Abstract
Previous research indicated that fine particulate matter (PM2.5) exposure affected both offspring neurodevelopment and the colonization of gut microbiota (GM), while the underlying mechanism remained unclear. Our study aimed to evaluate the impacts of prenatal PM2.5 exposure on child cognitive development and investigate the role of neonatal GM colonization in the association. Based on the Shanghai Maternal–Child Pairs Cohort, 361 maternal–child pairs were recruited. Prenatal PM2.5 exposure concentrations were estimated using a high-spatial-resolution prediction model, and child neurodevelopment was assessed by the Ages and Stages Questionnaire. Multivariable linear regression models, logistic regression models, linear discriminant analysis effect size, and random forest model were applied to explore the associations among PM2.5 exposure, GM colonization, and children’s neurodevelopment. The present study revealed a negative correlation between PM2.5 exposure throughout pregnancy and child neurodevelopment. Prenatal PM2.5 exposure was associated with an increased risk of suspected developmental delay (SDD) (OR = 1.683, 95% CI: 1.138, 2.489) in infants aged 2 months. Additionally, potential operational taxonomic unit markers were identified for PM2.5-related neurotoxicity, demonstrating promising classification potential for early SDD screening (AUC = 71.27%). Prenatal PM2.5 exposure might disrupt the composition, richness, and evenness of meconium GM, thereby influencing cognitive development and the occurrence of SDD in offspring. Seven PM2.5-related genera, Ruminococcus gnavus group, Romboutsia, Burkholderiaceae Caballeronia Paraburkholderia, Blautia, Alistipes, Parabacteroides, and Bacteroides, were validated as correlated with prenatal PM2.5 exposure and the occurrence of SDD. Moreover, alterations of GM related to PM2.5 exposure and SDD might be accompanied by changes in functional pathways of amino acid, lipid, and vitamin metabolism as indicated by differentially enriched species in the Kyoto Encyclopedia of Genes and Genomes.
Keywords: fine particulate matter, birth cohort, neurodevelopmental delay, gut microbiota
1. Introduction
Outdoor air pollution has long been a significant environmental health concern in China, resulting in an estimated 1.4 million deaths attributable to it in 2019.1 In response, China formally announced the Nationally Determined Contribution (NDC) in 2020, and China pledged to take substantial measures to reduce carbon dioxide emissions by 2030 and achieve carbon neutrality by 2060.2 However, based on the official observation network, the national mean annual fine particulate matter (PM2.5) concentration of China in 2020 was 33 μg/m3,3 6.6 times higher than the standard in the the latest World Health Organization (WHO) guideline.4 Even with the implementation of carbon neutrality policies to reduce PM2.5 pollution ideally, China still fell short of meeting air quality standards. Thus, more attention and efforts were required to address PM2.5 pollution both presently and in the future.
The establishment of the infant intestinal microbiota in parallel with a crucial period in early brain development, but few studies had explored their association with developmental and behavioral performance.5 Several longitudinal studies had reported that other genera within the Bacteroidetes phylum (i.e., Prevotella), depleted in late pregnancy, were linked to internalizing behaviors at 2 years old.6 However, little association was found between Bacteroides-predominant microbiome at 2 months and character in 6-month-old children.7 Moreover, a cross-sectional survey containing 77 toddlers aged 2 years revealed that increased intestinal bacterial diversity and relative abundance of the Bacteroidetes phylum (i.e., Parabacteroides) were related to infant temperament according to their parental report.8 Similarly, a cohort study recruiting 89 infants indicated that an abundance of Bacteroides in gut microbiota (GM) at 12 months of age was associated with enhanced cognitive development at 2 years of age.9 Additionally, a study involving 309 children revealed that a Bacteroides-predominant intestinal microbiome at 3–6 months of age was related to increased risk of a delayed fine motor domain evaluated by the Ages and Stages Questionnaire, Third Edition (ASQ-3), a prevalent screening tool, used to monitor child development across five domains.10 Overall, these findings highlighted the importance of further investigation into the function of the intestinal microbiome in promoting healthy neurological development in children.
Epidemiological literature has reported the adverse effects of prenatal exposure to PM2.5 on brain development, increasing the risk of cognitive impairment, neurodevelopment delay, and behavior disorders in children.11−13 An animal experiment demonstrated that prenatal PM2.5 exposure during pregnancy induced autism-like behavioral disorders in offspring during adulthood.14 A study conducted in the U.S. reported that prenatal PM2.5 exposure was significantly linked with an increased risk of neurological development delay in domains of problem solving and communication.15 However, some studies had reported insignificant association between prenatal PM2.5 exposure and child neurological development.16,17 More comprehensive studies are required to validate the interrelationship.
A study, profiling microbiome across fetal organs, demonstrated direct spatial colonization of microbial entities, localized with the lumen of growing fetal intestine during the second trimester of pregnancy.18 Furthermore, the colonization and composition of children’s intestinal flora in early life were shaped by prenatal environment exposure, host genetics, and delivery mode, etc.19−21 A review reported that outdoor air pollution was related to reduced intestinal microbial biodiversity in the population.22 An investigation on the American population found an association between outdoor air pollution and increased relative abundance of Coriobacteriaceae and reduced Bacteroidaceae in fecal samples.23 Moreover, an animal experiment revealed that exposure to PM2.5 pollution induced increased abundance of the Bacteroidetes phylum and a higher alpha diversity in the colon.24 More studies indicated that particulate matter, particularly PM2.5, was able to alter intestinal microbiota.25−27 PM2.5 reached the lungs after initial inhalation and then phagocytized into alveolar and bronchioles spaces.28,29 Subsequently, these PM2.5 were retained within macrophages and could quickly access the mucus layer, reaching the oropharynx and eventually being swallowed into the intestine. Overall, this process might contribute to the improvement of intestine permeability and alterations in the composition of GM.
Previous studies investigated the effect of air pollution on microbiota, as well as the association between air pollution and offspring neurodevelopment. However, studies on the relationship among prenatal air pollution, neonatal microbiota, and children’s neurodevelopment were limited, especially lacking sufficient evidence from human epidemiological studies. In this study, 361 mother–child pairs from Shanghai MCPC were included to explore the association between prenatal PM2.5 exposure and childhood neurodevelopment, as well as to assess the role of intestinal microbial dysbiosis in this association.
2. Material and Methods
2.1. Study Population
Participants in this study were maternal–infant pairs recruited from the Shanghai Maternal–Child Pairs Cohort (MCPC), a prospective birth cohort aiming to estimate the multifaceted impacts of prenatal and maternal environmental and sociopsychological factors, particularly their interactions, on offspring development and growth. More details of the cohort have been published previously.30 The eligibility criteria were as follows: (1) maternal age ≥18 years; (2) free from specific severe chronic diseases (e.g., hypertension, diabetes, and neurological disorders); (3) no alcohol consumption or smoking during pregnancy; (4) residency in Shanghai for more than one year and delivered in a local hospital. To explore the role of neonatal GM in the association between prenatal PM2.5 exposure and children’s neurodevelopment, 361 mother–child pairs were enrolled for whom they had collected neonatal feces and completed the neurodevelopment assessment before 24 months of age. Ethical approval of this study was granted by Fudan University’s Institutional Review Boards (IRB#2016-04-0587; IRB#2015-TYSQ-12-1). The gestational women and guardians of the infants had signed informed consent of this study, in accordance with the strengthening reporting guideline of the Reporting of Observational Studies in Epidemiology (STROBE).
2.2. Demographic Characteristics
Professional nurses administered a standard structured questionnaire to collect socioeconomic information (e.g., maternal education, annual family income), residential address, and lifestyle (e.g., physical activity, alcohol consumption, smoking). Physical state and medical history information, such as gestational weight gain, maternal age, prepregnancy body mass index (BMI), parity, last menstrual period (LMP, the first day of the last menstrual period before pregnancy), pregnancy complications, and delivery mode were obtained from medical records. Prepregnancy BMI (weight in kilograms divided by height in meters squared) was calculated based on prepregnancy weight and height, and gestational age was determined in weeks using the mother’s LMP. Infant developmental indices such as birth length, birth weight, infant gender, and gestational age were also retrieved from medical histories.
2.3. Assessment of PM2.5 Personal Exposure
The residential addresses of all participants were geocoded, and individual daily mean PM2.5 concentrations were estimated by a machine-learning-based prediction approach with 1 km × 1 km resolution. Details of the prediction model development process and exposure estimation could be found in previous publications.31 In brief, PM2.5 concentration was predicted by integrating land cover information, the aerosol depth (AOD), meteorological conditions, Normalized Difference Vegetation Index (NDVI), road networks, and other relevant factors. The model provided daily PM2.5 concentration predictions at a resolution of 1 km × 1 km with complete temporal and spatial coverage. This method cross-validation R2 between ground measurement and daily model prediction was 0.88. Exposure data were categorized into trimester 1 (from 1 to 13 gestational weeks), trimester 2 (from 14 to 26 gestational weeks), trimester 3 (gestational weeks 27 to delivery), and the whole pregnancy (from LMP to delivery). The subjects were stratified into high- and low-exposure groups based on the median level of PM2.5 exposure. Ambient humidity and temperature were estimated by daily mean relative humidity (%) and daily mean temperature (°C) obtained from the Shanghai Meteorological Data Sharing Service System (http://www.cnemc.cn/).
2.4. Childhood Neurological Development Measurements
Infant neurocognitive development was assessed using the Ages and Stages Questionnaire, Third Edition (ASQ-3), Chinese version, at ages 2, 6, 12, and 24 months. The ASQ-3 includes 30 items representing neurofunction across five domains: problem-solving, communication, gross motor, fine motor, and personal–social behavior. The questionnaire was completed by parents, and each domain was scored on a scale of 0–60,32 with higher scores reflecting better neurodevelopment in the corresponding domain. Suspected developmental delay (SDD) in children was defined as scores in any domain less than two standard deviations (SD) from the mean (mean-2SD) at the follow up time point. Additionally, if the score in any of the five domains or the ASQ-T (the total ASQ score of the child) was below the threshold, the child was considered to manifest SDD.33,34 The outcomes of child cognition development assessment are presented in Tables S2 and S3. Further details of this questionnaire and the criteria have been published previously.35
2.5. DNA Extraction and Sequence of the 16s rRNA Gene
Each fecal sample was collected from all newborn infants within 24 h after delivery (mean 6.5 h) via professional staff using commercial collection kits purchased from Second Genome, San Francisco, CA. These samples were gathered in a sterilized polypropylene tube through wooden depressors and a sterilized swab and immediately saved at −20 °C for less than 6 h. Finally, they were transferred to −80 °C within an average of 2 days after collection until analysis. Using the FastDNA Spin Kit for Stool from MP Biomedicals, Santa Ana, CA, all DNA extractions from the 200 mg of neonatal meconium samples were processed according to the manufacturer’s instructions. Subsequently, at Jiangnan University of Wuxi, China, the State Key Laboratory of Food Science and Technology amplified the 16s rRNA gene’s hypervariable region V3–V4. After extraction from 2.0% agarose gel, the polymerase chain reaction (PCR) products were further purified through a TIANgel Mini Purification Kit bought from TIANGEN, Beijing, China. Then the productions were quantified using a Qubit dsDNA HS Assay Kit from Life Technologies Corporation, Carlsbad, CA. Based on TruSeq DNA LT Sample Preparation Kit from Illumina, Santiago, CA, library preparation was performed and sequencing was carried out through the Illumina MiSeq PE300 platform.36
2.6. Bioinformatics Pipeline
Utilizing QIIME2, paired-end sequenced data derived from the MiSeq run were merged through FLASE and pooled,37 demultiplexed, and assigned operational taxonomic units (OTUs) at a 97% similarity threshold.38 Subsequently, in the aggregate of 520 5433 (min 1783, mean 24 908, max 138 828) sequences still existed, and samples were normalized based on the overall sun scaling approach for further analysis. Alpha diversity, assessed at the OTU level, was calculated in R (“vegan” package). Moreover, the Chao richness estimator was employed to estimate microbial richness, indicating the number of different taxa in every sample. Evenness, an estimation of the relative abundance of diverse taxa in each sample, was estimated through the Simpson diversity index, Shannon diversity index, and phylogenetic diversity (PD) index. Abundance data for each OTU from the genus to phylum levels were summarized using packages of R (“phyloseq” package). Furthermore, the 16S rRNA gene sequence from each sample was mapped to the KEGG database (https://www.kegg.jp/) for annotation, and the abundance of metabolic functional pathways was predicted through the Phylogenetic Investigation of Community through Reconstruction of Unobserved States 2 (PICRUSs 2) (https://github.com/picrust/picrust2/wiki).
2.7. Statistical Analysis
Descriptive data for lifestyle variables and demographic characteristics were presented as mean ± SD for continuous variables and frequency (%) for categorical variates. Comparisons between groups, including the boys–girls and low–high groups, were made using the t test and Wilcoxon test for continuous statistics, depending on the distribution of the statistics, and the Chi-Squared test for categorical data. Multivariable linear regression models were utilized to assess the association between prenatal personal PM2.5 exposure and ASQ scores, as well as alpha diversity indices in various domains, with adjustment for confounders. Logistic regression models were applied to evaluate the interrelationship between individual PM2.5 exposure during pregnancy and SDD in diverse domains, with adjustment for confounders. Additionally, two-pollutant models were employed, with each model further adjusted for one of the four gaseous pollutants (O3, SO2, NO2, CO) or inhalable particles (PM10), to estimate the association of PM2.5 concentration with ASQ scores in diverse domains and the risk of SDD. Two-pollutant models were conducted to validate the robustness of such associations. Linear Discriminant Analysis Effect Size (LEFSe) estimation, calculated on the Galaxy Web site (http://huttenhower.sph.harvard.edu/galaxy/, v1.0), was utilized to identify characteristic microbial taxa between low and high groups divided by median exposure level over the entire pregnancy. Moreover, Multivariate Analysis by Linear Models (MaAslin 2) was performed to verify the gut microbial KEGG functional pathways related to prenatal PM2.5 exposure. A random forest model incorporating gut microbes at the genus level was trained to predict the occurrence of SDD and identify SDD-related genera. A sparse model containing the related taxa was then trained on the set to predict the occurrence of SDD. To address data skewness, the relative abundance of the genus was subjected to natural log transformation. The association between genera and KEGG was estimated through Spearman’s correlation analysis.
Underlying confounders were selected according to existing literature and expert knowledge. Variables including child sex, maternal age, and child age known to be associated with children’s neurological development were identified as potential confounders.39−41 In the present study, maternal age, parity, maternal prepregnancy BMI, maternal physical activity, maternal education level, passive smoking states, season of conception, annual family income, child age, paternal age, child gender, premature birth, gestational weight gain (GWG), and feeding patterns were included in the primary analysis model. Moreover, according to the International Physical Activity Questionnaire (IPAQ), maternal physical activity was assessed during early and late pregnancy.42 The distribution of individual temperature, humidity, PM10, and other four pollutants exposure levels during pregnancy based on fixed-site monitoring stations in Shanghai is presented in Table S1. All analysis and calculations, except for LEFSe analysis, were performed using R, version 4.2.0.
3. Result
3.1. Characteristics of Study Population
A total of 361 mother–child pairs were enrolled in this study, and the characteristics of participants are summarized in Table 1. Notably, over 40% of mothers had completed at least a college education, with the average maternal age recorded as 29.15 ± 4.20 years. Detailed distributions of individual PM2.5 and five other air pollutants exposures throughout the entire pregnancy are presented in Table S1. The median individual PM2.5 exposure concentration over the entire pregnancy period was 43.37 μg/m3. Although girls generally exhibited higher ASQ scores across most domains than boys (except for the personal–social domain aged 12 months), the differences were not statistically significant. Meanwhile, there was no significant difference in SDD incidence between girls and boys. Further details regarding children’s neurodevelopment assessments are provided in Tables S2 and S3.
Table 1. Study Characteristics of the Mother–Child Pairs (N = 361)a.
| variable | total subjects (N = 361) | boys (N = 196) | girls (N = 165) | P value* |
|---|---|---|---|---|
| Maternal Characteristics | ||||
| age of pregnancy, years | 29.15 ± 4.20 | 29.08 ± 4.29 | 29.24 ± 4.10 | 0.723 |
| prepregnancy BMI, kg/m2 | 21.58 ± 3.06 | 21.52 ± 3.06 | 21.65 ± 3.08 | 0.674 |
| educational level | 0.529 | |||
| junior high school or below | 24 (6.65%) | 11 (5.61%) | 13 (7.88%) | |
| senior high school or junior college | 190 (52.63%) | 101 (51.53%) | 89 (53.94%) | |
| college degree or higher | 147 (40.72%) | 84 (42.86%) | 63 (38.18%) | |
| annual family income, yuan | 0.072 | |||
| <100 000 | 95 (26.31%) | 43 (21.94%) | 52 (31.52%) | |
| 100 000–300 000 | 239 (66.20%) | 135 (68.88%) | 104 (63.03%) | |
| >300 000 | 27 (7.48%) | 18 (9.18%) | 9 (5.45%) | |
| passive smoking status | 0.929 | |||
| yes | 90 (24.93%) | 48 (24.49%) | 42 (25.45%) | |
| no | 271 (75.07%) | 148 (75.51%) | 123 (74.55%) | |
| physical activityb | 0.97 | |||
| low | 142 (39.34%) | 78 (39.80%) | 64 (38.79%) | |
| moderate | 201 (55.69%) | 108 (55.10%) | 93 (56.36%) | |
| high | 18 (4.99%) | 10 (5.10%) | 8 (4.85%) | |
| energy intake in pregnancy, kcal | 2236.44 ± 1080.32 | 2136.41 ± 1068.65 | 2355.28 ± 1084.25 | 0.055 |
| GWG, kg | 13.53 ± 5.67 | 13.72 ± 5.39 | 13.31 ± 5.98 | 0.503 |
| mode of delivery (%) | 0.499 | |||
| vaginal | 159 (44.04%) | 90 (45.92%) | 69 (41.82%) | |
| Caesarean | 202 (56.96%) | 106 (54.08%) | 96 (58.18%) | |
| parity (%) | 0.415 | |||
| primipara | 202 (56.96%) | 114 (58.16%) | 88 (53.33%) | |
| multipara | 159 (44.04%) | 82 (41.84%) | 77 (46.67%) | |
| pregnancy syndromec | 0.508 | |||
| no | 220 (60.94%) | 123 (62.76%) | 97 (58.79%) | |
| yes | 141 (39.06%) | 73 (37.24%) | 68 (41.21%) | |
| gestational age, weeks | 39.32 (1.26) | 39.21 ± 1.29 | 39.47 ± 1.22 | 0.045 |
| Child Characteristics | ||||
| preterm birth (%) | 0.473 | |||
| no | 346 (95.84%) | 10 (5.10%) | 5 (3.03%) | |
| yes | 15 (4.16%) | 186 (94.90%) | 160 (96.97%) | |
| birth season (%) | 0.123 | |||
| cold-season | 168 (46.54%) | 99 (50.51%) | 69 (41.82%) | |
| warm-season | 193 (53.46%) | 97 (49.49%) | 96 (58.18%) | |
| feeding patterns | 0.575 | |||
| breast feeding | 182 (50.41%) | 97 (49.49%) | 85 (51.52%) | |
| mixed feeding | 99 (27.42%) | 58 (29.59%) | 41 (24.85%) | |
| artificial feeding | 80 (22.16%) | 41 (20.92%) | 39 (23.64%) | |
| birth weight, g | 3344.60 ± 446.14 | 3362.60 (475.60) | 3323.21 (407.57) | 0.397 |
| birth length, cm | 50.06 ± 0.94 | 50.08 ± 1.19 | 50.02 ± 0.49 | 0.538 |
| Ages and Stages Questionnaire, Third Edition (ASQ-3) | ||||
| ASQ-Ta-2M | 235.53 ± 38.18 | 233.19 ± 38.43 | 238.30 ± 37.82 | 0.205 |
| ASQ-Ta-6M | 220.70 ± 46.81 | 218.02 ± 50.73 | 223.87 ± 41.70 | 0.345 |
| ASQ-Ta-12M | 240.67 ± 41.51 | 236.84 ± 42.28 | 245.44 ± 40.19 | 0.099 |
| ASQ-Ta-24M | 246.80 ± 41.45 | 245.96 ± 40.44 | 247.87 ± 42.89 | 0.731 |
Abbreviations: GWG, gestation weight gain; BMI, body mass index.
Physical activity was assessed by the International Physical Activity Questionnaire-Short Form.
Pregnancy syndrome included anemia, hypertension, diabetes, and thyroid disease.
3.2. Association of Prenatal Individual PM2.5 Exposure and Early Child Neurodevelopment
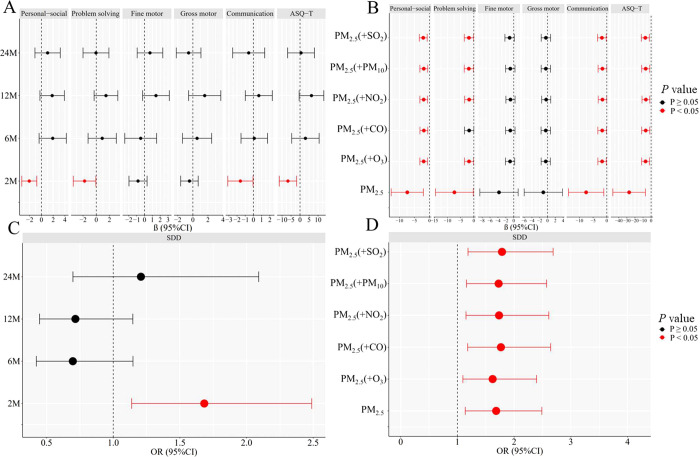
Figure 1A illustrates the association of individual PM2.5 exposure during the entire pregnancy with children’s ASQ scores at 2, 6, 12, and 24 months of age. Each SD μg/m3 increase in prenatal PM2.5 exposure was negatively linked with ASQ-T scores, the communication domain, problem-solving domain, and personal–social developmental domain (βAQT-T-2M = −6.717, 95% CI: −11.530, −1.904; βcommunication-2M = −1.595, 95% CI: −3.136, −0.053; βproblem-solving-2M = −1.771, 95% CI: −3.481, 0.060; βpersonal–social-2M = −2.074, 95% CI: −3.348, −0.800), respectively. In two-pollutant models (Figure 1B), these associations in infants aged 2 months remained robust after adjustment for gaseous pollutants and PM10.
Figure 1.
PM2.5 exposure during the entire pregnancy (each SD) was associated with the neurodevelopment of children. (A) Association between prenatal PM2.5 exposure and ASQ scores at 2, 6, 12, and 24 months of age. (B) Association of prenatal PM2.5 exposure and ASQ scores at 2 months of age in a two-pollutant model. (C) Association of prenatal PM2.5 exposure and SDD at 2, 6, 12, and 24 months of age. (D) Association of prenatal PM2.5 exposure and SDD at 2 months of age in a two-pollutant model. Results were presented as calculated values β, odds ratio (OR), and 95% confidence intervals (CI), and the highlighted associations in red are at P value <0.05. These models were adjusted for confounders of parental characteristics (parity, maternal age, maternal pregravid BMI, maternal physical activity, maternal educational level, annual family income, pregnancy syndrome, passive smoking status, season of conception, delivery mode, paternal age, premature birth, gestational weight gain (GWG), temperature, and relative humidity) and child characteristics (child age at ASQ test, birthweight, feeding patterns, and child sex).Abbreviations: BMI, body mass index; ASQ, Ages and Stages Questionnaire; SDD, suspected developmental delay at followup point.
Figure 1C demonstrates a significant association between PM2.5 exposure throughout the pregnancy and the incidence of SDD in children at 2, 6, 12, and 24 months of age. Each SD μg/m3 increase in PM2.5 exposure during pregnancy was positively related to SDD incidence (ORentire-pregnancy = 1.683, 95% CI: 1.138, 2.489). In two-pollutant models (Figure 1D), the magnitude (i.e., the size of the evaluated effect) of the association of individual prenatal PM2.5 exposure with SDD aged 2 months remained significant after adjustment for other pollutants.
3.3. PM2.5 Exposure throughout Pregnancy and Neurodevelopment Associated with Neonatal Microbial Alpha Diversity
Given the observed interrelationship of PM2.5 exposure during the entire pregnancy with the ASQ scores and the incident of SDD mainly at 2 months old in children, the meconium samples were applied to estimate the effect of prenatal PM2.5 exposure on GM and the difference in microbial alpha diversity between SDD and non-SDD infants. Table 2 presents the association between PM2.5 exposure during the entire pregnancy and GM alpha diversity. Per SD μg/m3 increase in PM2.5 exposure exhibited a negative association with the Shannon (β = −0.367, 95% CI: −0.543, −0.192), Chao1 (β = −9.381, 95% CI: −14.416, −4.347), phylogenetic diversity (PD) (β = −30.904, 95% CI: −46.362, −15.446), and Simpson (β = −0.054, 95% CI: −0.090, −0.017) indices.
Table 2. Association of PM2.5 Exposure during the Entire Pregnancy (Each SD) and the Alpha Diversity of Neonatal Fecesa.
| alpha diversity | β | upper | lower | P value |
|---|---|---|---|---|
| Shannon | –0.367 | –0.543 | –0.192 | <0.001 |
| Chao1 | –9.381 | –14.416 | –4.347 | <0.001 |
| PD | –30.904 | –46.362 | –15.446 | <0.001 |
| Simpson | –0.054 | –0.090 | –0.017 | 0.004 |
Adjustment for parental characteristics (parity, maternal age, maternal pregravid BMI, maternal physical activity, maternal educational level, annual family income, passive smoking status, season of conception, paternal age, premature birth, GWG, temperature, and relative humidity) and child characteristics (feeding patterns, birthweight, and child sex).
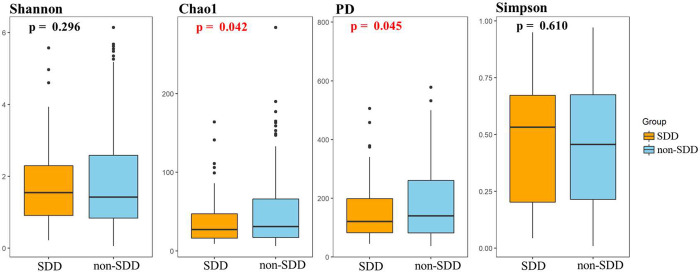
The association between PM2.5 exposure during different trimesters and the incidence of SDD at 2, 6, 12, and 24 months of age is depicted in Figure S1. The microbiome structures of neonatal feces samples from the low- and high-prenatal PM2.5 exposure groups are illustrated in Figure S2. Notably, children with SDD exhibited lower microbial richness (Chao1 index, P = 0.042) and lower bacterial evenness (PD index, P = 0.045) than neonates in the non-SDD group, suggesting an association between child cognitive development and neonatal feces alpha diversity.
Figure 2.
Comparison of neonatal meconium bacterial alpha diversity between SDD and non-SDD groups. Comparisons of alpha diversity indices between SDD and non-SDD groups using the t test (P value <0.05 was considered significant).
3.4. Individual PM2.5 Exposure throughout Pregnancy Associated with Neonatal Bacterial Taxa Colonization and Neurodevelopment in Children
3.4.1. Association between Prenatal PM2.5 Exposure and the Colonization of Microbial Genera
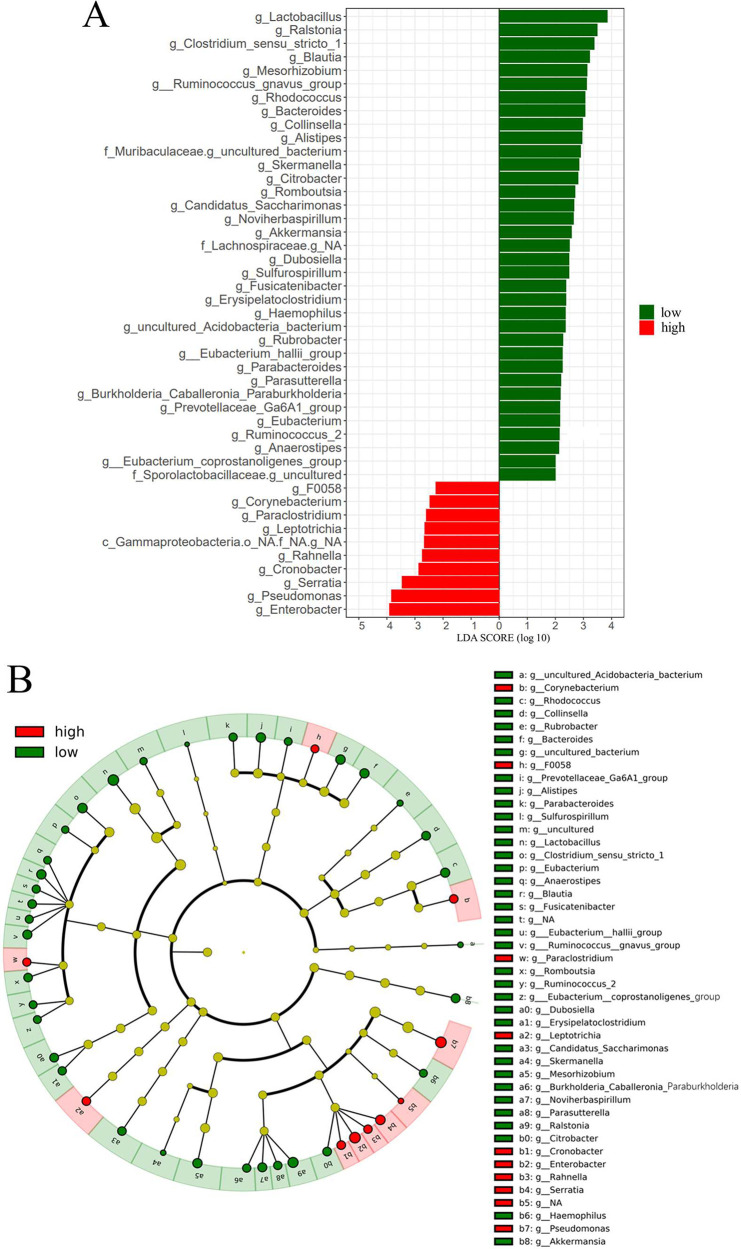
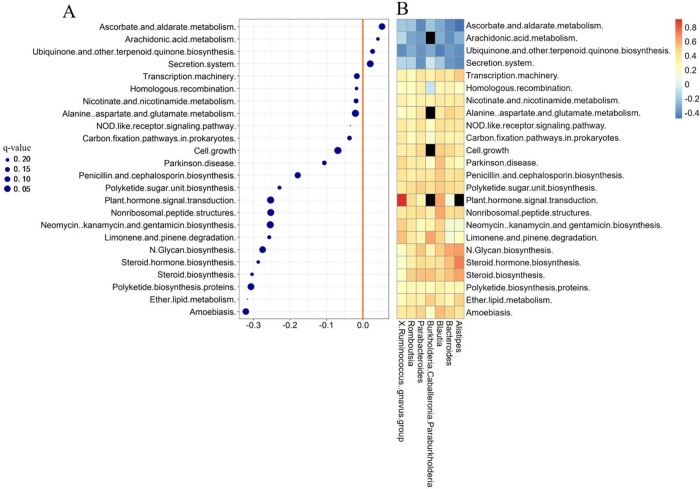
LEFSe analysis was conducted on the relative abundance of neonatal feces to identify differential abundant taxa between the low- and high-PM2.5 exposure groups. The results, depicted in Figure 3, revealed 102 microbial taxa exhibiting differential abundance (Table S4) between the two groups (linear discriminant analysis (LDA) score >2, P <0.05). Particularly, 45 bacterial genera, including Ruminococcus gnavus group, Romboutsia, Burkholderiaceae Caballeronia Paraburkholderia, Blautia, Alistipes, Parabacteroides, and Bacteroides, displayed differential abundance between the low- and high-PM2.5 exposure groups at the genus level (Figure 3A). Taxonomic representation of biological and statistically consistent diversity between neonatal feces from the low- and high-exposure group is illustrated in Figure 3B.
Figure 3.
LEFSe analysis for characteristic microbial genus in low- and high-individual PM2.5 exposure groups. Only microbial genera with LDA score >2 (P < 0.05). (A) The differently enriched taxa from low- and high-prenatal PM2.5 exposure groups are presented in the cladogram. (B) Genus, family, order, class, and phylum that were diversely presented between low- and high-prenatal PM2.5 exposure groups, as indicated by relative abundance using LEFSe analysis, are shown in Table S4.
3.4.2. Recognition and Validation of Neonatal Feces Bacterial OTUs-Based Markers for SDD
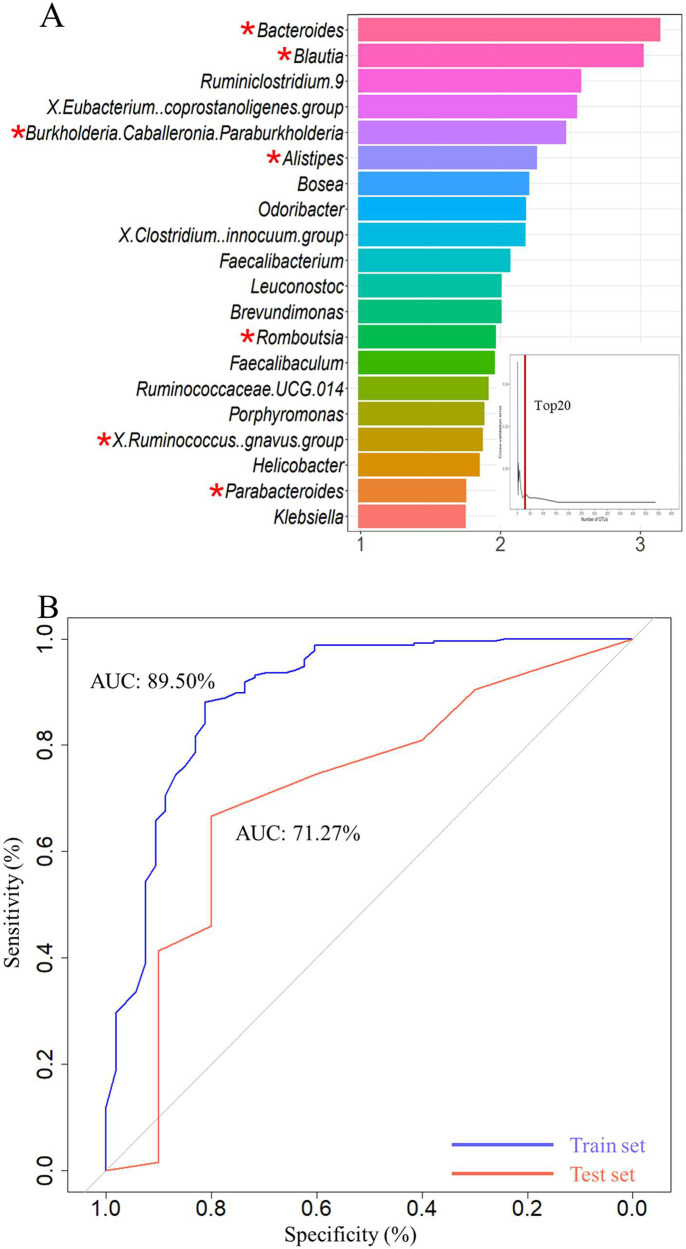
The microbiota structures of neonatal meconium samples between the SDD group and non-SDD group are shown in Figure S3. To estimate the classification potential of neonatal feces bacteria markers for SDD, a random forest model was constructed. Initially, the entire included population was segregated into training and validation sets. The training set, encompassing 80% of the samples (included 53 SDD and 235 non-SDD) underwent 10-fold cross-validation for training and modeling. During this cross-validation phase, the data set was systematically divided into 10 equivalent segments, adhering to a conventional protocol, where nine segments were cyclically utilized for training and one for validation. In the final phase, the model, refined through 10-fold cross-validation, underwent a further validation within the total population’s validation subset to evaluate its robustness and accuracy. The top 20 (at least 10% samples detected) differential abundant taxa were then verified as crucial markers between 53 SDD and 235 non-SDD (Figure 4A). Utilizing these 20 taxa, the model achieved an area under the curve (AUC) value of 89.50% and 71.27% in distinguishing between children with SDD and non-SDD in the training and testing phase, respectively (Figure 4B). Specifically, considering the previously identified 45 PM2.5 exposure-related genus and the validated 20 neurodevelopment OTU markers, seven genus, namely Ruminococcus gnavus group, Romboutsia, Burkholderiaceae Caballeronia Paraburkholderia, Blautia, Alistipes, Parabacteroides, and Bacteroides were validated as PM2.5-related neurotoxicity markers, denoted by “*” in Figure 4A.
Figure 4.
Classifiers derived from GM, verifying specific OTU markers, to predict the occurrence of SDD. (A) In the training phase, a 10-fold cross-validation was conducted on the random forest model, and the estimated top 20 differentially abundant markers were chosen as the optimal marker set according to the random forest model between 53 SDD and 235 non-SDD. (B) The AUC value between SDDs and non-SDD in the train and test set. The x-axis reflected the mean decrease accuracy to each microbial taxa, which presents the contribution to the accuracy of the model. “*” indicates the relative abundance difference originating from LEFSe analysis of 16s rRNA gene sequencing at the genus level in SDD-T versus non-SDD.
3.4.3. Association between PM2.5 Exposure during the Entire Pregnancy with KEGG Functional Pathways and the Identified Seven Microbial Taxa
The outcomes of functional analysis, elucidating the interrelationship between prenatal PM2.5 exposure throughout pregnancy and the relative abundance of the KEGG level 3 pathways, are presented in Figure 5A. A comprehensive summary of analysis outcomes using multivariate linear regression analysis is provided in Table S5. After adjusting for potential confounders, 24 different KEGG level 3 functional pathways were found to be statistically associated with prenatal PM2.5 exposure (q-value >0.25 considered significant). Furthermore, the association between these seven differentially abundant taxa and PM2.5-related KEGG functional pathways is depicted in a heatmap (Figure 5B). The results demonstrated that the reduced level of KEGG functional pathways derived from low prenatal PM2.5 exposure, including “Ascorbate and aldarate metabolism”, “Arachidonic acid metabolism”, “Secretion system”, and “Ubiquinone and other terpenoid quinone biosynthesis”, which were negatively associated with the relative abundance of certain genera enriched in non-SDD children. Additionally, the increased level of KEGG functional pathways including “Alanine aspartate and glutamate metabolism”, “Steroid biosynthesis”, and “Steroid hormone biosynthesis” was positively related to the abundance of taxa enriched in the low-prenatal PM2.5 exposure group. Therefore, the results indicated that alterations in KEGG functional pathways were associated with changes in the GM in neonatal feces. Meanwhile, these combined analyses might partially explain the potential pathogenesis of SDD. Additional details of the analysis results are presented in Table S6.
Figure 5.
Differential KEGG functional pathways and genus related to PM2.5 exposure during the entire pregnancy. (A) Differential KEGG functional pathways related to prenatal PM2.5 exposure. The association of KEGG functional pathways (KEGG level 3, using phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) data sets) and gestational PM2.5 exposure was assessed by Multivariate Analysis by Linear Models (q-value <0.25 was considered significant). (B) Association between seven differentially abundant taxa and PM2.5-related KEGG functional pathways. The “black hole” indicates P value >0.05.
4. Discussion
The current birth cohort study observed that PM2.5 exposure throughout pregnancy was associated with a reduction in ASQ scores in the problem-solving, communication, and personal–social developmental domains, as well as a higher risk of SDD at 2 months in offspring. Meanwhile, prenatal PM2.5 exposure exhibited a negative association with the alpha diversity indices of neonatal feces and 45 microbial genera associated with PM2.5 exposure. Based on the random forest model for predicting early SDD, the optimal 20 specific OTUs markers linked with SDD were identified. Seven genera (Ruminococcus gnavus group, Romboutsia, Burkholderiaceae Caballeronia Paraburkholderia, Blautia, Alistipes, Parabacteroides, and Bacteroides) exerted an effects on the association between prenatal PM2.5 exposure and the occurrence of SDD, suggesting their potential as early biomarkers of PM2.5-related neurotoxicity.
Available research studies indicated that in developing countries, approximately 10%–20% of children suffer from neurodevelopmental disorders.43 Our findings align with previous studies, indicating that prenatal PM2.5 exposure was related to early children’s neurodevelopmental delay, particularly in the problem-solving, communication, and personal–social domains.11,12,15 However, a meta-analysis study including six European cohorts found no significant relationship between prenatal exposure to PM2.5 and offspring’s psychomotor and cognition development.39 Similarly, a cohort study consisting of 1109 mother–child pairs in America reported no significant association between PM2.5 exposure during pregnancy and cognition performance decline.44 The inconsistency in findings might derive from discrepancies among studies including various confounders (e.g., infant feeding patterns and gestational age), methods of exposure estimation, outcome assessment (e.g., tools for neurodevelopment evaluation, age of neurodevelopment evaluation, and the definition of cases).
The mechanism underlying the effects of prenatal PM2.5 exposure on childhood neurodevelopment remains unclear. Some studies suggested that the intestinal flora may influence both neurodevelopmental disorders and neural behaviors via the gut–brain axis through immune, neuronal, and endocrine/systemic pathways, leading to cognitive and neurological alterations.45−49 Epidemiology evidence supported the role of GM in neurodevelopmental disorders, including anxiety, autism, and depression.50 According to the DOHaD hypothesis, the first 1000 days of life, from conception to 2 years after birth, are crucial for neurodevelopment and growth.51 During this period, fine particulate matter can enter systemic circulation and then penetrate into the gut epithelia through microfold cells on Peyer’s patches and via enterocytes due to immature barriers.52 Ambient particulate matter might reach the gut through the ingestion of inhaled particulate matter after mucociliary removal in the airway.25 Particulate matter could impair intestinal barrier integrity and enhance bacterial translocation, which leads to systemic and intestinal inflammatory reactions and oxidative stress,23,25 resulting in changes of short-chain fatty acids (SCFAs) production and hence may influence psychological functioning, including their neurol basis and cognitive processes through the gut–brain axis.53,54 The current study found that PM2.5 exposure was negatively linked with alpha diversity indices of the GM, as well as lower Simpson and Chao1 indices in the SDD group compared to the non-SDD group. This suggested that alpha diversity of GM may play a critical role in the interrelationship between prenatal PM2.5 exposure and SDD. This finding was consistent with previous research from the FinnBrain Birth Cohort Study, demonstrating a negative association between alpha diversity and fear reactivity as well as passive emotionality.7 Additionally, a previous study involving 410 child–mother pairs reported mediating effects of neonatal feces bacterial richness in the association between maternal emotional symptoms and an infant’s personal–social behavior.55
Seven genera were associated with both PM2.5 exposure throughout pregnancy and the risk of neurodevelopmental delay and were considered as predictive markers for SDD occurrence. Previous studies had linked these signature genera to neurodevelopment and neurological diseases.56,57 For instance, an animal experiment found that gut Ruminococcus affected the neonatal development, as reflected by brain N-acetylaspartate (NAA), medicated by serum cortisol.58 Epidemiological evidence indicated that Romboutsia was identified at significantly lower abundance in the optical spectrum disorders (NMOSD) patients, suggesting that the dysbiosis of GM contributes to the onset and progression of neurological diseases.59 Besides, certain association existed between intestinal microbiome, including Burkholderia Caballeronia Paraburkholderia, and neuroinflammation, which exerted adverse influence on child neurodevelopment.60Blautia, a well-known advantageous genus, produces butyric acid,61 which serves as a primary energy source for colonocytes.62 Studies claimed that a lower abundance of Blautia was related to compromised practical reasoning ability in toddlers.63 Averina et al. reported a significant decrease in the abundance of genes-encoding proteins involved in melatonin, GABA, and butyric acid production in the GM of individuals with autism spectrum disorder (ASD), most of which belong to Alistipes.64 Moreover, a case-control study revealed that the ASD group displayed a depletion of Bacteroides which may be involved in the pathogenesis of ASD through regulation of the DOPA signaling pathway.65 However, the function of Parabacteroides is still unknown. Some studies considered that Parabacteroides might be putative probiotics, negatively associated with major depressive disorder (MDD), and that the Parabacteroides genus produces SCFA.66,67 Nevertheless, a systematic review revealed that children with ASD present a significantly higher abundance of the Parabacteroides taxa, warranting further investigation into the function of Parabacteroides.
In the present study, KEGG pathways were applied to identify specific metabolic pathways related to the GM in offspring following prenatal PM2.5 exposure, aiming to elucidate the interrelationship between alterations in neonatal feces and influenced neurodevelopment. The association analysis revealed that prenatal PM2.5 exposure was linked to gene replication and repair, amino acid metabolism, and alterations of lipid metabolism. One experiment reported that PM2.5 exposure induces disrupted the microbiome in Alzheimer’s Disease (AD) mice, significantly associated with dysregulation of fundamental metabolic processes such as amino acid metabolism, lipid metabolism, carbohydrates, digestive system, and dysbiosis of the endocrine and neurodegenerative diseases, particularly AD.68 The analysis of KEGG pathways related to the seven signature genera in this study also indicated that the disturbance in the GM in SDD children was closely correlated with the alterations of several basic physiological metabolism processes, primarily involving amino acid, lipid, and vitamin metabolism. Moreover, some studies had found that these abnormal alterations further indicate changes in potential metabolic, environmental information processing, and genetic information processing in SDD children. For instance, the altered metabolism pathways (N-glycan biosynthesis and alanine aspartate and glutamate metabolism) in SDD children could affect the accumulation of lipopolysaccharides, metabolic disturbance, depletion of hormones, neurotransmitters (especially serotonin), and immune system modulators, which were linked to neurodegenerative disorders.69,70 The significant degradation of amino acids such as glutamate in the high-exposure group could induce an increase in neurotransmitter levels related to neurological diseases.71 Glutamate serves as a significant source of α-ketoglutarate (α-KG), which is involved in the metabolic process following isocitrate dehydrogenase (IDH) 1/2 mutation and is related to DNA methylation.72 Furthermore, alterations in nicotinate and nicotinamide metabolism affected the concentration of nicotinate in the gut, eliciting antioxidant and anti-inflammatory activity and displaying protective effects against neurodegenerative mechanisms associated with neurological problems.73 Changes in arachidonic acid metabolism with prenatal PM2.5 exposure might influence the production of arachidonic acid, crucial for brain development as it regulates ion channel activity, cell membrane fluidity, and optimal cognitive function, consistent with improved behavioral performance.74,75 Interventions targeting the microbiome might offer new preventive and therapeutic options for neurological disorders by modulating mediators of microbiome–gut–brain communication influenced by microbiome metabolism, including serotonin and SCFAs.76
Overall, this study possessed several advantages that substantiate the reliability and robustness of the current analysis. To our knowledge, this study was the first to evaluate the impact of prenatal PM2.5 exposure on meconium GM within a birth cohort and to explore the role of GM in the interrelation between prenatal PM2.5 exposure and childhood neurodevelopment. Second, repeated estimations of child neurodevelopment allowed us to determine the crucial window of susceptibility for PM2.5 exposure. Third, the validation of GM markers by the random forest model suggested that the altered intestinal microbiota may provide an underlying target to predict and prevent PM2.5-related neurological impairment via the gut–brain axis. Nevertheless, there are also some limitations to the present study. First, prenatal PM2.5 exposure estimation did not consider the individual spatial and temporal activity patterns, which might cause underlying exposure misclassification derived from outdoor and indoor variations. Moreover, due to a lack of sufficient fecal samples at 2 months of age, temporal changes in the gut microbiome of participants were not fully investigated. Finally, although this study provided an underlying therapeutic target based on epidemiological evidence, more conclusive and direct evidence is required. Further research studies are warranted to confirm our findings in toxicological and epidemiological studies in vivo and in vitro.
5. Conclusion
The current study was the first to demonstrate that prenatal PM2.5 exposure could induce dysbiosis in the neonatal gut microbiota, affecting bacterial composition, richness, and evenness of GM involved in various biological pathways. It also suggested an underlying association of prenatal PM2.5 exposure with the early life neurodevelopment in offspring. Additionally, the neonatal gut microbiome may play a role in children’s neurodevelopmental delays resulting from prenatal PM2.5 exposure, particularly involving genera including Ruminococcus gnavus group, Romboutsia, Burkholderiaceae Caballeronia Paraburkholderia, Blautia, Alistipes, Parabacteroides, and Bacteroides. Our findings suggested that the neonatal gut microbiome may serve as an early biomarker to predict neurodevelopmental toxicity associated with prenatal PM2.5 exposure. These results, including altered microbial colonization and KEGG functional pathways, provided novel insights into understanding the influence of prenatal PM2.5 on child neurodevelopment and potential directions for elucidating the underlying mechanism and therapeutic intervention for air pollution-related neurological toxicity effects. Furthermore, these results further support the prospective development of evidence-based intervention strategies targeting the GM to prevent or cure neurological developmental problems in early life. Overall, it is essential to further validate our conclusions through additional in vitro and in vivo research.
Acknowledgments
We thank the participating exposure estimators and the principal investigators and are very grateful for the contributions of all investigators and quality controllers who participated in the Shanghai MCPC study.
Data Availability Statement
Data is available in this published study and its additional information files, which include all database generated or analyzed in this work.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/envhealth.4c00050.
Demographic characteristics of the study subjects for 16sRNA sequencing; results of child neurodevelopmental assessments and child suspected neurodevelopment delay (SDD) by Ages and Stages Questionnaire (ASQ-3); prenatal individual PM2.5 exposure of the entire pregnancy and children’s ASQ scores; microbiota structures of neonatal feces samples from the prenatal PM2.5 high-exposure group and low-exposure group; microbiota structures of neonatal feces samples from the SDD group and non-SDD group; LEFSe analysis on GM between low- and high-PM2.5 exposure groups during pregnancy; association of KEGG functional pathways with prenatal PM2.5 exposure analyzed using MaAsLin2; Spearman’s correlation analysis between KEGG functional pathways and prenatal PM2.5 exposure (PDF)
Author Contributions
⊥ These authors contribute to this work equally.
Author Contributions
Y.L.: Writing-original draft, visualization, formal analysis, methodology, software, data curation; L.Z.: Methodology, investigation, software; J.W.: Modification, data curation, methodology; X.S.: Investigation, resources; J.L.: Methodology, supervision; Y.G.: Investigation, resources; H.W.: Investigation, resources; Y.Z.: Investigation, resources; Y.X.: Investigation, resources; W.C.: Investigation, resources; P.W.: Methodology, investigation, funding acquisition; Y.Z.: Methodology, conceptualization, data curation, resources, project administration, supervision, funding acquisition.
This study was supported by the National Natural Science Foundation of China (Grant No. 82273585, 81872581), the National Key Research and Development Program of China (Grant No. 2019YFE0114500, 2022YFC2705004), and the China Postdoctoral Science Foundation (Grant No. 2022T150127, 2021M700797).
The authors declare no competing financial interest.
Notes
Ethics approval of this work was granted by the Institutional Review Boards (IRB) of Fudan University (IRB#2015-TYSQ-12-1; IRB#2016-04-0587).
Supplementary Material
References
- Murray C. J. L.; Zheng P.; Abbafati C; Aravkin A. Y.; Abbas K. M.; Abbasi-Kangevari M.; Abd-Allah F.; Abdelalim A.; Abdollahi M.; Abdollahpour I.; et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396 (10258), 1223–1249. 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.; Zheng Y.; Lei Y.; Xue W.; Yan G.; Liu X.; et al. Air quality benefits of achieving carbon neutrality in China. Sci. Total Environ. 2021, 795, 148784. 10.1016/j.scitotenv.2021.148784. [DOI] [PubMed] [Google Scholar]
- Cheng J.; Tong D.; Zhang Q.; Liu Y.; Lei Y.; Yan G.; et al. Pathways of China’s PM2.5 air quality 2015–2060 in the context of carbon neutrality. Natl. Sci. Rev. 2021, 8 (12), nwab078. 10.1093/nsr/nwab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO Global Air Quality Guideline. 2021
- Tamana S. K.; Tun H. M.; Konya T.; Chari R. S.; Field C. J.; Guttman D. S.; et al. Bacteroides-dominant gut microbiome of late infancy is associated with enhanced neurodevelopment. Gut Microbes 2021, 13 (1), 1–17. 10.1080/19490976.2021.1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughman A.; Ponsonby A. L.; O’Hely M.; Symeonides C.; Collier F.; Tang M. L. K.; et al. Gut microbiota composition during infancy and subsequent behavioural outcomes. EBioMedicine 2020, 52, 102640. 10.1016/j.ebiom.2020.102640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aatsinki A. K.; Lahti L.; Uusitupa H. M.; Munukka E.; Keskitalo A.; Nolvi S.; et al. Gut microbiota composition is associated with temperament traits in infants. Brain Behav Immun 2019, 80, 849–858. 10.1016/j.bbi.2019.05.035. [DOI] [PubMed] [Google Scholar]
- Christian L. M.; Galley J. D.; Hade E. M.; Schoppe-Sullivan S.; Kamp Dush C.; Bailey M. T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav Immun 2015, 45, 118–27. 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A. L.; Xia K.; Azcarate-Peril M. A.; Goldman B. D.; Ahn M.; Styner M. A.; et al. Infant Gut Microbiome Associated With Cognitive Development. Biol. Psychiatry 2018, 83 (2), 148–159. 10.1016/j.biopsych.2017.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordillo J. E.; Korrick S.; Laranjo N.; Carey V.; Weinstock G. M.; Gold D. R.; et al. Association of the Infant Gut Microbiome With Early Childhood Neurodevelopmental Outcomes: An Ancillary Study to the VDAART Randomized Clinical Trial. JAMA Netw Open 2019, 2 (3), e190905. 10.1001/jamanetworkopen.2019.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk H. E.; Perera F.; Braun J. M.; Kingsley S. L.; Gray K.; Buckley J.; et al. Prenatal air pollution exposure and neurodevelopment: A review and blueprint for a harmonized approach within ECHO. Environ. Res. 2021, 196, 110320. 10.1016/j.envres.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas I.; Basagana X.; Cirach M.; Lopez-Vicente M.; Suades-Gonzalez E.; Garcia-Esteban R.; et al. Association between Early Life Exposure to Air Pollution and Working Memory and Attention. Environ. Health Perspect. 2019, 127 (5), 57002. 10.1289/EHP3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagalan L.; Bickford C.; Weikum W.; Lanphear B.; Brauer M.; Lanphear N.; et al. Association of Prenatal Exposure to Air Pollution With Autism Spectrum Disorder. JAMA Pediatr 2019, 173 (1), 86–92. 10.1001/jamapediatrics.2018.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church J. S.; Tijerina P. B.; Emerson F. J.; Coburn M. A.; Blum J. L.; Zelikoff J. T.; et al. Perinatal exposure to concentrated ambient particulates results in autism-like behavioral deficits in adult mice. Neurotoxicology 2018, 65, 231–240. 10.1016/j.neuro.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha S.; Yeung E.; Bell E.; Insaf T.; Ghassabian A.; Bell G.; et al. Prenatal and early life exposures to ambient air pollution and development. Environ. Res. 2019, 174, 170–175. 10.1016/j.envres.2019.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lertxundi A.; Andiarena A.; Martinez M. D.; Ayerdi M.; Murcia M.; Estarlich M.; et al. Prenatal exposure to PM2.5 and NO2 and sex-dependent infant cognitive and motor development. Environ. Res. 2019, 174, 114–121. 10.1016/j.envres.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Lertxundi A.; Baccini M.; Lertxundi N.; Fano E.; Aranbarri A.; Martinez M. D.; et al. Exposure to fine particle matter, nitrogen dioxide and benzene during pregnancy and cognitive and psychomotor developments in children at 15 months of age. Environ. Int. 2015, 80, 33–40. 10.1016/j.envint.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Lai G. C.; Yao L. J.; Aung T. T.; Shental N.; Rotter-Maskowitz A.; et al. Microbial exposure during early human development primes fetal immune cells. Cell 2021, 184 (13), 3394. 10.1016/j.cell.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.; Zhou Y.; Qin Y.; Li Y.; Yu L.; Li R.; et al. Sex-specific effects of PM2.5 maternal exposure on offspring’s serum lipoproteins and gut microbiota. Sci. Total Environ. 2020, 739, 139982. 10.1016/j.scitotenv.2020.139982. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Forster S. C.; Tsaliki E.; Vervier K.; Strang A.; Simpson N.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574 (7776), 117–121. 10.1038/s41586-019-1560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittinger K.; Zhao C.; Li Y.; Ford E.; Friedman E. S.; Ni J.; et al. Bacterial colonization reprograms the neonatal gut metabolome. Nat. Microbiol 2020, 5 (6), 838–847. 10.1038/s41564-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. J.; Lee S. Y.; Kim H. B.; Lee E.; Hong S. J. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res. 2014, 6 (5), 389–400. 10.4168/aair.2014.6.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete T. L.; Jones R. B.; Chen Z.; Kim J. S.; Habre R.; Lurmann F.; et al. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ. Res. 2018, 161, 472–478. 10.1016/j.envres.2017.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu E. A.; Comba I. Y.; Cho T.; Engen P. A.; Yazici C.; Soberanes S.; et al. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ. Pollut. 2018, 240, 817–830. 10.1016/j.envpol.2018.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S. Y.; Kaplan G. G.; Madsen K. L. Air pollution effects on the gut microbiota: a link between exposure and inflammatory disease. Gut Microbes 2014, 5 (2), 215–9. 10.4161/gmic.27251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld C. S. Gut Dysbiosis in Animals Due to Environmental Chemical Exposures. Front. Cell. Infect. Microbiol. 2017, 7, 396. 10.3389/fcimb.2017.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Yang J.; Saffari A.; Jacobs J.; Baek K. I.; Hough G.; et al. Ambient Ultrafine Particle Ingestion Alters Gut Microbiota in Association with Increased Atherogenic Lipid Metabolites. Sci. Rep. 2017, 7, 42906. 10.1038/srep42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Lung dosimetry: pulmonary clearance of inhaled particles. Aerosol Sci. Technol. 1993, 18, 279. 10.1080/02786829308959605. [DOI] [Google Scholar]
- Kreyling W. G.; Blanchard J. D.; Godleski J. J.; Haeussermann S.; Heyder J.; Hutzler P.; et al. Anatomic localization of 24- and 96-h particle retention in canine airways. J. Appl. Physiol (1985) 1999, 87 (1), 269–84. 10.1152/jappl.1999.87.1.269. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Wang P.; Zhou Y.; Xia B.; Zhu Q.; Ge W.; et al. Prenatal fine particulate matter exposure, placental DNA methylation changes, and fetal growth. Environ. Int. 2021, 147, 106313. 10.1016/j.envint.2020.106313. [DOI] [PubMed] [Google Scholar]
- Meng X.; Liu C.; Zhang L.; Wang W.; Stowell J.; Kan H.; Liu Y. Estimating PM2.5 concentrations in Northeastern China with full spatiotemporal coverage, 2005–2016. Remote Sens Environ 2021, 253, 112203. 10.1016/j.rse.2020.112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. Squires D. B.Ages & Stages Questionnaires[R], Third ed. (ASQ-3[TM]): A Parent-Completed Child-Monitoring System; Brookes Publishing Company: Baltimore, MD, 2009. [Google Scholar]
- Zheng S.; Fang J.; Bai G.; He X.; Hua M.; Zhu B.; et al. The association between parental risks and childhood development: findings from a community-based survey in East China. BMC Public Health 2023, 23 (1), 878. 10.1186/s12889-023-15702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C.; Wu X.; Huang K.; Yan S.; Li Z.; Xia X.; et al. Domain- and sex-specific effects of prenatal exposure to low levels of arsenic on children’s development at 6 months of age: Findings from the Ma’anshan birth cohort study in China. Environ. Int. 2020, 135, 105112. 10.1016/j.envint.2019.105112. [DOI] [PubMed] [Google Scholar]
- Wang P.; Zhao Y.; Li J.; Zhou Y.; Luo R.; Meng X.; et al. Prenatal exposure to ambient fine particulate matter and early childhood neurodevelopment: A population-based birth cohort study. Sci. Total Environ. 2021, 785, 147334. 10.1016/j.scitotenv.2021.147334. [DOI] [PubMed] [Google Scholar]
- Kozich J. J.; Westcott S. L.; Baxter N. T.; Highlander S. K.; Schloss P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79 (17), 5112–20. 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoc T.; Salzberg S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27 (21), 2957–63. 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso J. G.; Kuczynski J.; Stombaugh J.; Bittinger K.; Bushman F. D.; Costello E. K.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7 (5), 335–6. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guxens M.; Garcia-Esteban R.; Giorgis-Allemand L.; Forns J.; Badaloni C.; Ballester F.; et al. Air pollution during pregnancy and childhood cognitive and psychomotor development: six European birth cohorts. Epidemiology 2014, 25 (5), 636–47. 10.1097/EDE.0000000000000133. [DOI] [PubMed] [Google Scholar]
- Guxens M.; Lubczynska M. J.; Muetzel R. L.; Dalmau-Bueno A.; Jaddoe V. W. V.; Hoek G.; et al. Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biol. Psychiatry 2018, 84 (4), 295–303. 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Yorifuji T.; Kashima S.; Diez M. H.; Kado Y.; Sanada S.; Doi H. Prenatal exposure to outdoor air pollution and child behavioral problems at school age in Japan. Environ. Int. 2017, 99, 192–198. 10.1016/j.envint.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Tan L.; Zou J.; Zhang Y.; Yang Q.; Shi H. A Longitudinal Study of Physical Activity to Improve Sleep Quality During Pregnancy. Nat. Sci. Sleep 2020, 12, 431–442. 10.2147/NSS.S253213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogundele M. O.; Morton M. Classification, prevalence and integrated care for neurodevelopmental and child mental health disorders: A brief overview for paediatricians. World J. Clin Pediatr 2022, 11 (2), 120–135. 10.5409/wjcp.v11.i2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. H.; Gold D. R.; Rifas-Shiman S. L.; Melly S. J.; Zanobetti A.; Coull B. A.; et al. Prenatal and Childhood Traffic-Related Pollution Exposure and Childhood Cognition in the Project Viva Cohort (Massachusetts, USA). Environ. Health Perspect 2015, 123 (10), 1072–8. 10.1289/ehp.1408803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J. A.; Mazmanian S. K. Emerging evidence linking the gut microbiome to neurologic disorders. Genome Med. 2018, 10 (1), 98. 10.1186/s13073-018-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson T. R.; Mazmanian S. K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17 (5), 565–76. 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. Y.; Kim J. M.; Lee H. L.; Go M. J.; Joo S. G.; Kim J. H. Codium fragile Suppresses PM(2.5)-Induced Cognitive Dysfunction by Regulating Gut-Brain Axis via TLR-4/MyD88 Pathway. Int. J. Mol. Sci. 2023, 24 (16), 12898. 10.3390/ijms241612898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q.; Yang Q.; Liu X. The microbiota-gut-brain axis and neurodevelopmental disorders. Protein Cell 2023, 14 (10), 762–775. 10.1093/procel/pwad026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudino Dos Santos J. C.; Lima M. P. P.; Brito G. A. C.; Viana G. S. B. Role of enteric glia and microbiota-gut-brain axis in parkinson disease pathogenesis. Ageing Res. Rev. 2023, 84, 101812. 10.1016/j.arr.2022.101812. [DOI] [PubMed] [Google Scholar]
- Cryan J. F.; O’Riordan K. J.; Sandhu K.; Peterson V.; Dinan T. G. The gut microbiome in neurological disorders. Lancet Neurol 2020, 19 (2), 179–194. 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- Barker D. J. The origins of the developmental origins theory. J. Intern Med. 2007, 261 (5), 412–7. 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Lai S. K.; Wang Y. Y.; Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv Rev. 2009, 61 (2), 158–71. 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalile B.; Van Oudenhove L.; Vervliet B.; Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol Hepatol 2019, 16 (8), 461–478. 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- Keogh C. E.; Kim D. H. J.; Pusceddu M. M.; Knotts T. A.; Rabasa G.; Sladek J. A.; et al. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav Immun 2021, 91, 437–450. 10.1016/j.bbi.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Q.; Jiang Z.; Shi H.; Zou J.; Lu W.; Xiao X.; et al. Associations of maternal prenatal emotional symptoms with neurodevelopment of children and the neonatal meconium microbiota: A prospective cohort study. Psychoneuroendocrinology 2022, 142, 105787. 10.1016/j.psyneuen.2022.105787. [DOI] [PubMed] [Google Scholar]
- Maldonado Weng J.; Parikh I.; Naqib A.; York J.; Green S. J.; Estus S.; LaDu M. J. Synergistic effects of APOE and sex on the gut microbiome of young EFAD transgenic mice. Mol. Neurodegener 2019, 14 (1), 47. 10.1186/s13024-019-0352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie A.; Ensink E.; Li P.; Gordevicius J.; Marshall L. L.; George S.; et al. Bacterial Butyrate in Parkinson’s Disease Is Linked to Epigenetic Changes and Depressive Symptoms. Mov Disord 2022, 37 (8), 1644–1653. 10.1002/mds.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd A. T.; Berding K.; Wang M.; Donovan S. M.; Dilger R. N. Serum cortisol mediates the relationship between fecal Ruminococcus and brain N-acetylaspartate in the young pig. Gut Microbes 2017, 8 (6), 589–600. 10.1080/19490976.2017.1353849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z.; Qiu Y.; Wang J.; Fang Y.; Zhang Y.; Chen H.; et al. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: A cross sectional study. J. Neuroimmunol 2020, 339, 577126. 10.1016/j.jneuroim.2019.577126. [DOI] [PubMed] [Google Scholar]
- Lv Z.; Liu R.; Su K.; Gu Y.; Fang L.; Fan Y.; et al. Acupuncture ameliorates breast cancer-related fatigue by regulating the gut microbiota-gut-brain axis. Front Endocrinol (Lausanne) 2022, 13, 921119. 10.3389/fendo.2022.921119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q.; Li X.; Wang M.; Qiao X.; Huang F.; Hu C.; et al. Walnut green husk ethanol extract improves gut microbiota and their metabolites associated with NLRP3 in non-alcoholic steatohepatitis. Food Funct 2022, 13 (11), 6387–6403. 10.1039/D2FO00012A. [DOI] [PubMed] [Google Scholar]
- Miquel S.; Martin R.; Rossi O.; Bermudez-Humaran L. G.; Chatel J. M.; Sokol H.; et al. Faecalibacterium prausnitzii and human intestinal health. Curr. Opin Microbiol 2013, 16 (3), 255–61. 10.1016/j.mib.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Guzzardi M. A.; Ederveen T. H. A.; Rizzo F.; Weisz A.; Collado M. C.; Muratori F.; et al. Maternal pre-pregnancy overweight and neonatal gut bacterial colonization are associated with cognitive development and gut microbiota composition in pre-school-age offspring. Brain Behav Immun 2022, 100, 311–320. 10.1016/j.bbi.2021.12.009. [DOI] [PubMed] [Google Scholar]
- Averina O. V.; Kovtun A. S.; Polyakova S. I.; Savilova A. M.; Rebrikov D. V.; Danilenko V. N. The bacterial neurometabolic signature of the gut microbiota of young children with autism spectrum disorders. J. Med. Microbiol 2020, 69 (4), 558–571. 10.1099/jmm.0.001178. [DOI] [PubMed] [Google Scholar]
- Dan Z.; Mao X.; Liu Q.; Guo M.; Zhuang Y.; Liu Z.; et al. Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes 2020, 11 (5), 1246–1267. 10.1080/19490976.2020.1747329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y.; Zhang L.; Wang X.; Yi Y.; Shan Y.; Liu B. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369 (1), fnac072 10.1093/femsle/fnac072. [DOI] [PubMed] [Google Scholar]
- Maes M.; Vasupanrajit A.; Jirakran K.; Klomkliew P.; Chanchaem P.; Tunvirachaisakul C.; Payungporn S. Adverse childhood experiences and reoccurrence of illness impact the gut microbiome, which affects suicidal behaviours and the phenome of major depression: towards enterotypic phenotypes. Acta Neuropsychiatr 2023, 12, 1240. 10.3390/cells12091240. [DOI] [PubMed] [Google Scholar]
- Fu P.; Bai L.; Cai Z.; Li R.; Yung K. K. L. Fine particulate matter aggravates intestinal and brain injury and affects bacterial community structure of intestine and feces in Alzheimer’s disease transgenic mice. Ecotoxicol Environ. Saf 2020, 192, 110325. 10.1016/j.ecoenv.2020.110325. [DOI] [PubMed] [Google Scholar]
- van der Lee S. J.; Teunissen C. E.; Pool R.; Shipley M. J.; Teumer A.; Chouraki V.; et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement 2018, 14 (6), 707–722. 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Duran-Aniotz C.; Hetz C. Glucose Metabolism: A Sweet Relief of Alzheimer’s Disease. Curr. Biol. 2016, 26 (17), R806–9. 10.1016/j.cub.2016.07.060. [DOI] [PubMed] [Google Scholar]
- Tran S. M.; Mohajeri M. H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13 (3), 732. 10.3390/nu13030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y.; Yang H.; Chen L. Metabolic regulation on the immune environment of glioma through gut microbiota. Semin. Cancer Biol. 2022, 86 (2), 990–997. 10.1016/j.semcancer.2021.05.005. [DOI] [PubMed] [Google Scholar]
- Xicoy H.; Wieringa B.; Martens G. J. M. The Role of Lipids in Parkinson’s Disease. Cells 2019, 8 (1), 27. 10.3390/cells8010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A.; Metherel A. H.; Fiabane L.; Buddenbaum N.; Bazinet R. P.; Shaikh S. R. Do Eicosapentaenoic Acid and Docosahexaenoic Acid Have the Potential to Compete against Each Other?. Nutrients 2020, 12 (12), 3718. 10.3390/nu12123718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L.; Cuccioloni M.; Gong C.; Cecarini V.; Spina M.; Zheng Y.; et al. Gut microbiota modulation in Alzheimer’s disease: Focus on lipid metabolism. Clin Nutr 2022, 41 (3), 698–708. 10.1016/j.clnu.2022.01.025. [DOI] [PubMed] [Google Scholar]
- Liu P.; Wu L.; Peng G.; Han Y.; Tang R.; Ge J.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 2019, 80, 633–643. 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available in this published study and its additional information files, which include all database generated or analyzed in this work.