all trials.
Discussion
We identified 13 completed studies involving 1154 participants comparing either LIA or ACB alone to a combination of both interventions for patients undergoing total knee arthroplasty. These studies were all deemed to have either a high risk of bias or to have some concerns. There is therefore a need for high-quality low-risk of bias studies assessing the use of LIA and ACB in combination. The principal finding was that participants receiving a combination of LIA and ACB reported lower pain scores at rest and with activity in the first 24 hours following surgery with no difference in pain scores seen at 48 hours at rest and 72 hours with activity. No difference in morphine consumption, distance walked, range of movement or length of stay was detected between groups. Adverse events were poorly reported . None of the published studies were considered at low risk of bias.
We only included English language reports in this review, which may limit the generalisability of our results. However, only one article was explicitly excluded on this basis where an English language translation was not available. Due to the small number of included studies, we were unable to explore the heterogeneity between studies and so a random-effects meta-analysis had to be used.
While a combination of LIA and ACB resulted in statistically significant improvements in pain, the clinical relevance of this remains uncertain. The difference in pain did not translate into improvements in function as assessed by distance walked or range of movement nor did it reduce the length of stay following TKR. While LIA and ACB appear to be safe in combination, it is unclear whether this represents a cost-effective intervention as both take time to perform and the benefit appears to be time limited. However, there is limited data following the first 3 days.
Previous systematic reviews have assessed the comparison of LIA or ACB alone compared with the combination.45 46 These reviews differed in their eligibility criteria by including a broader definition of the interventions, allowing ACB to be delivered postoperatively out of theatre and including non-randomised studies. Both these reviews concluded that the combination had reduced pain scores and morphine usage compared with an individual modality. Our study shared similar results with the combined intervention having lower pain scores at rest and on movement over the first 72 hours. We also found reduced morphine usage over the first 72 hours with the combined compared with either individual modality; however, the results were not statistically significant.
TKR is one of the most commonly performed elective surgical procedures with the number of procedures expected to increase due to an ageing population with increased levels of obesity. Pain is common after TKR and is the main reason for inpatient admission,47 as well as seeking further medical advice following discharge.3 Despite multimodal approaches to pain management, breakthrough pain is common, particularly with activity, and at present, managed with strong opioids. However, the use of opioids is not benign both in the short term with around a fifth of patients reporting inpatient opioid-related adverse events48 as well as longer term with issues around chronic opioid use. As such optimising peri-operative analgesia is of critical importance both for patients, healthcare professionals and healthcare payers.
Conclusions
We found low-quality evidence that combination LIA and ACB reduces postoperative pain. Further high-quality trials are needed to confirm if combination LIA and ACB is superior to either alone for postoperative pain and other patient outcomes.
supplementary material
Professor HP and Professor CH are National Institute for Health Research (NIHR) Senior Investigators. HP is supported in part by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre (BRC) (NIHR203331). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care .
Footnotes
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-080555).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Data availability free text: Relevant additional documentation and information are available on Figshare.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Andrew Mott, Email: andrew.mott@york.ac.uk.
Samantha Brady, Email: samantha.gascoyne@york.ac.uk.
Isabelle Briggs, Email: isabelle.briggs@york.ac.uk.
Maggie Barrett, Email: maggie.barrett@york.ac.uk.
Helen Fulbright, Email: helen.fulbright@york.ac.uk.
Thomas William Hamilton, Email: thomas.hamilton@ndorms.ox.ac.uk.
Catherine Hewitt, Email: catherine.hewitt@york.ac.uk.
Jeya Palan, Email: jeya.palan@nhs.net.
Hemant Pandit, Email: H.Pandit@leeds.ac.uk.
Data availability statement
Data are available upon reasonable request.
References
- 1.Abbott TEF, Fowler AJ, Dobbs TD, et al. Frequency of surgical treatment and related hospital procedures in the UK: a national ecological study using hospital episode statistics. Br J Anaesth. 2017;119:249–57. doi: 10.1093/bja/aex137. [DOI] [PubMed] [Google Scholar]
- 2.NJR NJR annual report 2022. The National Joint Registry. 2022. [17-Aug-2023]. https://www.njrcentre.org.uk/njr-annual-report-2022/ Available. Accessed.
- 3.Chan EY, Blyth FM, Nairn L, et al. Acute postoperative pain following hospital discharge after total knee arthroplasty. Osteoarthr Cartil. 2013;21:1257–63. doi: 10.1016/j.joca.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Lo LWT, Suh J, Chen JY, et al. Early Postoperative Pain After Total Knee Arthroplasty Is Associated With Subsequent Poorer Functional Outcomes and Lower Satisfaction. J Arthroplasty. 2021;36:2466–72. doi: 10.1016/j.arth.2021.02.044. [DOI] [PubMed] [Google Scholar]
- 5.Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop. 2011;82:577–81. doi: 10.3109/17453674.2011.618911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burn E, Liddle AD, Hamilton TW, et al. Cost-effectiveness of unicompartmental compared with total knee replacement: a population-based study using data from the National Joint Registry for England and Wales. BMJ Open. 2018;8:e020977. doi: 10.1136/bmjopen-2017-020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton TW, Athanassoglou V, Mellon S, et al. Liposomal bupivacaine infiltration at the surgical site for the management of postoperative pain. Cochrane Database Syst Rev. 2017;2:CD011419. doi: 10.1002/14651858.CD011419.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamilton TW, Knight R, Stokes JR, et al. Efficacy of Liposomal Bupivacaine and Bupivacaine Hydrochloride vs Bupivacaine Hydrochloride Alone as a Periarticular Anesthetic for Patients Undergoing Knee Replacement: A Randomized Clinical Trial. JAMA Surg. 2022;157:481–9. doi: 10.1001/jamasurg.2022.0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strickland LH, Kelly L, Hamilton TW, et al. Early recovery following lower limb arthroplasty: Qualitative interviews with patients undergoing elective hip and knee replacement surgery. Initial phase in the development of a patient-reported outcome measure. J Clin Nurs. 2018;27:2598–608. doi: 10.1111/jocn.14086. [DOI] [PubMed] [Google Scholar]
- 10.Hasabo EA, Assar A, Mahmoud MM, et al. Adductor canal block versus femoral nerve block for pain control after total knee arthroplasty: A systematic review and Meta-analysis. Medicine (Baltimore) 2022;101:e30110. doi: 10.1097/MD.0000000000030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mott A. Full search strategy and search results. 2023. Available. [DOI]
- 13.The EndNote Team EndNote. 2013
- 14.Covidence. 2022. www.covidence.org Available.
- 15.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12:55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JTJ, Chandler J, Cumpston M, editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2023. www.training.cochrane.org/handbook Available. [Google Scholar]
- 19.Mott A. Supplementary material - excluded studies. 2023. Available. [DOI]
- 20.Atchabahian A, Cuff G, Cuevas R. ESRA19-0264 delayed attainment of physical therapy milestones with the addition of an adductor canal block to local infiltration analgesia following total knee arthroplasty. E-Poster Viewing Abstracts. 2019 doi: 10.1136/rapm-2019-ESRAABS2019.417. [DOI] [Google Scholar]
- 21.Biswas A, Perlas A, Ghosh M, et al. Relative Contributions of Adductor Canal Block and Intrathecal Morphine to Analgesia and Functional Recovery After Total Knee Arthroplasty: A Randomized Controlled Trial. Reg Anesth Pain Med. 2018;43:154–60. doi: 10.1097/AAP.0000000000000724. [DOI] [PubMed] [Google Scholar]
- 22.Rajkumar N, Karthikeyan M, Soundarrajan D, et al. Comparison of Efficacy of Adductor Canal Block, Local Infiltration Analgesia and Both Combined in Postoperative Pain Management After Total Knee Arthroplasty: A Randomized Controlled Trial. Indian J Orthop. 2021;55:1111–7. doi: 10.1007/s43465-021-00482-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goytizolo EA, Lin Y, Kim DH, et al. Addition of Adductor Canal Block to Periarticular Injection for Total Knee Replacement: A Randomized Trial. J Bone Joint Surg Am. 2019;101:812–20. doi: 10.2106/JBJS.18.00195. [DOI] [PubMed] [Google Scholar]
- 24.Grosso MJ, Murtaugh T, Lakra A, et al. Adductor Canal Block Compared with Periarticular Bupivacaine Injection for Total Knee Arthroplasty: A Prospective Randomized Trial. J Bone Joint Surg Am. 2018;100:1141–6. doi: 10.2106/JBJS.17.01177. [DOI] [PubMed] [Google Scholar]
- 25.Luo Z-Y, Yu Q-P, Zeng W-N, et al. Adductor canal block combined with local infiltration analgesia with morphine and betamethasone show superior analgesic effect than local infiltration analgesia alone for total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2022;23:468. doi: 10.1186/s12891-022-05388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nader A, Kendall MC, Manning DW, et al. Single-Dose Adductor Canal Block With Local Infiltrative Analgesia Compared With Local Infiltrate Analgesia After Total Knee Arthroplasty: A Randomized, Double-Blind, Placebo-Controlled Trial. Reg Anesth Pain Med. 2016;41:678–84. doi: 10.1097/AAP.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 27.Sawhney M, Mehdian H, Kashin B, et al. Pain After Unilateral Total Knee Arthroplasty: A Prospective Randomized Controlled Trial Examining the Analgesic Effectiveness of a Combined Adductor Canal Peripheral Nerve Block with Periarticular Infiltration Versus Adductor Canal Nerve Block Alone Versus Periarticular Infiltration Alone. Anesth Analg. 2016;122:2040–6. doi: 10.1213/ANE.0000000000001210. [DOI] [PubMed] [Google Scholar]
- 28.Tziona D, Papaioannou M, Mela A, et al. Local infiltration analgesia combined with a standardized multimodal approach including an adductor canal block in total knee arthroplasty: a prospective randomized, placebo-controlled, double-blinded clinical trial. J Anesth. 2018;32:326–32. doi: 10.1007/s00540-018-2476-x. [DOI] [PubMed] [Google Scholar]
- 29.Nicolino TI, Costantini J, Carbó L. Complementary Saphenous Nerve Block to Intra-Articular Analgesia Reduces Pain After Total Knee Arthroplasty: A Prospective Randomized Controlled Trial. J Arthroplasty. 2020;35:S168–72. doi: 10.1016/j.arth.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Abstracts and Highlight Papers of the 32nd Annual European Society of Regional Anaesthesia & Pain Therapy (ESRA) Congress 2013. Reg Anesth Pain Med. 2013;38:E1–121. doi: 10.1097/AAP.0b013e3182a6a39d. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q, Yue Y, Li D, et al. Efficacy of Single-Shot Adductor Canal Block Combined With Posterior Capsular Infiltration on Postoperative Pain and Functional Outcome After Total Knee Arthroplasty: A Prospective, Double-Blind, Randomized Controlled Study. J Arthroplasty. 2019;34:1650–5. doi: 10.1016/j.arth.2019.03.076. [DOI] [PubMed] [Google Scholar]
- 32.Zhou M, Ding H, Ke J. Adductor canal block in combination with posterior capsular infiltration on the pain control after TKA. Ir J Med Sci. 2018;187:465–71. doi: 10.1007/s11845-017-1647-3. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Fan L. Comparison adductor canal block combined with local infiltration analgesia and adductor canal block alone for pain management after total knee arthroplasty. Medicine (Baltimore) 2020;99:e21881. doi: 10.1097/MD.0000000000021881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren Y, Liao J, Qin X, et al. Adductor canal block with periarticular infiltration versus periarticular infiltration alone after total knee arthroplasty. Medicine (Baltimore) 2020;99:e20213. doi: 10.1097/MD.0000000000020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adductor canal block. [15-Sep-2023]. https://classic.clinicaltrials.gov/ct2/show/NCT04513145 Available. Accessed.
- 36.CTG labs - NCBI. [15-Sep-2023]. https://www.clinicaltrials.gov/study/NCT05427019 Available. Accessed.
- 37.Can single-injection adductor canal blocks improve postop pain relief in patients undergoing total knee arthroplasty? [15-Sep-2023]. https://classic.clinicaltrials.gov/ct2/show/NCT02276495 Available. Accessed.
- 38.中国临床试验注册中心 - 世界卫生组织国际临床试验注册平台一级注册机构. [15-Sep-2023]. https://www.chictr.org.cn/showproj.html?proj=21222 Available. Accessed.
- 39.中国临床试验注册中心 - 世界卫生组织国际临床试验注册平台一级注册机构. [15-Sep-2023]. https://www.chictr.org.cn/showproj.html?proj=46627 Available. Accessed.
- 40.CTRI https://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=49276&EncHid=&userName=CTRI/2021/08/03536 Available.
- 41.CTG labs - NCBI. [15-Sep-2023]. https://clinicaltrials.gov/ct2/keydates/NCT04258241 Available. Accessed.
- 42.How does the addition of adductor canal block to local infiltration affect recovery in patients undergoing total knee arthroplasty? A feasibility study. Clinicaltrials.gov. [15-Sep-2023]. https://www.clinicaltrials.gov/ct2/show/NCT04648072 Available. Accessed.
- 43.CTG labs - NCBI. https://clinicaltrials.gov/ct2/show/results/NCT02740192 n.d. Available.
- 44.CTRI [15-Sep-2023]. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=42221 Available. Accessed.
- 45.Mingdeng X, Yuzhang A, Xiaoxiao X, et al. Combined application of adductor canal block and local infiltration anesthesia in primary total knee arthroplasty: an updated meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg. 2022;142:913–26. doi: 10.1007/s00402-020-03706-x. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Li A, Zhang Y. The efficacy of combined adductor canal block with local infiltration analgesia for pain control after total knee arthroplasty. Medicine (Baltimore) 2018;97:e13326. doi: 10.1097/MD.0000000000013326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Husted H, Lunn TH, Troelsen A, et al. Why still in hospital after fast-track hip and knee arthroplasty? Acta Orthop. 2011;82:679–84. doi: 10.3109/17453674.2011.636682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Patanwala AE, Naylor JM, et al. Impact of modified-release opioid use on clinical outcomes following total hip and knee arthroplasty: a propensity score-matched cohort study. Anaesthesia. 2023;78:1237–48. doi: 10.1111/anae.16070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rauf Does saphenous nerve block improve analgesia after total knee replacement when used in combination with local infiltration analgesia a prospective randomised double blinded controlled trial. Reg Anesth Pain Med. 2013;38 doi: 10.1097/AAP.0b013e3182a6a39d. [DOI] [Google Scholar]