Key Points
Question
What are the risk factors for, characteristics of, and clinical consequences of amyloid-related imaging abnormalities-edema (ARIA-E) in phase 3 trials of gantenerumab in early Alzheimer disease (AD)?
Findings
Severity of amyloid-beta (Aβ) neuropathology and comorbid cerebrovascular pathology may be important baseline risk factors for ARIA-E, in addition to apolipoprotein E ε4. ARIA-E had no long-term impact on cognitive/functional scale performance in most participants.
Meaning
These results demonstrate that clinicians may wish to consider potential risk factors for ARIA-E (eg, severity of Aβ neuropathology and comorbid cerebrovascular pathology) when prescribing anti-Aβ monoclonal antibodies for early AD and developing individualized safety monitoring plans.
This study describes the characteristics of amyloid-related imaging abnormalities and risk factors and clinical consequences of amyloid-related imaging abnormalities-edema.
Abstract
Importance
Data from 2 phase 3 studies of gantenerumab, GRADUATE I/II, and their open-label extensions represent a resource to further characterize amyloid-related imaging abnormalities (ARIA), including long-term sequelae.
Objectives
To describe the characteristics of ARIA and risk factors and clinical consequences of ARIA-edema (ARIA-E).
Design, Setting, and Participants
Secondary data collection from the GRADUATE I/II phase 3 randomized, double-blind, placebo-controlled, 116-week parallel-group studies and their open-label extensions, including PostGraduate, with up to 210 (mean, 125) weeks of total gantenerumab treatment were conducted between 2018 and 2023. The study included multicenter trials at 288 sites across 30 countries. GRADUATE I/II enrolled 985 and 980 participants, respectively, with early symptomatic Alzheimer disease (AD) and amyloid-beta (Aβ) pathology who were aged 50 to 90 years. PostGraduate enrolled 1382 participants (671 previously randomized to gantenerumab). Data were analyzed from November 2, 2022, to October 10, 2023.
Interventions
GRADUATE I/II participants were randomized 1:1 to gantenerumab or placebo. Nine-month uptitration was used to mitigate ARIA risk.
Main outcomes and measures
Postbaseline safety monitoring, including brain magnetic resonance imaging (MRI) findings, and adverse events and cognitive assessments.
Results
The safety-evaluable MRI population of GRADUATE I/II comprised 1939 participants (mean age, 71.7 years; 1105 female [57.0%]). Severity of AD–related Aβ neuropathology (lower cerebrospinal fluid [CSF] Aβ42, hazard ratio [HR] for CSF Aβ42: 0.4; 95% CI, 0.2-0.7) and comorbid cerebrovascular pathology (Fazekas score: HR, 1.6; 95% CI, 1.3-2.0; total superficial siderosis count: HR, 1.9; 95% CI, 1.3-2.6; total microhemorrhage count: HR, 1.3; 95% CI, 1.0-1.5) may be important baseline risk factors for ARIA-E, in addition to apolipoprotein E (APOE) ε4 status (APOE ε4 heterozygous carrier: HR, 2.0; 95% CI, 1.4-2.8 and APOE ε4 homozygous carrier: HR, 4.7; 95% CI, 3.2-6.7). At the group level, ARIA-E did not impact long-term cognitive and functional performance (relative difference in adjusted means for Clinical Dementia Rating–Sum of Boxes was −9% in pooled GRADUATE analysis at week 116 and when censored at first ARIA-E). While taking gantenerumab, ARIA-E and ARIA-hemosiderin occurred in 24.9% (247 of 993) and 22.9% (227 of 993) participants, respectively; first ARIA-E occurred by week 64 in 86.2% (213 of 247) of participants with ARIA-E. Narratives are provided for all serious symptomatic ARIA-E cases.
Conclusions and Relevance
These results show that in addition to APOE ε4 allele count, severity of Aβ neuropathology and comorbid cerebrovascular pathology may be relevant for clinicians prescribing anti-Aβ monoclonal antibodies for early AD and developing individualized safety monitoring plans. Evaluation of these risk factors in other anti-Aβ monoclonal antibodies is recommended.
Trial registrations
ClinicalTrials.gov Identifiers: NCT03444870, NCT03443973, NCT04374253.
Introduction
Amyloid-beta (Aβ) processing and deposition play a critical role in the Alzheimer disease (AD) pathogenesis. Anti-Aβ monoclonal antibodies (mAbs) lecanemab and donanemab demonstrated significant reductions vs placebo in clinical progression of early symptomatic AD (mild cognitive impairment due to AD and mild AD) in phase 3 clinical trials, in parallel with significant Aβ plaque clearance. These drugs are US Food and Drug Administration licensed for the treatment of early symptomatic AD.
Amyloid-related imaging abnormalities (ARIA) comprise vasogenic edema and/or sulcal effusions (ARIA-edema [ARIA-E]; best visualized on magnetic resonance imaging [MRI] fluid-attenuated inversion recovery sequences) and microhemorrhages and/or superficial siderosis (SS) (ARIA-hemosiderin [ARIA-H]; on iron-sensitive MRI sequences, such as gradient-recalled echo/T2*-weighted or susceptibility-weighted imaging). While ARIA may develop spontaneously in relation to AD or cerebral amyloid angiopathy (CAA), it is also the main adverse effect of anti-Aβ mAbs (absolute rates vary between molecules) and was reported for a microglial-receptor triggering receptor expressed on myeloid cells 2–binding mAb in AD. ARIA pathophysiology hypotheses include Aβ clearance-related temporarily increased cerebral vascular permeability; reactive immune cell–mediated inflammation, and complement cascade activation by anti-Aβ mAb-vascular Aβ complexes.
Since ARIA represents a class effect of first-generation anti-Aβ mAbs, understanding ARIA risk factors and symptomatology may inform future clinical decision-making and MRI monitoring. It is also important for ensuring individuals with AD are informed of anti-Aβ mAbs risks.
Key efficacy and safety outcomes from 2 global phase 3 gantenerumab studies, GRADUATE I and II (NCT03444870/NCT03443973), and their open-label rollover studies, including PostGraduate (NCT04374253), have been reported previously. Although gantenerumab was not associated with a significant reduction in clinical decline in participants with early AD (difference in Clinical Dementia Rating–Sum of Boxes [CDR-SB] at week 116 vs placebo: GRADUATE I, −0.31; 95% CI, −0.66 to 0.05 and GRADUATE II, −0.19; 95% CI, 0.55-0.17; favoring gantenerumab), these studies provide a rich resource to further characterize ARIA and its longer-term consequences. While in GRADUATE I/II incidence of ARIA-H was higher in gantenerumab than placebo arms, incidence of new isolated ARIA-H was comparable, similar to observations for other in-class mAbs; the higher incidence of ARIA-H in the active arms is driven by ARIA-H concurrent with ARIA-E. As such, while we report key ARIA-H characteristics, we focus on the risk factors, descriptive characteristics, and clinical consequences of ARIA-E.
Methods
The design of the identical phase 3 randomized, double-blind, placebo-controlled, parallel-group GRADUATE I and II trials has been previously published. Briefly, GRADUATE I and II enrolled 985 and 980 participants, respectively, with early symptomatic AD and confirmed Aβ pathology who were aged 50 to 90 years. More than 5 microhemorrhages and/or focal SS areas (though also more than 3 focal SS areas), overall Fazekas score of 3, and anticoagulation constituted exclusion criteria. Single- or dual-antiplatelet therapy was allowed. PostGraduate (NCT04374253) was an open-label, multicenter, rollover study evaluating the long-term safety, tolerability, and efficacy of gantenerumab in participants from the GRADUATE I/II trials. Trial conduct followed the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use E6 guideline for Good Clinical Practice and the Declaration of Helsinki and applicable laws and regulations. An independent data monitoring committee provided oversight. All trial participants provided written informed consent.
In GRADUATE I/II, participants were randomized 1:1 to subcutaneously administered gantenerumab or placebo for up to 116 weeks with a 9-month uptitration regimen to the 510-mg every 2 weeks target dose to mitigate dose-dependent ARIA risk. Safety MRIs were performed at baseline, prior to each dose uptitration, regularly on target dose, and as determined by investigators and evaluated by a single central neuroradiologist (Figure 1). ARIA dosing intervention rules are provided in eFigure 1 in Supplement 1. Participants followed an identical dosing regimen and MRI frequency regardless of APOE ε4 status. ARIA-E radiological severity was determined using Barkhof Grand Total Score ([BGTS] range, 0-60).
Figure 1. GRADUATE I and II: Study Drug Dosing Regimen and Routine Magnetic Resonance Imaging (MRI) Schedule.
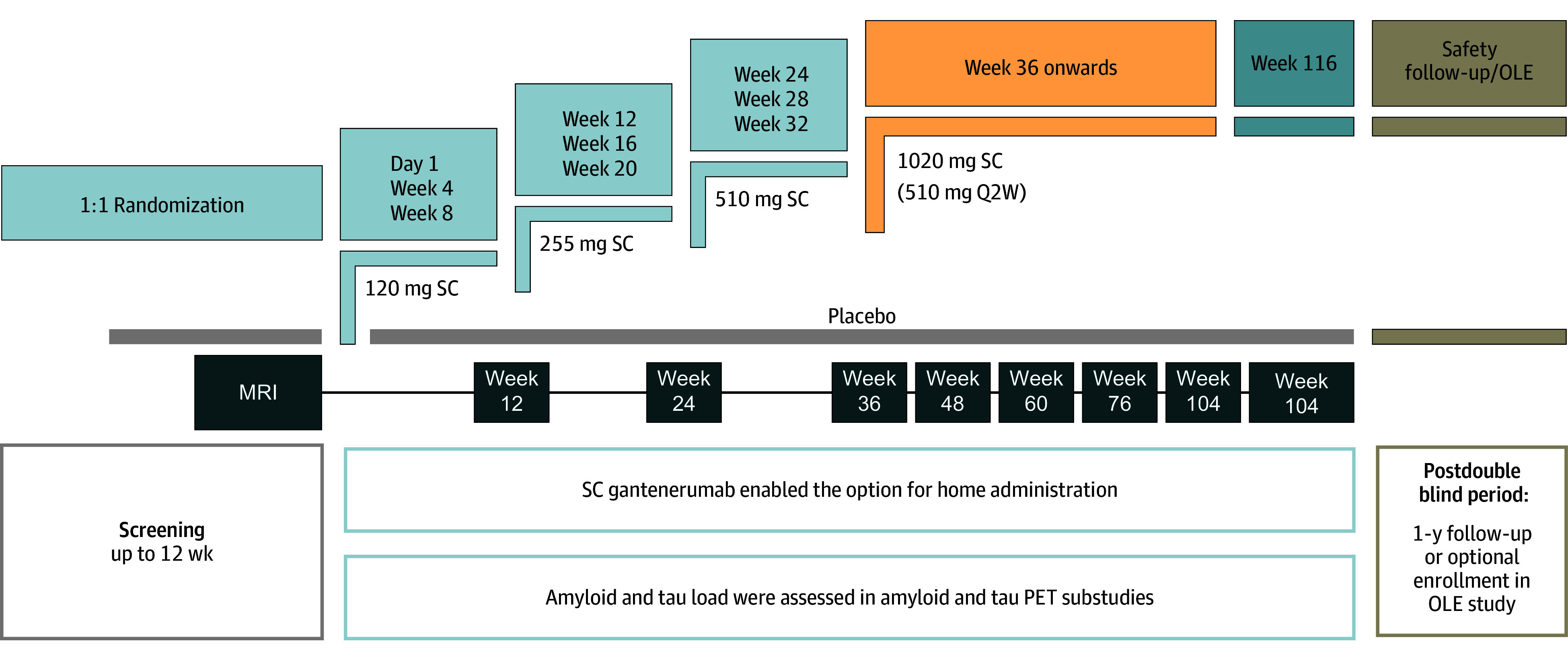
OLE indicates open-label extension; PET, positron emission tomography; Q2W, every 2 weeks; SC, subcutaneous.
After 116 weeks of double-blind treatment, eligible participants could receive open-label gantenerumab, initiated under the GRADUATE protocol or after directly entering PostGraduate (eFigure 2 in Supplement 1) with up to 210 (mean, 125) weeks of gantenerumab treatment in participants randomized to gantenerumab in the double-blind phase. Otherwise, 14- and 50-week postdosing follow-up was required.
Safety outcomes included adverse events (AEs), serious AEs, and MRI findings. Since only selected ARIA MRI findings were reportable as AEs (ie, symptomatic, leading to dosing intervention, or otherwise clinically significant), ARIA analyses reported are based on the MRI safety-evaluable dataset. Symptomatic ARIA-E was defined by central nervous system (CNS) AE(s) temporally associated with ARIA-E MRI findings, regardless of the investigator’s causality attribution.
Efficacy scales, including CDR-SB, the Alzheimer’s Disease Assessment Scale, Cognition Subscale 13 (ADAS-Cog13), Alzheimer’s Disease Cooperative Study Group Activities of Daily Living (ADCS-ADL), and Functional Activities Questionnaire (FAQ), and amyloid positron emission tomography, volumetric MRI, and plasma and cerebrospinal fluid (CSF) biomarkers were collected and reported. Measures were implemented to mitigate ARIA-related bias in efficacy assessments.
Statistical Analysis
As GRADUATE I/II were identically designed with efficacy and safety results concordant, the pooled dataset from the double-blind study periods was used that included all participants who received at least 1 study drug dose, followed by at least 1 MRI (safety-evaluable MRI population). Postbaseline data covered the double-blind period from the first study drug dose up to 14 weeks after the last dose, but no later than the day before the first open-label gantenerumab dose for participants who entered the open-label period. Data from the open-label period and PostGraduate were used to repeat the assessment of time to first ARIA-E event in participants in the gantenerumab arm in the double-blind period. Analyses were prespecified in a statistical analysis plan, except the analyses of baseline risk factors. Additional descriptive ARIA analyses are provided beyond those reported previously, including stratification by APOE ε4 allele count or baseline microhemorrhage/SS presence. We used SAS version 9.4 (SAS Institute) r 4.3.1 (The R Project for Statistical Computing) for analysis.
For the analysis of risk factors for ARIA-E/ARIA-E with temporally concurrent new ARIA-H, a broad set of baseline variables for testing was developed (eMethods in Supplement 1). For the assessment of the long-term impact of ARIA-E on CDR-SB (pooled GRADUATE dataset, reported previously by study), ADAS-Cog13, ADCS-ADL, and FAQ, a mixed-effect model of repeated measures was used, with all outcome data included as well as with outcome data censored upon first ARIA-E.
Results
The safety-evaluable MRI population of the GRADUATE trials comprised 1939 participants (placebo, 946; gantenerumab, 993). Baseline characteristics were comparable between treatment groups, with a mean age of 71.7 (SD, 7.7) years (eTable 1 in Supplement 1). Most participants were female (1105 of 1939 [57.0%]), had mild cognitive impairment (1062 of 1939 [54.8%]), and received symptomatic AD treatment (1240 of 1939 [64.0%]). Half of the participants were heterozygous APOE ε4 carriers (964 of 1939 [49.7%]), followed by noncarriers (645 of 1939 [33.3%]) and homozygous carriers (330 of 1939 [17.0%]). At baseline, 97 of 993 participants (9.8%) in the gantenerumab arm had microhemorrhages and/or SS (80 of 993 [8.1%] had 1 to 5 microhemorrhages and 25 of 993 [2.5%] 1 to 3 focal areas of SS). Participant flow and baseline demographics/characteristics for PostGraduate are provided in eFigure 3 and eTable 2 in Supplement 1.
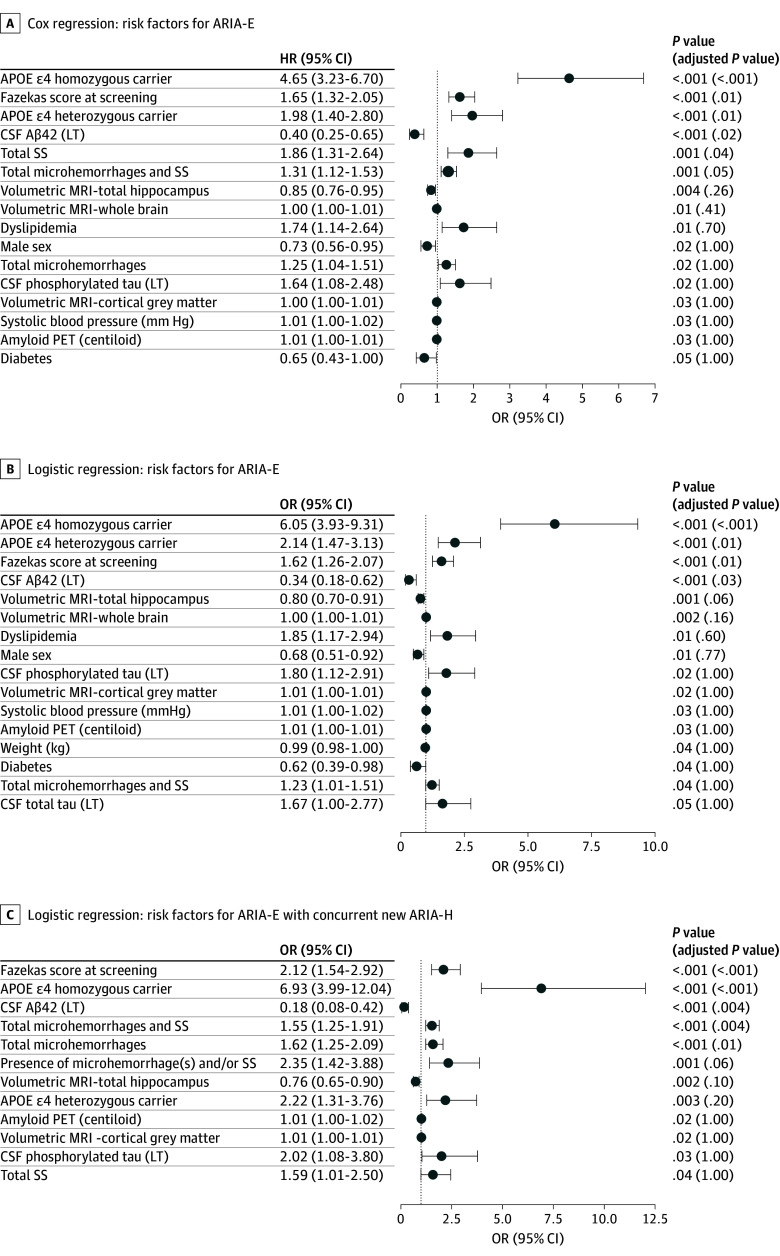
In univariate models, higher APOE ε4 allele number, higher Fazekas score, and lower CSF Aβ42 were associated with increased risk of ARIA-E (Figure 2A and B) and ARIA-E with concurrent ARIA-H (Figure 2C), and remained significant following Bonferroni correction. In proportional hazards modeling, higher number of SS at baseline was associated with higher risk of ARIA-E (Figure 2A). In models of ARIA-E with concurrent new ARIA-H, larger baseline numbers of microhemorrhages and/or SS and microhemorrhages alone were associated with increased risk (Figure 2C; descriptive statistics for all 57 potential baseline risk factors in eTable 3 in Supplement 1).
Figure 2. Risk Factors for Amyloid-Related Imaging Abnormalities-Edema: Univariate Analyses for Baseline Variables.
All the variables are measured at baseline unless indicated otherwise. Univariate analyses for baseline variables with an unadjusted P value of less than .05. Apolipoprotein E (APOE) ε4, dyslipidemia, sex, diabetes, and presence of amyloid-related imaging abnormalities-hemosiderin are treated as categorical variables with associated reference levels of APOE ε4 noncarrier, no dyslipidemia, female, no diabetes, and absent. Aβ indicates amyloid-beta; CSF, cerebrospinal fluid; HR, hazard ratio; LT, log 2 transformed; MRI, magnetic resonance imaging; OR, odds ratio; PET, positron emission tomography; SS, superficial siderosis.
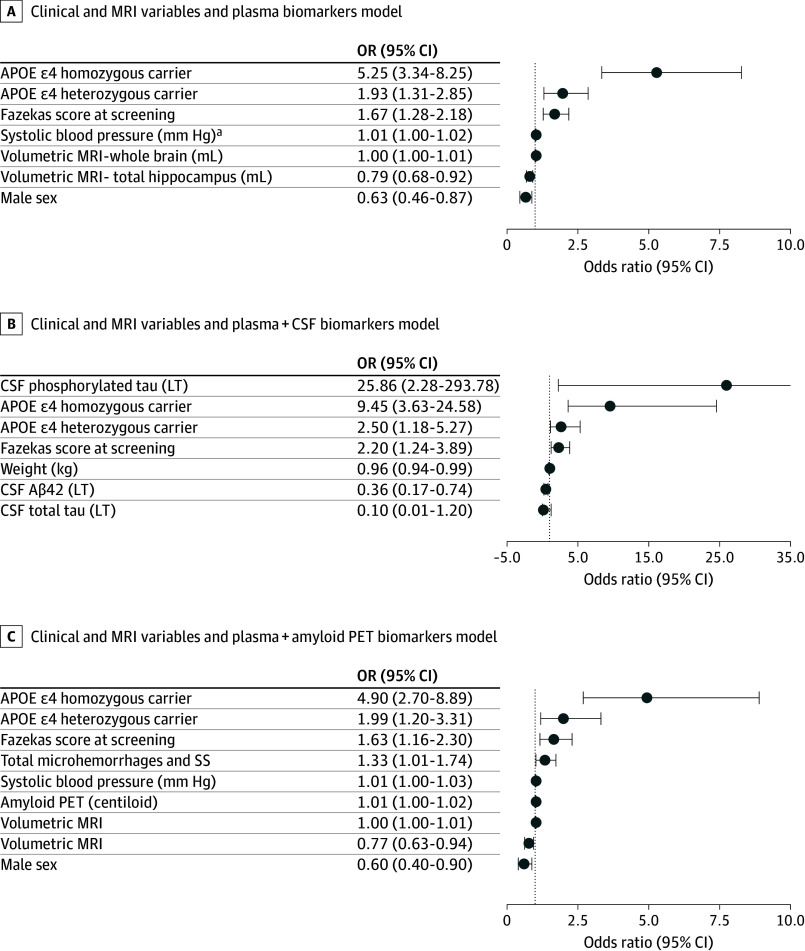
Multivariate models were built from variables with an unadjusted P value of less than .05 to optimize model fit; retained variables contain additional information on ARIA-E risk relative to other variables in the model. Variables remaining in multivariate models of ARIA-E risk following stepwise selection are shown in Figure 3. For multivariate model results for ARIA-E with concurrent ARIA-H, see eTable 4 in Supplement 1.
Figure 3. Multivariate Modeling With Stepwise Logistic Regression for Baseline Variables.
Apolipoprotein E (APOE) ε4 and sex are treated as categorical variables with APOE ε4 noncarrier and female as the reference levels. In addition to those meeting criteria for univariate significance, hippocampal volume, whole-brain volume, systolic blood pressure, and sex were retained in multivariate models of amyloid-related imaging abnormalities-edema risk. In models containing cerebrospinal fluid (CSF) measures, CSF pTau, CSF tTau, and weight were also retained; whereas in amyloid positron emission tomography (PET) models, amyloid PET centiloid, and total number of amyloid-related imaging abnormalities-hemosiderin remained in multivariate models. OR indicates odds ratio.
aPer 1 mm Hg; per 10 mm Hg, the OR would be 1.1.
In the GRADUATE gantenerumab dataset, ARIA-E and ARIA-H occurred in 247 of 993 (24.9%) and 227 of 993 (22.9%) participants, respectively. Overall incidences of ARIA-E and symptomatic ARIA-E at the study-population level increased with the APOE ε4 count. This pattern was also observed for ARIA-E with new concurrent ARIA-H and recurrent ARIA-E (Table 1). However, within individuals who developed ARIA-E, there was no association between the number of APOE ε4 copies and symptomatic or serious symptomatic ARIA-E rate. The characteristics of serious symptomatic ARIA-E cases are presented in eTable 5 and eMethods in Supplement 1.
Table 1. Selected Descriptive Characteristics of Amyloid-Related Imaging Abnormalities (ARIA) Magnetic Resonance Imaging (MRI) Findings by Apolipoprotein E (APOE) ε4 Status.
| MRI findings by APOE genotype | No. (%) of participants | |||||
|---|---|---|---|---|---|---|
| Placebo (n = 946) | Gantenerumab (n = 993) | |||||
| APOE ε4 noncarrier (n = 310) | APOE ε4 heterozygous (n = 486) | APOE ε4 homozygous (n = 150) | APOE ε4 noncarrier (n = 335) | APOE ε4 heretozygous (n = 478) | APOE ε4 homozygous (n = 180) | |
| ARIA-E | ||||||
| ARIA-E MRI findings by APOE genotype | ||||||
| Totala | 9 (2.9) | 10 (2.1) | 7 (4.7) | 44 (13.1) | 117 (24.5) | 86 (47.8) |
| No. of episodes | 10 | 11 | 8 | 66 | 170 | 144 |
| Symptomatic ARIA-E | ||||||
| Totalb,a | 1 (0.3) | 1 (0.2) | 0 (0) | 13 (3.9) | 18 (3.8) | 19 (10.6) |
| Those with ARIA-E | 1/9 (11.1) | 1/10 (10.0) | 0/7 (0) | 13/44 (29.5) | 18/117 (15.4) | 19/86 (22.1) |
| Serious symptomatic ARIA-E | ||||||
| Totalb,c | 0 (0) | 0 (0) | 0 (0) | 6 (1.8) | 2 (0.4) | 3 (1.7) |
| Those with ARIA-E | 0/9 (0) | 0/10 (0) | 0/7 (0) | 6/44 (13.6) | 2/117 (1.7) | 3/86 (3.5) |
| ARIA-E with concurrent new ARIA-Hd | ||||||
| Total | 1 (0.3) | 4 (0.8) | 2 (1.3) | 20 (6.0) | 59 (12.3) | 55 (30.6) |
| Those with ARIA-E | 1/9 (11.1) | 4/10 (40.0) | 2/7 (28.6) | 20/44 (45.5) | 59/117 (50.4) | 55/86 (64.0) |
| ARIA-E with concurrent new microhemorrhaged | ||||||
| Total | 1 (0.3) | 2 (0.4) | 0 (0.0) | 17 (5.1) | 43 (9.0) | 50 (27.8) |
| Those with ARIA-E | 1/9 (11.1) | 2/10 (20.0) | 0/7 (0) | 17/44 (38.6) | 43/117 (36.8) | 50/86 (58.1) |
| ARIA-E with concurrent new superficial siderosisd | ||||||
| Total | 1 (0.3) | 2 (0.4) | 2 (1.3) | 14 (4.2) | 38 (7.9) | 25 (13.9) |
| Those with ARIA-E | 1/9 (11.1) | 2/10 (20.0) | 2/7 (28.6) | 14/44 (31.8) | 38/117 (32.5) | 25/86 (29.1) |
| Recurrent ARIA-E | ||||||
| Total | 1 (0.3) | 1 (0.2) | 1 (0.7) | 14 (4.2) | 40 (8.4) | 41 (22.8) |
| Those with ARIA-E at risk of recurrence | 1/6 (16.7) | 1/7 (14.3) | 1/7 (14.3) | 14/30 (46.7) | 40/98 (40.8) | 41/66 (62.1) |
| Time to onset of first ARIA-E, mean (SD), wk | 59.4 (39.7) | 60.9 (34.8) | 49.6 (35.9) | 49.6 (25.6) | 48.8 (20.7) | 45.1 (19.0) |
| Radiological severity of all ARIA-E episodes (BGTS) | ||||||
| Mean (SD) | 3.8 (3.8) | 4.2 (2.9) | 2.9 (2.2) | 8.5 (7.9) | 7.9 (6.1) | 10.5 (8.8) |
| ARIA-E episodes of severity ≥4e | 3/10 (30.0) | 5/11 (45.5) | 3/8 (37.5) | 46/66 (69.7) | 131/170 (77.1) | 116/144 (80.6) |
| Resolution time of all ARIA-E episodes | ||||||
| No. all resolved ARIA-E episodes | 10 | 10 | 8 | 62 | 164 | 139 |
| Mean resolution time (SD), wk | 8.7 (7.1) | 8.8 (4.7) | 4.4 (1.5) | 10.9 (7.3) | 10.3 (8.2) | 13.0 (9.7) |
| Median resolution time, wk | 5.9 | 8.0 | 4.0 | 8.6 | 8.3 | 11.7 |
| ARIA-H | ||||||
| Any new ARIA-H MRI findings by APOE genotype | ||||||
| No. (%) | 31 (10) | 61 (12.6) | 24 (16.0) | 58 (17.3) | 95 (19.9) | 74 (41.1) |
| Mean No. of new findings (SD) | 1.8 (2.8) | 1.7 (1.3) | 1.9 (1.4) | 4.4 (6.8) | 4.3 (5.3) | 9.9 (15.1) |
| Median No. of new findings | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 5.0 |
| Any new microhemorrhage | ||||||
| No. (%) | 27 (8.7) | 49 (10.1) | 18 (12.0) | 47 (14.0) | 71 (14.9) | 67 (37.2) |
| Mean No. of new findings (SD) | 1.7 (1.8) | 1.5 (1.0) | 2.0 (1.5) | 3.4 (6.3) | 3.6 (5.0) | 9.3 (15.8) |
| Median No. of new findings | 1.0 | 1.0 | 1.0 | 1.0 | 2.0 | 4.0 |
| Any new superficial siderosis | ||||||
| No. (%) | 5 (1.6) | 16 (3.3) | 8 (5.3) | 25 (7.5) | 54 (11.3) | 37 (20.6) |
| Mean No. of new findings (SD) | 2.2 (2.7) | 1.7 (1.5) | 1.3 (0.5) | 3.8 (4.0) | 2.8 (2.8) | 3.1 (1.9) |
| Median No. of new findings | 1.0 | 1.0 | 1.0 | 2.0 | 2.0 | 3.0 |
Abbreviations: ARIA-E, amyloid-related imaging abnormalities-edema; ARIA-H, amyloid-related imaging abnormalities-hemosiderin; BGTS, Barkhof Grand Total Score.
Reported previously.
Symptomatic ARIA-E was defined as ARIA-E temporally associated with central nervous system symptoms.
Serious symptomatic ARIA-E was defined as ARIA-E associated with central nervous system symptoms where the ARIA-E adverse event and/or the central nervous system symptom adverse event was reported as a serious adverse event.
ARIA-E and ARIA-H concurrence was defined as temporal co-occurrence, with new ARIA-H MRI finding(s) detected at the time of emerging or continuing ARIA-E MRI finding(s).
BGTS 4 or higher was the protocol-mandated threshold for study drug dosing suspension; dosing could continue if ARIA-E event was BGTS less than 4 and asymptomatic.
In the double-blind period of the GRADUATE trials, the mean time to first ARIA-E in gantenerumab-treated participants was 35.7 (SD, 13.0) weeks and 49.2 (SD, 21.4) weeks in participants with and without baseline microhemorrhages and/or SS, respectively. A numerically shorter time to first ARIA-E onset and increased mean radiological severity, resolution time, and likelihood of recurrence in those who had an opportunity for recurrence (ie, initial episode’s resolution followed by gantenerumab dosing and MRI) were observed in APOE ε4 homozygotes compared with noncarriers and heterozygotes (Table 1). In those who developed ARIA-E, homozygotes were more likely to develop concurrent microhemorrhages but not SS compared with heterozygotes and noncarriers (Table 1). Most gantenerumab-treated participants with ARIA-E had their first ARIA-E episode by week 64 (213 of 247 [86.2%]). However, first ARIA-E occurred up to week 116 and, including the open-label period, first ARIA-E occurred up to week 168, albeit at lower incidence than in the double-blind period (eFigures 4 through 7 in Supplement 1 including by APOE ε4 stratifications).
In the GRADUATE gantenerumab dataset, concurrent new ARIA-H was reported for 142 of 324 participants (43.8%) with asymptomatic ARIA-E and 36 of 56 (64.3%) with symptomatic ARIA-E. The mean radiological ARIA-E severity of the most severe episode per participant in BGTS was 9.0 (SD, 6.7), 17.5 (SD, 12.0), and 21.3 (SD, 8.3) for asymptomatic, nonserious symptomatic, and serious symptomatic ARIA-E, respectively. Of participants with ARIA-E who had an opportunity for ARIA-E recurrence in the double-blind period, 15 of 21 (71.4%) with and 80 of 173 (46.2%) without baseline microhemorrhages and/or SS had recurrent ARIA-E.
In gantenerumab-treated participants in the double-blind period who had recurrent ARIA-E, the mean radiological severity of the first ARIA-E was 9.8 (SD, 7.8) vs 9.3 (SD, 7.1) for the second ARIA-E episode. There was no evidence for a change in symptomatic status between first and second ARIA-E episode (eTable 6 in Supplement 1).
Of gantenerumab-treated participants, 11 of 993 had serious symptomatic ARIA-E (1%). The most common symptoms were confusion, headache, and aphasia; less common symptoms included seizures and status epilepticus (eTable 5 in Supplement 1). Eight of 11 participants were treated with corticosteroids. None received thrombolytics. There were no ARIA-related fatalities. Summaries for all participants who experienced serious symptomatic ARIA-E (involving hospitalization) are reported in the eMethods in Supplement 1. APOE ε4 status did not substantially impact the incidence of serious symptomatic ARIA-E (Table 1).
In the GRADUATE dataset, at week 116, comparable results were observed from models including all available efficacy measure outcome data vs models where available outcome data were censored at first ARIA-E, as measured by difference and relative difference in adjusted means on any of the efficacy scales tested (Table 2).
Table 2. GRADUATE I and II: Adjusted Mean Changes From Baseline and Differences in Selected Efficacy Scales.
| Efficacy scale | Overall at wk 116 | Censored at time of first ARIA-E | ||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted mean (SE) [No.] | Difference in adjusted means (SE) | Relative difference in adjusted means (%) | Adjusted mean (SE) [No.] | Difference in adjusted means (SE) | Relative difference in adjusted means (%) | |||
| Placebo | Gantenerumab | Placebo | Gantenerumab | |||||
| CDR-SB | 3.3 (0.1) [752] | 3.0 (0.1) [728] | −0.3 (0.1) | −9 | 3.3 (0.1) [733] | 3.0 (0.1) [562] | −0.3 (0.2) | −9 |
| ADAS-Cog13 | 8.8 (0.3) [730] | 7.4 (0.3) [707] | −1.4 (0.5) | −15 | 8.8 (0.3) [711] | 7.7 (0.4) [544] | −1.1 (0.5) | −12 |
| ADCS-ADL | −10.8 (0.4) [751] | −9.8 (0.4) [729] | 1.1 (0.6) | 10 | −10.8 (0.4) [733] | −9.9 (0.5) [561] | 0.9 (0.7) | 9 |
| FAQ | 7.4 (0.2) [749] | 6.5 (0.2) [n = 726] | −0.9 (0.3) | −13 | 7.4 (0.2) [732] | 6.7 (0.3) [560] | −0.7 (0.3) | −10 |
Abbreviations: ADAS-Cog13, Alzheimer Disease Assessment Scale—Cognitive Subscale 13; ADCS-ADL, Alzheimer Disease Cooperative Study—Activities of Daily Living; ARIA-E, amyloid-related imaging abnormalities-edema; CDR-SB, Clinical Dementia Rating—Sum of Boxes; FAQ, Functional Activities Questionnaire.
Discussion
ARIA is an important adverse effect of anti-Aβ mAbs tested in AD, including gantenerumab, with some of these agents available in clinical practice for the treatment of early symptomatic AD.
Reports describing and characterizing ARIA with anti-Aβ mAbs have previously been published. As noted in the Introduction, findings from studies of gantenerumab and other in-class mAbs suggest that the imbalance in ARIA-H between active treatment and placebo arms is driven by ARIA-H co-occurring with ARIA-E. For this reason, we focused on the risk factors, descriptive characteristics, and clinical consequences of ARIA-E associated with gantenerumab treatment in the GRADUATE studies.
Higher anti-Aβ mAb dose, increased number of APOE ε4 alleles, and microhemorrhage presence are well established as nonmodifiable baseline risk factors for ARIA-E. Analyses of the GRADUATE dataset, including 57 baseline variables of interest—to our knowledge, the broadest published set of baseline variables assessed—confirmed the increasing APOE ε4 number as a significant baseline ARIA-E risk factor. Additionally, the number of SS, higher Fazekas score, and lower CSF Aβ42 emerged as potential baseline risk factors for ARIA-E. Total microhemorrhage and SS number, higher amyloid burden on positron emission tomography, more cardiovascular risk factors, lower hippocampal volume, female sex, and higher CSF phosphorylated tau showed some association that, while not statistically significant when corrected for multiple comparisons, should be explored further. Overall, these findings suggest that severity of AD amyloid-related neuropathology (lower CSF Aβ42) and comorbid cerebrovascular pathology (Fazekas score, total SS, and total microhemorrhage counts) may be important ARIA-E risk factors in addition to APOE ε4 number.
Multiple mechanisms have been proposed to explain the association between the APOE ε4 allele and increased ARIA-E risk: (1) reduced cerebrovascular integrity, (2) increased neuroinflammation and immune dysregulation, and (3) elevated levels of CAA. Total SS is also associated with CAA, while white matter hyperintensities may reflect reduced cerebrovascular integrity or CAA, particularly when in a multispot pattern, suggesting these risk factors mediate ARIA risk through shared mechanisms.
Increasing number of APOE ε4 alleles was also associated with higher incidence of ARIA-E with new ARIA-H. Numerically shorter time to first ARIA-E onset and increased mean radiological severity, resolution time, and likelihood of recurrence (in those with a recurrence opportunity) were observed in APOE ε4 homozygotes compared with heterozygotes and noncarriers; apart from similar findings for donanemab concerning ARIA-E radiological severity, these associations between ARIA-E characteristics and number of ε4 copies have not been published for other mAbs. The apparent lack of impact of 1 APOE ε4 copy on these ARIA-E features vs the potential impact of 2 APOE ε4 copies requires further investigation.
Approximately 1 in 5 individuals with ARIA-E had temporally associated CNS symptoms, most commonly headache and dizziness. Although incidence of any ARIA-E and symptomatic ARIA-E increased with APOE ε4 allele count, despite APOE ε4 allele count being associated with greater radiological severity, among individuals who developed ARIA-E, there was a similar rate of symptomatic cases across APOE ε4 allele counts. Notably, two-thirds of symptomatic ARIA-E events, about 50% more than of asymptomatic ARIA-E events, co-occurred with new ARIA-H. However, greater radiological severity of symptomatic ARIA-E, and ARIA-E with concurrent new ARIA-H (eTable 7 in Supplement 1) makes it difficult to determine whether the relationship with symptomatology is directly or indirectly related to greater radiological severity.
The specific ARIA incidence varies between anti-Aβ mAbs (ARIA-E, 13% to 35% and symptomatic ARIA-E, 3% to 9%; ARIA-H microhemorrhage, 14% to 27% and SS, 6% to 16%) due to intrinsic molecular characteristics, targeted amyloid species, trial designs, and population characteristics, including baseline amyloid positron emission tomography burden and distribution, or definition of symptomatic ARIA-E. However, generally similar patterns regarding rates according to APOE ε4 status, rates of symptomatic ARIA-E within overall ARIA-E rates, or imbalances in ARIA-H driven by those with ARIA-E have been reported for these mAbs, including gantenerumab.
Most gantenerumab-treated participants with ARIA-E had their first ARIA-E episode within 16 months of initiating gantenerumab; however, first ARIA-E cases also occurred much later, up to 42 months from treatment initiation. This was later than for other mAbs and unexpected based on previous open-label studies with a similar gantenerumab dosing regimen and could be due to the longer titration period and slower and lower than predicted Aβ removal in the GRADUATE studies. Baseline microhemorrhages and/or SS were associated with an approximately 3-month earlier onset of first ARIA-E.
Radiological ARIA-E severity on gantenerumab was approximately 2 times higher in participants with CNS symptoms than in those without and higher with serious than nonserious symptomatic ARIA-E. However, there also was a significant overlap between the groups and large variability in radiological-clinical relationship, with some radiologically severe ARIA-E cases remaining asymptomatic. Further work is required to understand the relationship between radiological features and symptomatology, including lesion location. Radiological severity did not increase in recurrent events, although this analysis carries a risk for retention bias.
There was no evidence for ARIA-E impact on longitudinal cognitive (CDR-SB, ADAS-Cog13) and functional (ADCS-ADL, FAQ) outcomes at the group level, supporting that ARIA-E do not generally result in long-term detrimental effects on cognition or function. However, ARIA-E might have long-term sequelae in individual cases (eMethods in Supplement 1). For example, in a participant who experienced a serious symptomatic ARIA-E, a residual significant decline in activities of daily living following ARIA-E resolution was considered by the investigator as a potential ARIA-E sequela, albeit confounded by significant Mini-Mental State Examination decline trajectory that started before ARIA-E onset. The lack of impact of APOE ε4 status on serious symptomatic ARIA-E incidence in this series differs from other reports with serious events primarily in APOE ε4 carriers and may be due to the limited number of serious events.
While serious symptomatic ARIA-E remains understudied due to its relative rarity, to our knowledge, ours is the first publication that provides case narratives for 11 such cases (eMethods in Supplement 1). Several of the cases presented with temporal focal neurologic deficits. Since the initial workup in individuals on anti-Aβ mAbs exhibiting such symptoms may focus on and lead to an inadvertent diagnosis of suspected ischemic stroke and treatment with thrombolytics, it is critical that emergency clinicians are aware that patients are receiving an Aβ-lowering antibody, ARIA is an important differential diagnosis, computed tomography is insensitive in detecting ARIA and MRI is required, and use of thrombolytics should be restricted unless ARIA is excluded. Although GRADUATE I/II were not designed to assess the safety of a treatment rechallenge post–ARIA, treatment reintroduction following severe radiological/symptomatic ARIA should be considered carefully.
Limitations
Limitations of our study include the unknown generalizability of our findings to a real-world early symptomatic AD population given the underrepresentation of racial and ethnic minorities and exclusion of certain individuals (eg, with more than 5 microhemorrhages and/or focal SS areas, though also more than 3 focal SS areas, significant white matter pathology, unstable or clinically significant cardiovascular disease, individuals on anticoagulation) from GRADUATE I/II. The number of symptomatic and serious symptomatic ARIA-E events is low; therefore, conclusions should be interpreted cautiously. Time to first ARIA-E-event analyses relied on routine MRI time points (although investigators could perform unscheduled MRIs), which might lead to delayed recording of ARIA-E onset. Additionally, PostGraduate was terminated early after the GRADUATE studies missed their primary end point. ARIA-E severity was expressed in BGTS because that determined clinical actions in the GRADUATE trials, but BGTS does not map 1:1 to other scales. Although ARIA is a class effect of first-generation anti-Aβ mAbs, these findings should be considered molecule specific and requiring further evaluation for other in-class molecules, particularly concerning ARIA-E baseline risk factors.
While gantenerumab lacked sufficient clinical benefit for continued development, Roche is developing trontinemab, a novel, distinct monoclonal antibody. Trontinemab uses a Brainshuttle technology to gain enhanced brain access via transferrin receptor 1–mediated transcytosis at the capillary level, in combination with a gantenerumab-derived immunoglobulin G framework as an anti-Aβ binder. It is hypothesized that the ability to use low doses and to deliver the therapy more directly into the brain parenchyma may result in additional safety benefits, such as reduced ARIA risk. This is supported by preliminary results from an ongoing phase 1b/2a study (NCT04639050) suggesting rapid and robust amyloid lowering by trontinemab with low ARIA incidence.
Conclusions
Data from 1939 participants in 2 phase 3 studies with gantenerumab in early symptomatic AD allowed for characterization of ARIA-E with gantenerumab. Severity of AD–related amyloid neuropathology (lower CSF Aβ42) and comorbid cerebrovascular pathology (Fazekas score, total SS, and total microhemorrhage counts) may be important baseline ARIA-E risk factors, in addition to APOE ε4 allele count, and, in part, may increase risk because of higher baseline CAA burden. Symptomatic ARIA-E events tended to be radiologically more severe and were more frequently associated with ARIA-H vs asymptomatic cases. Although APOE ε4 allele count is associated with increased ARIA-E risk and increased radiological severity, it did not appear to influence symptomatic status among those who developed ARIA-E. In most participants, ARIA-E did not have a long-term impact on cognitive and functional performance.
These findings may be considered in clinical practice when prescribing anti-Aβ mAbs for early AD and developing individualized safety monitoring plans. Further evaluation of the identified potential ARIA-E risk factors in the context of other anti-Aβ mAbs is recommended.
eTable 1. GRADUATE I and II: Baseline Characteristics of Pooled Safety-evaluable MRI Population
eTable 2. PostGraduate: Demographic and Baseline Characteristics
eTable 3. Analysis for Risk Factors of ARIA-E in GRADUATE I and II, Demographic and Baseline Characteristics of Participants
eTable 4. Risk Factors for ARIA-E With Concurrent ARIA-H: Multivariate Modeling With Stepwise Logistic Regression for Baseline Variables With Univariate P <.05
eTable 5. GRADUATE I and II: Nature and Severity of CNS Symptoms in Serious Symptomatic ARIA-E Cases
eTable 6. GRADUATE I and II: Summary of Changes in Clinical Symptomatology From First to Second ARIA-E Episode, Gantenerumab Arm
eTable 7. GRADUATE I and II: Summary of Radiological Severity of ARIA-E MRI Findings by Concurrence with New ARIA-H MRI Findings
eFigure 1. GRADUATE I and II: ARIA-Related Dosing Intervention Rules
eFigure 2. PostGraduate: Study Design
eFigure 3. PostGraduate: Participant Flow
eFigure 4. Kaplan-Meier Plot of Time-to-Onset of First ARIA-E Event in the Double-Blind Period of GRADUATE Trials
eFigure 5. Kaplan-Meier Plot of Time-to-Onset of First ARIA-E Event from First Gantenerumab Dose in Double-Blind Period of GRADUATE Including Open-Label Period and PostGraduate Data
eFigure 6. GRADUATE I and II: Kaplan–Meier Plot of Time-to-Onset of First ARIA-E in the Double-Blind Period by APOE ε4 Status
eFigure 7. GRADUATE I and II and PostGraduate: Kaplan–Meier Plot of Time-to-Onset of First ARIA-E From First Dose of Gantenerumab in Double-Blind Phase, Including Open-Label Extension Data by APOE ε4 Status
eMethods. Case Narratives. GRADUATE I and II: Serious Symptomatic ARIA-E Cases
Trial protocol
Data sharing statement
References
- 1.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595-608. doi: 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sims JR, Zimmer JA, Evans CD, et al. ; TRAILBLAZER-ALZ 2 Investigators . Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi: 10.1001/jama.2023.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 4.Widera EW, Brangman SA, Chin NA. Ushering in a new era of Alzheimer disease therapy. JAMA. 2023;330(6):503-504. doi: 10.1001/jama.2023.11701 [DOI] [PubMed] [Google Scholar]
- 5.Eisai . LEQEMBI prescribing information. Accessed October 15, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269Orig1s001lbl.pdf
- 6.Eli Lilly . KISUNLA (donanemab-azbt) prescribing information. Accessed October 15, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/761248s000lbl.pdf
- 7.Sperling RA, Jack CR Jr, Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7(4):367-385. doi: 10.1016/j.jalz.2011.05.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H, Elhage A, Cho M, Apostolova LG, Nicoll JAR, Atri A. Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain. 2023;146(11):4414-4424. doi: 10.1093/brain/awad188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salloway S, Sperling R, Gilman S, et al. ; Bapineuzumab 201 Clinical Trial Investigators . A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061-2070. doi: 10.1212/WNL.0b013e3181c67808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling R, Salloway S, Brooks DJ, et al. Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol. 2012;11(3):241-249. doi: 10.1016/S1474-4422(12)70015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostrowitzki S, Lasser RA, Dorflinger E, et al. ; SCarlet RoAD Investigators . A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg SM, Bacskai BJ, Hernandez-Guillamon M, Pruzin J, Sperling R, van Veluw SJ. Cerebral amyloid angiopathy and Alzheimer disease—one peptide, two pathways. Nat Rev Neurol. 2020;16(1):30-42. doi: 10.1038/s41582-019-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salloway S, Chalkias S, Barkhof F, et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 2022;79(1):13-21. doi: 10.1001/jamaneurol.2021.4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong SY, Suh CH, Shim WH, Lim JS, Lee JH, Kim SJ. Incidence of amyloid-related imaging abnormalities in patients with Alzheimer disease treated with anti-β-amyloid immunotherapy: a meta-analysis. Neurology. 2022;99(19):e2092-e2101. doi: 10.1212/WNL.0000000000201019 [DOI] [PubMed] [Google Scholar]
- 15.Honig LS, Barakos J, Dhadda S, et al. ARIA in patients treated with lecanemab (BAN2401) in a phase 2 study in early Alzheimer’s disease. Alzheimers Dement (N Y). 2023;9(1):e12377. doi: 10.1002/trc2.12377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock J, Vasilopoulou F, Svensson E, Cosker K. Microglia and TREM2. Neuropharmacology. 2024;257:110020. doi: 10.1016/j.neuropharm.2024.110020 [DOI] [PubMed] [Google Scholar]
- 17.Aldea R, Grimm HP, Gieschke R, et al. In silico exploration of amyloid-related imaging abnormalities in the gantenerumab open-label extension trials using a semi-mechanistic model. Alzheimers Dement (N Y). 2022;8(1):e12306. doi: 10.1002/trc2.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista AF, Khan KA, Papavergi MT, Lemere CA. The importance of complement-mediated immune signaling in Alzheimer’s disease pathogenesis. Int J Mol Sci. 2024;25(2):817. doi: 10.3390/ijms25020817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov . Efficacy and safety study of gantenerumab in participants with early Alzheimer’s disease (AD). Accessed October 15, 2024. https://clinicaltrials.gov/study/NCT03444870
- 20.ClinicalTrials.gov . Safety and efficacy study of gantenerumab in participants with early Alzheimer’s disease (AD). Accessed October 15, 2024. https://clinicaltrials.gov/study/NCT03443973
- 21.ClinicalTrials.gov . A study to evaluate the safety, tolerability, and efficacy of long-term gantenerumab administration in participants with Alzheimer’s disease (AD). Accessed October 15, 2024. https://clinicaltrials.gov/study/NCT04374253
- 22.Bateman RJ, Smith J, Donohue MC, et al. ; GRADUATE I and II Investigators and the Gantenerumab Study Group . Two phase 3 trials of gantenerumab in early Alzheimer’s disease. N Engl J Med. 2023;389(20):1862-1876. doi: 10.1056/NEJMoa2304430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutmacher M, Hu C, Guenzler-Pukall V, et al. Pharmacokinetic-pharmacodynamic modeling of amyloid-related imaging abnormalities of edema following intravenous administration of bapineuzumab to subjects with mild to moderate Alzheimer’s disease. J Pharmacokint Pharmacodyn. 2013;40:Abstract W-040.
- 24.Retout S, Gieschke R, Serafin D, Weber C, Frey N, Hofmann C. Disease modeling and model-based meta-analyses to define a new direction for a phase III program of gantenerumab in Alzheimer’s disease. Clin Pharmacol Ther. 2022;111(4):857-866. doi: 10.1002/cpt.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkhof F, Daams M, Scheltens P, et al. An MRI rating scale for amyloid-related imaging abnormalities with edema or effusion. AJNR Am J Neuroradiol. 2013;34(8):1550-1555. doi: 10.3174/ajnr.A3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall JN, Mormino E, Ng A, et al. Six recurrent amyloid-related imaging abnormality episodes in a patient treated with aducanumab. JAMA Neurol. 2022;79(1):87-89. doi: 10.1001/jamaneurol.2021.3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippi M, Cecchetti G, Spinelli EG, Vezzulli P, Falini A, Agosta F. Amyloid-related imaging abnormalities and β-amyloid-targeting antibodies: a systematic review. JAMA Neurol. 2022;79(3):291-304. doi: 10.1001/jamaneurol.2021.5205 [DOI] [PubMed] [Google Scholar]
- 28.Honig LS, Sabbagh MN, van Dyck CH, et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimers Res Ther. 2024;16(1):105. doi: 10.1186/s13195-024-01441-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson C, Estergard W, Oh J, et al. Prevalence of asymptomatic vasogenic edema in pretreatment Alzheimer’s disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimers Dement. 2011;7(4):396-401. doi: 10.1016/j.jalz.2011.05.2353 [DOI] [PubMed] [Google Scholar]
- 30.DiFrancesco JC, Longoni M, Piazza F. Anti-Aβ autoantibodies in amyloid related imaging abnormalities (ARIA): candidate biomarker for immunotherapy in Alzheimer’s disease and cerebral amyloid angiopathy. Front Neurol. 2015;6:207. doi: 10.3389/fneur.2015.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pankiewicz JE, Sadowski MJ. APOE genotype and Alzheimer’s immunotherapy. Oncotarget. 2017;8(25):39941-39942. doi: 10.18632/oncotarget.17990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyons M, Neve A, Huang Z, Das B, Wojtowicz J, Bullain S. Baseline risk factors for developing ARIA-E from the SCarlet RoAD and Marguerite RoAD open-label extension studies. Alzheimers Dement. 2022;18(S10):e065856. doi: 10.1002/alz.065856 [DOI] [Google Scholar]
- 33.Loomis SJ, Miller R, Castrillo-Viguera C, et al. Genome-wide association studies of ARIA from the aducanumab phase 3 ENGAGE and EMERGE studies. Neurology. 2024;102(3):e207919. doi: 10.1212/WNL.0000000000207919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60(4):652-656. doi: 10.1212/01.WNL.0000046581.81650.D0 [DOI] [PubMed] [Google Scholar]
- 35.Foley KE, Wilcock DM. Three major effects of APOEε4 on Aβ immunotherapy induced ARIA. Front Aging Neurosci. 2024;16:1412006. doi: 10.3389/fnagi.2024.1412006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charidimou A, Boulouis G, Frosch MP, et al. The Boston criteria version 2.0 for cerebral amyloid angiopathy: a multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol. 2022;21(8):714-725. doi: 10.1016/S1474-4422(22)00208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardlaw JM, Makin SJ, Valdés Hernández MC, et al. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimers Dement. 2017;13(6):634-643. doi: 10.1016/j.jalz.2016.09.006 [DOI] [Google Scholar]
- 38.Biogen . ADUHELM (aducanumab-avwa) prescribing information. Accessed October 15, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761178s011lbl.pdf
- 39.Neve A, Das B, Wojtowicz J, et al. Long-term safety of gantenerumab in participants with Alzheimer’s disease: a phase III, double-blind, and open-label extension study (Marguerite RoAD). Journal of Alzheimer’s Disease. 2024;101(1):353-367. doi: 10.3233/JAD-240221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howe MD, Britton KJ, Joyce HE, et al. Initial experiences with amyloid-related imaging abnormalities in patients receiving aducanumab following accelerated approval. J Prev Alzheimers Dis. 2023;10(4):765-770. [DOI] [PubMed] [Google Scholar]
- 41.Klein G, Scelsi MA, Barakos J, et al. Comparing ARIA-E severity scales and effects of treatment management thresholds. Alzheimers Dement (Amst). 2022;14(1):e12376. doi: 10.1002/dad2.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm HP, Schumacher V, Schäfer M, et al. Delivery of the Brainshuttl amyloid-beta antibody fusion trontinemab to non-human primate brain and projected efficacious dose regimens in humans. MAbs. 2023;15(1):2261509. doi: 10.1080/19420862.2023.2261509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulic L, Alcaraz F, Thanasopoulou A, et al. Rapid dose-dependent amyloid plaque depletion with trontinemab, a novel BrainshuttleTM antibody in development for the treatment of Alzheimer’s disease. Presented at AD/P, March 5-9, 2024; Lisbon, Portugal. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. GRADUATE I and II: Baseline Characteristics of Pooled Safety-evaluable MRI Population
eTable 2. PostGraduate: Demographic and Baseline Characteristics
eTable 3. Analysis for Risk Factors of ARIA-E in GRADUATE I and II, Demographic and Baseline Characteristics of Participants
eTable 4. Risk Factors for ARIA-E With Concurrent ARIA-H: Multivariate Modeling With Stepwise Logistic Regression for Baseline Variables With Univariate P <.05
eTable 5. GRADUATE I and II: Nature and Severity of CNS Symptoms in Serious Symptomatic ARIA-E Cases
eTable 6. GRADUATE I and II: Summary of Changes in Clinical Symptomatology From First to Second ARIA-E Episode, Gantenerumab Arm
eTable 7. GRADUATE I and II: Summary of Radiological Severity of ARIA-E MRI Findings by Concurrence with New ARIA-H MRI Findings
eFigure 1. GRADUATE I and II: ARIA-Related Dosing Intervention Rules
eFigure 2. PostGraduate: Study Design
eFigure 3. PostGraduate: Participant Flow
eFigure 4. Kaplan-Meier Plot of Time-to-Onset of First ARIA-E Event in the Double-Blind Period of GRADUATE Trials
eFigure 5. Kaplan-Meier Plot of Time-to-Onset of First ARIA-E Event from First Gantenerumab Dose in Double-Blind Period of GRADUATE Including Open-Label Period and PostGraduate Data
eFigure 6. GRADUATE I and II: Kaplan–Meier Plot of Time-to-Onset of First ARIA-E in the Double-Blind Period by APOE ε4 Status
eFigure 7. GRADUATE I and II and PostGraduate: Kaplan–Meier Plot of Time-to-Onset of First ARIA-E From First Dose of Gantenerumab in Double-Blind Phase, Including Open-Label Extension Data by APOE ε4 Status
eMethods. Case Narratives. GRADUATE I and II: Serious Symptomatic ARIA-E Cases
Trial protocol
Data sharing statement