Abstract
Background
Sugars Will Eventually be Exported Transporter (SWEET) gene family is a unique type of sugar transporter that plays a vital role in metabolic regulation, growth, development, and stress response in multiple species. This study aimed to systematically identify the SWEET gene family members and detect the regulation of gene expression and their potential roles of the SWEET gene family in Brassica juncea.
Results
A total of 66 BjSWEET (Brassica juncea Sugar Will Eventually be Exported Transporter) genes distributed across 17 chromosomes were identified. The gene structure and motifs were relatively conserved, with all members containing the MtN3/saliva domain. Phylogenetic analysis revealed that the SWEET gene family can be classified into four subfamilies (Clades I, II, III, and IV). Collinearity analysis revealed that there were 118 pairs of segment duplicates, indicating that some BjSWEET genes were obtained via segmental duplication. The promoter regions of the BjSWEET genes contained many plant hormone-related response elements, stress-related response elements, growth and development elements, and light-responsive regulatory elements. Furthermore, analysis of the expression profiles revealed that the expression levels of the BjSWEET genes differed among the eight different tissues. qRT‒PCR analysis of six selected BjSWEET genes revealed that the expression levels of BjSWEET17.2, BjSWEET17.4, BjSWEET12.2, and BjSWEET12.3 were significantly upregulated under drought treatment, suggesting that these genes may respond to drought stress in B. juncea.
Conclusion
This study systematically identified and analyzed the SWEET gene family members in B. juncea for the first time, laying the foundation for further research on the molecular mechanisms of drought resistance in B. juncea and providing theoretical guidance for the application of these genes in other species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05815-w.
Keywords: B. juncea, SWEET gene, Sugar transporter protein, Abiotic stress, Expression profile
Background
As the main carbon source and energy substances, soluble sugars such as glucose, fructose and galactose play essential roles in cytoskeleton formation, plant growth and development, energy metabolism, signal transduction, osmoregulation, and other processes [1, 2]. Sugar transporters are key mediators of sugar transport in plants, and sugars are transported from source to reservoir across the membrane, this process depends on sugar transporters. They can be widely found in fruits, veins, seeds, roots, pollen, and embryos [3]. To date, three main types of sugar transporters have been found in higher plants: SWEETs, monosaccharide transporters (MTs) and sucrose transporters (SUTs). Among them, SUT1 only exists in dicotyledonous plants, while SUT2 and SUT4 coexist in monocotyledonous and dicotyledonous plants, SUT3 and SUT5 only exist in monocotyledons. MTs are a protein superfamily, and gene expression can be regulated by environmental factors during cell development and can exhibit specific expression at different stages [4].
The SWEET gene family comprises bidirectional transmembrane transporters that can transport fructose, sucrose, and hexose in plants [5]. The typical SWEETs consist of seven transmembrane helices, which contain two tandem 3-TM repeat units, constituting the MtN3-slv domain or the PQ-loop domain, and the middle is connected by a less conserved transmembrane helix (TMH), thus comprising the TM SWEET structure of “3–1–3” [6]. In plants, the SWEET gene family can be classified into four subfamilies. The evolutionary sequences of the four subfamilies were as follows: Clade II, Clade I, Clade III and Clade IV [7]. Studies have shown that Clades I and II of the SWEET gene family mainly transport monosaccharides and preferentially transport hexose, while clade III transports disaccharides and mainly transports sucrose, Clade IV SWEETs function as fructose transporters in the vacuole [8]. To date, genome-wide analysis have identified SWEET gene family members in many plants, such as 17 in Arabidopsis thaliana, 21 in rice (Oryza sativa) [9], 52 in soybean (Glycine max) [10], 35 in potato (Solanum tuberosum) [11], 29 in tomato (Solanum lycopersicum) [12], and 23 in sorghum (Sorghum bicolor) [13]. Biochemical and functional analysis of the SWEET gene family revealed that SWEET proteins in plants were involved mainly in growth and development [14], host interactions, sugar compound distribution, transportation and storage, abiotic and biotic stress responses, and other expression regulatory processes [15]. For example, AtSWEET13 can contribute to pollen development [16], AtSWEET16 and AtSWEET17 have been found to be involved in the regulation of root growth and development and the transport of vacuolar sugars in Arabidopsis, and AtSWEET10 was reported to maintain cell viability in the Arabidopsis salt stress environment [17, 18]. SlSWEET15 in S. lycopersicum was considered as an essential regulator of salt tolerance [19], and SWEET9 in tobacco (Nicotiana attenuata) and Chinese cabbage (Brassica rapa) reportedly regulated nectar secretion [20].
Biotic and abiotic stresses affect plant growth and development and metabolic processes in many ways. In response to stress, plants alter their molecular structure and physiological and biochemical processes through a variety of signaling pathways. Under stress, soluble sugars play an important role in plants. Abiotic stress mainly includes drought, high temperature, low temperature, and ultraviolet radiation. Drought stress affects many physiological and biochemical processes in plants, including damage to the cell membrane, a decrease in the relative water content and chlorophyll content in leaves, and an increase in osmotic adjustment substances. Ultimately, plant performance is reduced in terms of yield and quality [21]. Therefore, studying the mechanism of plant growth under drought stress is important for B. juncea breeding. Previous studies have shown that AtSWEET17 was transcriptionally upregulated under drought stress [22] and thus involved in the drought stress response in Arabidopsis. The Arabidopsis sucrose transporters SWEET11 and SWEET12 were phosphorylated under drought stress, and their gene expression increased significantly when the environment recovered, after which their expression remained constant [23].
With the development of genome sequencing technology, an increasing number of SWEET genes in Brassicaceae have been genome-wide identified. In B. rapa, after genome-wide analysis of the SWEET gene family, BrSWEET genes have been identified [24, 25]. BoSWEET genes in B. oleracea involved in response to chilling and clubroot disease [26]. A total of 68 members of the SWEET gene family have been identified in B. napus, and BnSWEET12 was upregulated under drought stress [27]. B. juncea is an allotetraploid of Brassica, that is generated by spontaneous chromosome doubling after hybridization between two progenitor species, Brassica nigra and Brassica rapa [28]. It has been widely grown as an oilseed crop, economic vegetable, and condiment worldwide. However, genome-wide identification and analysis of the SWEET gene family have not been performed in B. juncea, and whether the BjSWEET genes participate in the drought stress response and the regulatory mechanism remain unknown. Therefore, understanding and studying the SWEET genes and their biological functions are essential for Brassicaceae breeding and cultivation of resistant varieties. In this study, we performed a genome-wide analysis of SWEET genes in B. juncea and the gene was renamed as BjSWEET. We subsequently analyzed the chromosome-based gene mapping, transmembrane helical structure, gene structure, conserved motifs, cis-acting elements in the promoters, phylogenetic relationships, and expression profiles of these genes. These findings lay a solid foundation for further elucidation of the biological function of SWEET genes in B. juncea.
Results
Identification and bioinformatics analysis of BjSWEET genes
A total of 66 SWEET gene family members were identified in B. juncea according to their respective orthologous genes in Arabidopsis (Table S1). These genes encode proteins with lengths ranging from 85 (BjSWEET8.4) to 411 aa (BjSWEET14.2), protein isoelectric points ranging from 4.77 to 9.72, and protein relative molecular weights between 9.68 (BjSWEET8.4) and 45.35 kDa (BjSWEET14.2). Approximately 92.42% of the BjSWEET proteins had isoelectric points higher than 7, indicating that most of the members were alkaline proteins. Subcellular localization prediction can help us to better explore molecular function. The results showed that BjSWEET8.3 was located in the nucleus, and that BjSWEET6.1 was located in the cell membrane and nucleus. BjSWEET8.4, BjSWEET8.5, BjSWEET10.1, BjSWEET10.2, and BjSWEET11.5 were localized in the cell membrane and chloroplasts. BjSWEET14.1 was localized in the cell membrane and peroxisomes. BjSWEET14.7 was localized in the cell membrane, chloroplast, Golgi and peroxisomes. BjSWEET17.2 was localized in the cell membrane and Golgi, while the remaining proteins were all localized in the cell membrane. These results indicated that BjSWEETs could perform biological functions in different subcellular organelles.
Phylogenetic and multiple sequence alignment analysis of BjSWEETs
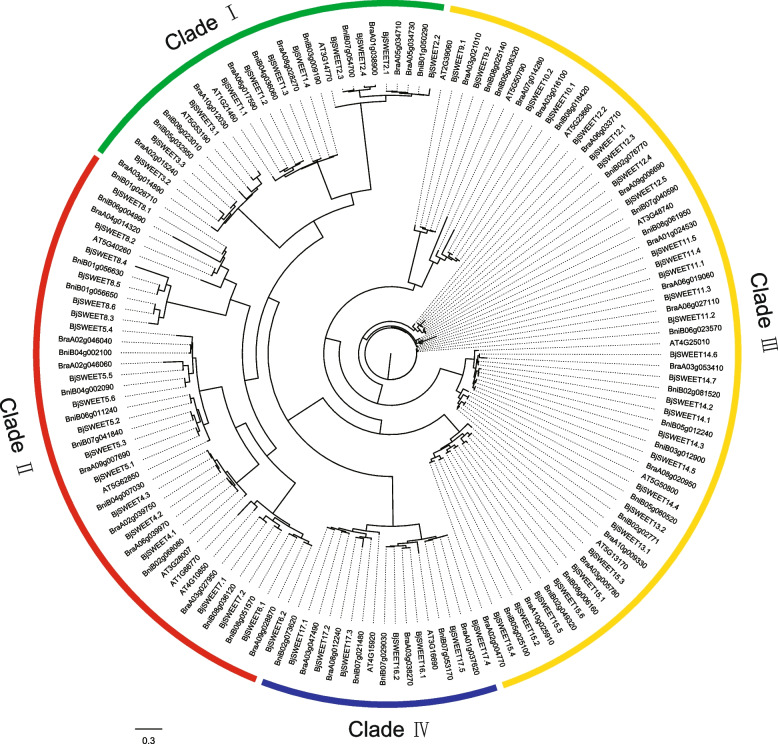
To better understand the evolutionary relationships and functional implications of BjSWEET genes, we constructed the phylogenetic tree of SWEET genes from B. juncea, B. rapa, B. nigra and A. thaliana (Fig. 1). A total of 34 SWEET genes have been identified from B. rapa and 37 SWEET genes have been identified from B. nigra, respectively. The analysis revealed that the SWEET gene family members can be classified into four distinct subfamilies, namely Clades I-IV. Among them, Clade I had 11 BjSWEETs, 8 BrSWEETs, 6 BniSWEETs, and 3 AtSWEETs genes. Clade II had 19 BjSWEETs, 8 BrSWEETs, 12 BniSWEETs and 5 AtSWEETs genes. Clade III had 29 BjSWEETs, 14 BrSWEETs, 15 BniSWEETs and 7 AtSWEETs genes. Clade IV had 7 BjSWEETs, 4 BrSWEETs, 4 BniSWEETs and 2 AtSWEETs genes. The SWEET genes of the three Brassica species were clustered with their homologous genes from A. thaliana. Notably, Clades II and III contained a greater number of SWEET genes than Clades I and IV. And few BjSWEETs genes from Clade I, Clade II and Clade IV were lost during the evolution of B. juncea. The phylogenetic tree accurately depicted that the SWEET genes with close evolutionary relationships had little differentiation and high homology, suggesting that these genes may have functional redundancy. Multiple sequence alignment analysis of BjSWEET genes using MEGA 6.06 and GeneDoc software showed that all 66 BjSWEET genes had a segment of conserved sequence, constituting the conserved MtN3-slv or PQ-loop superfamily (Fig. S1). Furthermore, the conserved domain of the BjSWEET proteins was located at the N-terminus. This result was consistent with the localization of the conserved domain of the typical SWEET proteins.
Fig. 1.
Phylogenetic tree of B. juncea SWEETs. The ML tree was constructed using MEGA6.06 software based on the SWEET protein sequences. Bootstrap values from 1000 replicates are displayed at each node. Four subfamilies were marked with different colors
Gene structure, conserved motifs and transmembrane helices of BjSWEETs
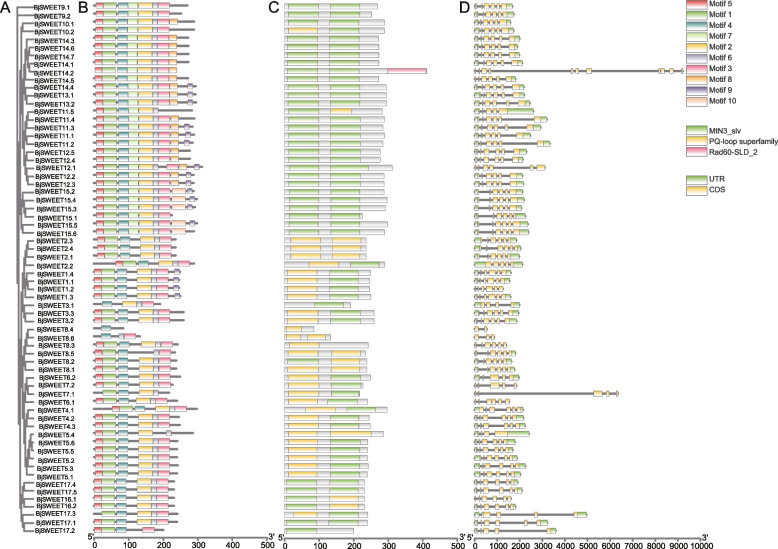
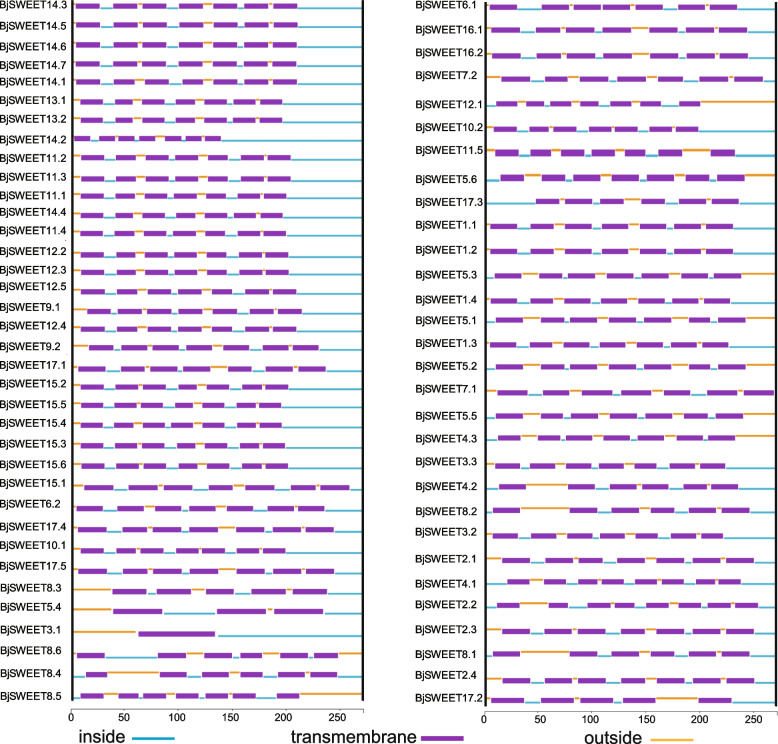
The BjSWEET proteins in the same subfamily had similar motifs, which was consistent with the results of the phylogenetic analysis. This indicated that these proteins may have similar functions (Fig. 2A). Conserved motifs can provide insights into the structural characteristics of BjSWEETs, and 10 conserved motifs were predicted (Fig. 2B, Table S2). The results found that all the BjSWEET proteins had 1 to 9 conserved motifs and all possessed motif 4, indicating that this motif is a unique SWEET domain of the SWEET gene family. Additionally, the same subfamily members contained similar conserved motifs, and certain motifs showed apparent specificity. For example, motif 7 and motif 8 were unique to Clade I (Fig. S2). However, BjSWEET3.1, BjSWEET8.3, BjSWEET8.4 and BjSWEET17.3 only possessed MtN3-slv domain or PQ-loop superfamily domain. This discrepancy may result in non-functionalization, sub-functionalization or neofunctionalization of these SWEET genes. Additionally, by analyzing the conserved domains of the SWEET gene family members, we observed that approximately 93.9% of BjSWEETs contained two domains, highlighting the essentiality of these domains for the normal expression of SWEET genes (Fig. 2C). The analysis of gene structure found that the number of introns of the BjSWEET genes differed (Fig. 2D). The SWEET genes in B. juncea exhibit structural diversity. The number of exons ranged from 2 to 11, and approximately 81.8% of the BjSWEET genes exhibited six exons. Most of the BjSWEET genes in Clade I subfamily had 6 exons expect BjSWEET2.2 and BjSWEET3.1. The BjSWEET genes in Clade II subfamily varied greatly, only 2 exons have been found in BjSWEET8.4. Most of the BjSWEET genes in Clade III subfamily had 6 exons except BjSWEET14.2 and BjSWEET11.5. The BjSWEET17.3 gene had 4 exons, while other BjSWEET genes in Clade III subfamily had 6 exons. The analysis of transmembrane helical structures showed that the BjSWEET8.4 protein contained only 1 transmembrane helix (TMH), BjSWEET8.6 contained 3 TMHs, BjSWEET3.1 and BjSWEET17.2 contained 5 TMHs, BjSWEET4.1 and BjSWEET2.2 contained 8 TMHs. Six proteins (BjSWEET17.3, BjSWEET4.2, BjSWEET8.1, BjSWEET8.2, BjSWEET8.3 and BjSWEET8.5) contained 6 TMHs, and the remaining BjSWEETs all contained 7 TMHs (Fig. 3). These results revealed significant variation among the 66 SWEET gene family members.
Fig. 2.
Detailed structure of SWEET family proteins in B. juncea. A Phylogenetic tree, (B) conserved motif, (C) conserved domain, (D) gene structure
Fig. 3.
Transmembrane helices of SWEET family proteins in B. juncea. The whole sequence of each BjSWEET protein was labeled as inside transmembrane with blue color, transmembrane with purple color, and transmembrane outside with orange color
Chromosomal localization and gene duplication of BjSWEETs
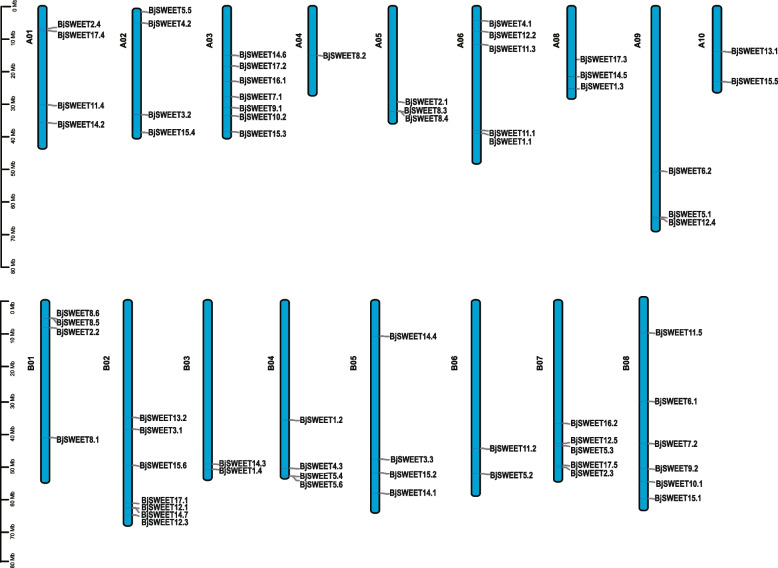
The chromosomal distribution of BjSWEETs was studied according to their physical location in the B. juncea genome. The results exhibited that a total of 66 SWEET gene family members in B. juncea were not evenly distributed across 17 chromosomes (Fig. 4). It was found that 32 genes were located in the A subgenome, 34 were located in the B subgenome. And 7 BjSWEET genes were found on the A03 and B02 chromosomes, but only one BjSWEET8.2 gene was identified on the A04 chromosome, while no BjSWEET gene was located on chromosome A07. Furthermore, we studied gene duplications within 66 BjSWEET genes by analyzing their collinearity (Fig. S3, Table S3). There were 118 pairs of segment duplicates, and some BjSWEET genes were repeatedly involved in the gene duplication events. Among these gene pairs, 6 pairs were found on the same chromosome and 112 pairs were distributed on diverse chromosomes, indicating that segmental duplication is the primary expansion model of SWEET gene family in B. juncea, and that some BjSWEETs were probably obtained by gene segmental duplication.
Fig. 4.
The distribution of SWEET genes on chromosomes in B. juncea. Chromosome size is indicated by its relative length. The scale bar represents megabases (Mb). The physical locations of BjSWEETs are drawn on each chromosome
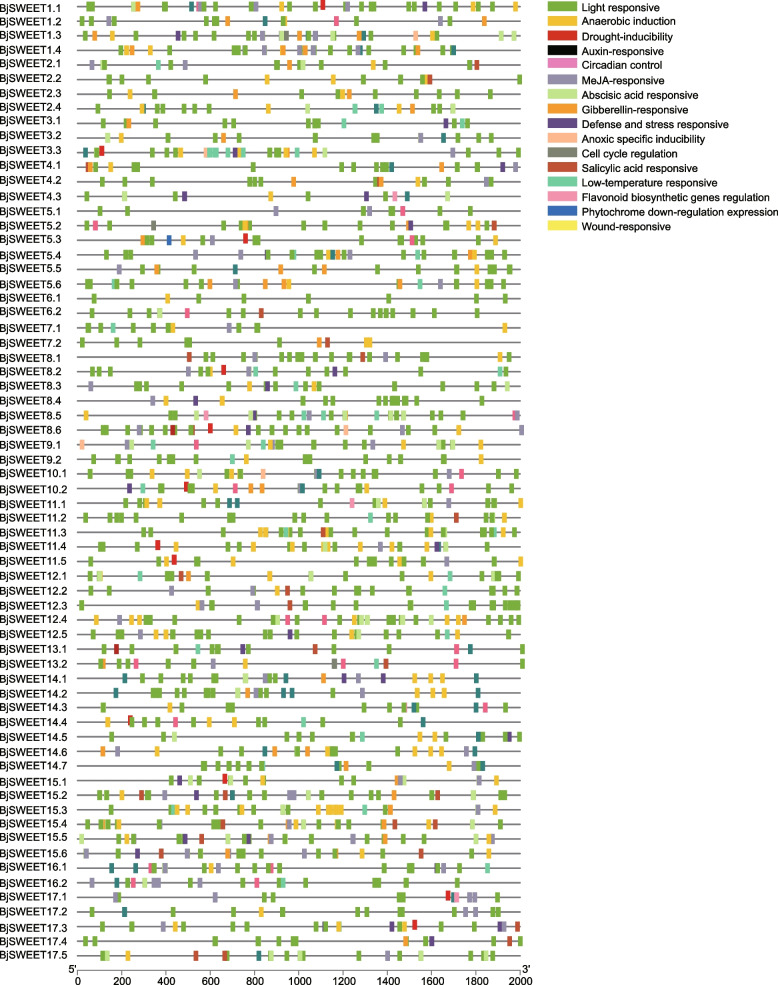
Cis-elements in the promoters of BjSWEET genes
To further analyze the potential regulatory mechanisms of the BjSWEET genes, we predicted the cis-elements in the promoters of these genes using the online tool PlantCARE. We finally selected 20 cis-elements (Fig. 5, Table S4). Among them, there were 5 plant hormone-related response elements, including gibberellin (GA), auxin, abscisic acid (ABA), salicylic acid (SA) and methyl jasmonate (MeJA). And 8 stress-related response elements, including anaerobic, defense and stress response, drought, hypoxia-specific, wound, circadian control, low temperature-responsive elements, and gene regulation of flavonoid biosynthesis, were identified. Additionally, light responsive elements and cis-elements related to plant growth and development, such as meristem expression, endosperm expression, seed-specific regulation, and differentiation of palisade mesophyll cells were detected. Compared with other cis-elements, light response elements were present in the promoters of all 66 BjSWEET genes, implying the universal involvement of BjSWEET genes in light control expression. These results indicated that BjSWEET genes likely play essential roles in plant growth and development, abiotic and biotic stress responses, hormone regulation, and light-controlled expression.
Fig. 5.
The prediction results of the cis-elements of the SWEET gene family in B. juncea. The cis-elements analysis was performed with the 2.0 kb upstream region using the online tool PlantCARE. Different plant hormone-related response elements, stress-responsive elements, light-responsive elements and cis-elements related to growth and development were showed in different colors
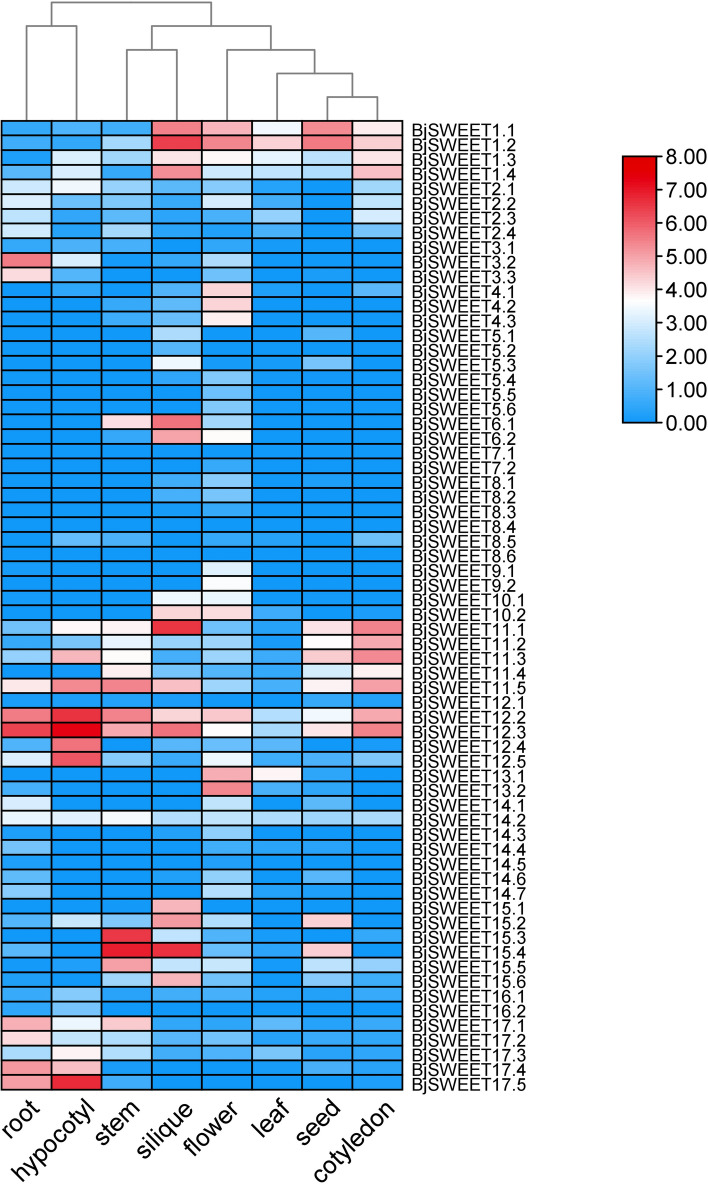
Expression pattern analysis of BjSWEET genes
Based on the transcriptome data, we constructed an expression heatmap of the BjSWEET genes in eight different tissues, including root, hypocotyl, stem, silique, flower, leave, seed and cotyledon (Fig. 6, Table S5). The expression patterns were further investigated to elucidate the potential biological functions of the BjSWEET genes. The results showed that the expression of the BjSWEET genes was generally low in the leaves. BjSWEET7.1, BjSWEET8.6 and BjSWEET8.4 were not expressed in eight tissues. Certain genes were specifically expressed in tissues, for example, BjSWEET15.4 presented the highest expression in stems, but its expression was much lower in other tissues. BjSWEET1.2 and BjSWEET13.2 were only highly expressed in flowers. And BjSWEET17.4 was more highly expressed in roots and hypocotyls than in other tissues. These results suggested that genes highly expressed in specific tissues may play a crucial role in the functional expression of these tissues.
Fig. 6.
Heatmap of the expression profiles of all the BjSWEET genes in 8 different tissues of B. juncea. The expression abundance of each transcript is represented by the normalized fragments per kilobase pair per million (FPKM) value and displayed as colored boxes from green (lower expression) to red (higher expression)
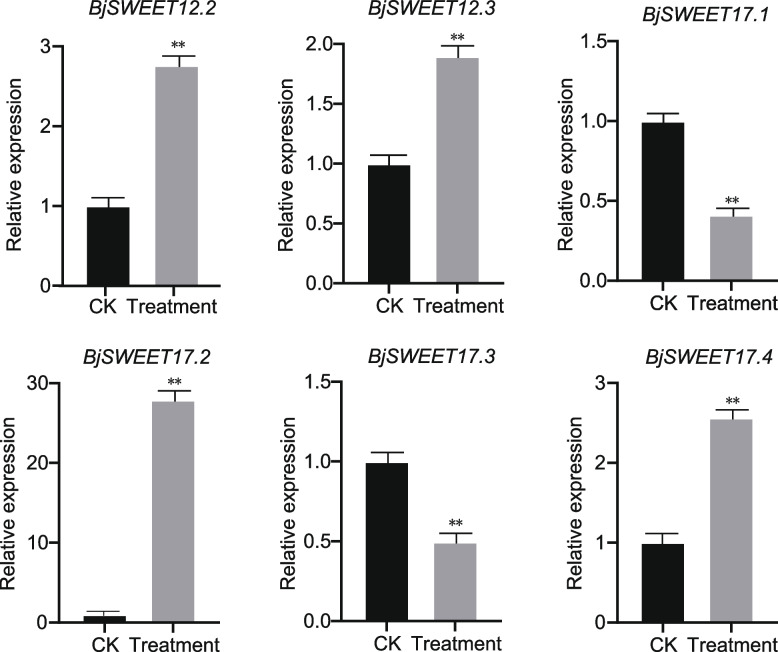
Expression profiles of BjSWEET genes under drought treatment via qRT-PCR
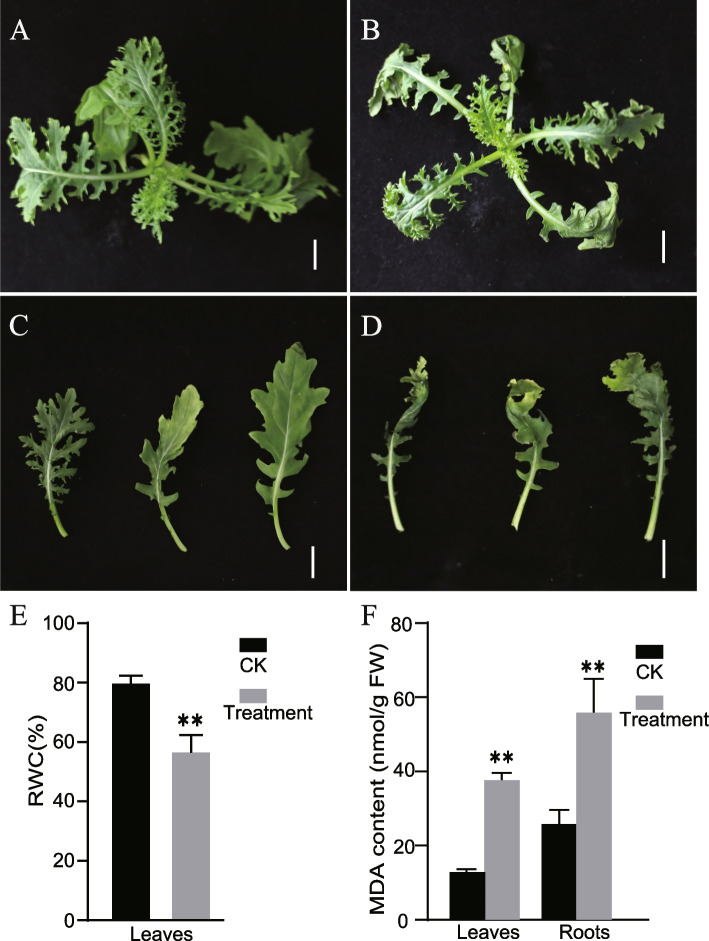
To further identify candidate BjSWEET genes involved in drought response, PEG6000 was used to simulate drought stress. Leaves from well-watered plants grow well (Fig. 7A, C). However, the leaves from drought treated mustard showed wilting and leaf edge damage (Fig. 7B, D). Drought stress significantly reduced the leaf relative water content when compared it with the well-watered mustard (Fig. 7E). Under drought stress, the MDA content in leaves increased significantly when compared it with the well-watered leaves (Fig. 7F). Although no significant phenotypic variation have found in the roots, the root showed an impressive capacity to influence its architecture in response to drought. The MDA content in the roots also increased significantly after drought stress treatment (Fig. 7F). The analysis of the BjSWEETs expression levels in eight tissues showed that BjSWEET17.1, BjSWEET17.2, BjSWEET17.3, BjSWEET17.4, BjSWEET12.2, and BjSWEET12.3 were highly expressed in roots. SWEET proteins are known to participate in vacuolar sugar transport in roots to exert their drought resistance. Thus, the relative expression of these genes under drought stress was measured using qRT-PCR (Fig. 8, Table S6). The results showed that the expression profiles of the BjSWEET genes differed under drought stress. Compared with those in the control, the expression of BjSWEET17.1 and BjSWEET17.3 decreased, while the expression of BjSWEET17.2, BjSWEET17.4, BjSWEET12.2, BjSWEET12.3 and BjSWEET12.3 were significantly up-regulated under drought treatment. These results suggested that BjSWEET17.2, BjSWEET17.4, BjSWEET12.2, and BjSWEET12.3 were likely involved in the response to drought stress.
Fig. 7.
Phenotypes, physiological, and biochemical difference between mustard XC under drought stress treatment and sterile water treatment. A The phenotype of XC under well sterile watered condition (CK). B The phenotype of XC under drought stress. C Representative leaves of XC under well sterile watered condition (CK). D Representative leaves of XC under drought stress. E The leaf relative water content (RWD) between drought stress and sterile water treatment (CK). F The malondialdehyde (MDA) content in leaves and roots between drought stress treatment and sterile water treatment (CK). Bar = 1 cm
Fig. 8.
Expression analysis of BjSWEET12 and BjSWEET17 under drought treatment by qRT‒PCR. The data are presented as the means ± standard deviations (STDEV) (n = 3). Asterisks indicate significant differences between sterile watered (CK) and drought stress treatment (Student's t test, ** p < 0.01)
Discussion
SWEET genes have exhibited a widespread presence in plants, animals, and prokaryotes. They play an important role in many physiological processes, such as the regulation of metabolism, plant growth and development, plant‒pathogen interactions, and stress responses [9]. The identification and functional studies of SWEET genes in Arabidopsis [3], rice [29, 30], maize [31] and microorganisms have revealed a wide range of biological functions of SWEET genes in different plant species [32, 33]. The regulatory function of the SWEET gene family is variable in different species, hence, more species-specific information is required to determine these roles. In this study, 66 BjSWEET genes have been identified in B. juncea. Additionally, 34 BrSWEET genes and 37 BniSWEET genes have been identified in B. rapa and B. nigra (Table S7). Phylogenetic analysis showed that SWEETs may originate from Clade II, this is consistent with previously result that plant SWEETs have originated from Clade II [34]. Most of the BjSWEET genes have 2 orthologous copies. While, BjSWEET5, BjSWEET8, and BjSWEET14 have 6 orthologous copies. Whole-genome duplication/triplication events were intertwined with segregation and rediploidization in plants, and had different effects on gene families. The expanded SWEET gene family in B. rapa following whole-genome triplication events showed complex regulation [25].
Eukaryotes possess multiple copies of SWEET genes, which can facilitate the diversification of metabolic regulation. Subcellular localization analysis showed that most of BjSWEET proteins were localized in the cell membrane. While, some BjSWEET proteins were co-localized proteins with different signal peptides. The analysis of gene structure, conserved motif, and multiple sequence alignment analysis of the BjSWEETs showed that 81.8% of the BjSWEETs had seven transmembrane helices (TMHs), and all the SWEET gene family members contained the MtN3/saliva domain, suggesting that the BjSWEET genes were relatively conserved during evolution. Gene structure analysis found that most of the BjSWEETs had six exons, which was consistent with the finding that the last common ancestor of angiosperms (LCA) had six exons. According to the analysis of transmembrane helices, approximately 81.8% of BjSWEET proteins contained seven typical transmembrane α-helical domains. The number of THMs varied in the remaining BjSWEET proteins, and these results were consistent with the finding that the number of THMs increased or decreased in MtN3/saliva/SWEET proteins [9]. The phylogenetic evolutionary analysis indicated that the BjSWEET genes could be classified into 4 subfamilies. The evolutionary sequence of the 4 subfamilies is as follows: Clade I, Clade II, Clade III and Clade IV [35]. The expression of genes is mostly related to the cis-elements in the promoter regions. Cis-element analysis of the promoter regions revealed that the promoters of BjSWEET genes contained a large number of plant hormone-related response elements, stress-related response elements, growth and development elements, and light-responsive regulatory elements, indicating the possible involvement of these genes in related functional expression in B. juncea. It is widely known that sugar transporters are affected by exogenous hormones. In apple, abscisic acid (ABA) signal could enhance sugar accumulation by activating the expression of MdSWEET9b [36]. HcSWEET genes from Hemerocallis citrina may response to various hormones, light, and stresses [37]. Light responsive elements were the most diverse and abundant in the promoter regions of BjSWEET genes, implying the universal involvement of BjSWEET genes in light control expression. In Capsicum annuum, the light responsive elements are the most abundant cis-elements in the promoters of CaSWEET genes [38].
One of the crucial functions of roots is to extract water from the soil to provide water to plants. The root shows an impressive ability to influence its architecture in response to drought [39]. In Arabidopsis, AtSWEET17, which acted as a sugar transporter, participated in the sugar transport of root vacuoles and played an important role in drought stress [8]. AtSWEET11 and AtSWEET12 are induced in leaves, while AtSWEET11-15 have been upregulated in roots under drought stress [40]. SWEET17 is mainly expressed in the vasculature and meristem cells of roots, and SWEET12 is more highly expressed in roots. The BjSWEET genes may be involved in the expression of genes related to roots. The transcript expression analysis of BjSWEETs genes in eight tissues showed that BjSWEET17.1, BjSWEET17.2, BjSWEET17.3, BjSWEET17.4, BjSWEET12.2, and BjSWEET12.3 were highly expressed in roots, and some SWEET genes in B. juncea were specifically expressed in flowers, roots and stems, indicating that these genes play a role in reproductive processes and metabolic regulation in roots and stems. However, apart from light responsive elements, MeJA-responsive, abscisic acid responsive, salicylic acid responsive, and defense and stress responsive elements have been found in the promoters of BjSWEET genes. To further understand whether the BjSWEET12 and BjSWEET17 are involved in drought resistance, this study analyzed the expression profiles of SWEET12 and SWEET17 under drought treatment. The results showed that the expression of SWEET17.2, SWEET17.4, SWEET12.2 and SWEET12.3 were significantly up-regulated under drought treatment, suggesting that these genes were likely involved in the response to drought stress. These results lay a solid foundation for further study of the related molecular functions and mechanisms of SWEET genes in B. juncea.
Materials and methods
Identification and bioinformatics analysis of BjSWEET genes
Arabidopsis genome annotation information and SWEET protein sequences were obtained from TAIR (www.arabidopsis.org). Based on the homology of the Arabidopsis SWEET genes, using the AtSWEET protein sequence as the query sequence, we originally obtained the BjSWEET genes from the B. juncea ‘Xuecai’ (XC) genome (PRJCA015688) from the National Genomics Data Center (https://ngdc.cncb.ac.cn/gwh). The SWEET genes from B. rapa genome (Brara_Chiifu_V4.1/) and B. nigra genome (Brani_Ni100_V2/) were obtained from BRAD (http://brassicadb.cn) by the BLAST QUI Wrapper (Two Sequences Sets) function of TBtools [41], with an E-value threshold of 1e-5. Then we used the Venn and UpSet plot functions of TBtools to deduplicate these SWEET genes with minimum overlap size is 1. Interpro (www.ebi.ac.uk/interpro/) was used to screen the conserved domains (PF03083), and the SWEET gene family members were ultimately obtained from B. juncea. The Expasy tool (https://web.expasy.org/protparam/) was utilized to predict and analyze a variety of parameters of BjSWEET proteins, including encoded amino acids, relative molecular mass, and theoretical isoelectric points. Additionally, we predicted the subcellular localization of the SWEET protein in B. juncea using the online tool Plant-mPLoc (www.csbio.sjtu.edu.cn/bioinf/plant-multi/).
Phylogenetic and multiple sequence alignment analysis of SWEET sequences in B. juncea and Arabidopsis
The MAFFT tool was used to compare SWEET protein sequences from A.thaliana, B. rapa, B. nigra, and B. juncea with 1,000 bootstrap replicates [42]. Additionally, the phylogenetic tree of SWEET protein was constructed using IQ-TREE [43], employing the maximum-likelihood (ML) approach with 1000 replications with the parameters –m, TEST, -redo, -n, 1000.
Gene structure, conserved motifs and transmembrane helices of BjSWEETs
The SWEET protein sequences were submitted to the One Step Build a ML Tree function of TBtools to determine phylogenetic relationships. Using the Simple MEME wrapper function to predict the conserved motifs of the BjSWEET proteins with the maximum number of motifs 10, and e-value 10. The conserved domain information of the BjSWEET proteins was analyzed using the online tool NCBI Batch CD-search (www.ncbi.nlm.nih.gov). Then, the B. juncea SWEET genome annotation files, phylogenetic relationship information, conserved motif information, and domain information were submitted to the Gene Structure View function for visualization. The FASTA files of the BjSWEET protein sequences were submitted to the online tool Tmhmm 2.0 Service (https://services.healthtech.dtu.dk/services/TMHMM-2.0/) to obtain transmembrane helix structure information.
Chromosomal localization and gene duplication analysis of BjSWEETs
The genome annotation files and IDs of the BjSWEET genes were obtained from the sequenced B. juncea genome. The gene location visualize function of the GFF/GTF function of TBtools was used to visualize the chromosomal location of SWEET gene family members in B. juncea. The collinearity analysis of the SWEET gene family members in B. juncea was performed using the MCScanX function of TBtools with an E-value threshold of 1e-10.
Cis-elements in the promoters of BjSWEET genes
We obtained the 2000 bp upstream of the promoters of BjSWEETs by Gtf/Gff3 sequences extraction and fasta extraction function of TBtools. The online tool PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) was used to predict the promoter cis-elements. Then, we performed data screening based on the obtained Tab file and displayed it by the gene structure view function of TBtools selected with “Fill in gradient mode” option.
Gene expression pattern analysis of BjSWEETs
The SWEET gene expression data for eight different tissues were derived from our previous study, and the XC genome was used as a reference [44]. Fragments per kilobase pair of transcript per million mapped reads (FPKM) values of these BjSWEET genes across 8 different tissues have been listed in Table S5. The heatmap function of TBtools was subsequently used to visualize the expression of BjSWEET genes in root, hypocotyl, stem, silique, flower, leave, seed and cotyledon.
Drought treatment
The potherb mustard ‘Xuecai’ (XC) is widely used as a fresh and a pickled vegetable [44]. The selfed seeds of B. juncea inbred line XC used in this study were harvested from the experimental field at Xinyang Normal University (Xinyang, China). The seed of B. juncea inbred line XC was used in this study. Brassica juncea XC was planted in 1/2 MS agar at 25°C with 16 h of light and 8 h of darkness for 2 weeks. Then, 2 ml of sterile water (CK) or 10% (w/v) PEG6000 was injected evenly into the culture medium near the roots of each plant, respectively. Each group had 16 mustard plants for replication. The mustard XC plants treated with sterile water (CK) were used as the control plants. For phenotypic observation, samples were photographed after 7 days of treatment. Then, we sampled the roots and leaves of the plants in the CK and PEG6000 treatment.
Measurement of relative water content and malondialdehyde (MDA) content
The leaf relative water content (RWC) was measured according to a previously described method [45]. Mustard leaves with well-watered and drought treatment were collected to record the fresh weight (FW). Then, the dry weight (DW) was determined after the leaves were dried at 105°C for 2 h and 80°C until constant weight. The RWC was calculated with the formula: RWC (%) = (FW-DW)/(TW-DW) × 100%. The malondialdehyde (MDA) content was further examined with the thiobarbituric acid method [46]. The samples for RWC and MDA estimation were performed with 3 biological replicates. And 4 mustard plants were used for each replicate.
RNA extraction and qRT‒PCR
Total RNA was extracted with a TaKaRa MiniBEST Plant RNA Extraction Kit, and a RevertAid First Strand cDNA Synthesis Kit was used for cDNA synthesis. Quantitative fluorescence primers were designed using the batch qRT‒PCR primer design function and the primer check function of TBtools to check primers specificity. Based on the general principles of primer designation, for example the length of the primer is approximately 20 bp, the Tm is between 58 and 60 degrees Celsius, and the GC content is 40–60% as appropriate, to determine the final primer sequence (Table S8). The transcript expression levels of BjSWEET genes were quantified by qRT–PCR [47], and analyzed by using the 2−ΔΔCT method [48]. The BjActin gene was used as the internal control to normalize the transcript levels [49]. Each sample was performed with three technical replicates. Each reaction (total volume of 20 μL) consisted of 8.4 μL of diluted cDNA, 0.8 μL of forward primer, 0.8 μL of reverse primer, and 10 μL of 2 × SYBR qRT‒PCR Mix. The PCR cycle conditions were as follows: 95℃ for 2 min, 95℃ for 15 s, and 60℃ for 30 s. After return to the second step, 40 cycles were performed, a melting curve was generated to obtain the qRT‒PCR data. Then, the gene expression data was processed by IBM SPSS Statistics version 22.0. The Shapiro–Wilk test was used to evaluate whether the variables gene expression data between different groups were normally distributed. The gene expression data obtained by qRT‒PCR were presented as the means ± STDEV (standard deviation) and visualized using GraphPad Prism 8 software. The Student's t test was used to compare the significant difference between transcript expression levels of BjSWEET genes between drought stress and sterile water treatment. Asterisk denotes statistically significant differences between sterile watered (CK) and drought stress treatment (Student's t test, ** p < 0.01).
Supplementary Information
Additional file 1: Figure S1. Multiple sequence alignment of SWEET family proteins in Arabidopsis and B. juncea. The conserved domain of the BjSWEET proteins was localized at the N-terminus. The box showed that all 66 BjSWEET genes have a segment of conserved sequence constituting the conserved MtN3-slv or PQ-loop superfamily.
Additional file 2: Figure S2. Specific-conserved motifs of SWEET family proteins in B. juncea. The colored boxes represent different conserved motifs with different sequences and sizes.
Additional file 3: Figure S3. Gene duplication of BjSWEETs on chromosomes. Colored lines indicate duplicated SWEET gene pairs on the same chromosome.
Additional file 4: Table S1. Characteristics of the SWEET genes identified in B. juncea. Table S2. Conserved motifs and functional annotation of BjSWEETs. Table S3. The gene duplication pairs of SWEET genes in B. juncea. Table S4 Analysis of cis-acting elements in the SWEET gene family of B. juncea. Table S5 FPKM values of BjSWEET genes in 8 different tissues of B. juncea. Table S6 BjSWEET genes analyzed by qRT‒PCR. Table S7 SWEET genes in B. nigra and B. rapa. Table S8 Primers used for qRT‒PCR.
Acknowledgements
Not applicable.
Authors’ contributions
SPH conceived and designed all the experiments of the project. JJH, XYZ, JYC, and MKF performed experiments and analyzed the data. JJH performed bioinformatics analysis. JJH and SPH wrote the manuscript. SPH, SHZ, WZ, FX and GZM contributed materials and reagents. All authors read and approved the final manuscript.
Funding
This research was supported by grants from the National Natural Science Foundation of China (32002056), Henan Provincial Natural Science Foundation Project (232300420021), and the Nanhu Scholars Program for Young Scholars of XYNU.
Data availability
The transcriptome data was deposited in National Genomics Data Center with accession number PRJCA015688. Other datasets used and analyzed during the current study are contained within the article or supplementary files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–94. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Zhou L, Li T, Ruan Y, Zhang A, Dong X, Zhu Y, Li C, Fan J. Genome-wide investigation and characterization of SWEET gene family with focus on their evolution and expression during hormone and abiotic stress response in maize. Genes (Basel). 2022;13(10):1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L-Q, Hou B-H, Lalonde S, Takanaga H, Hartung ML, Qu X-Q, Guo W-J, Kim J-G, Underwood W, Chaudhuri B, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468(7323):527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eom JS, Chen LQ, Sosso D, Julius BT, Lin IW, Qu XQ, Braun DM, Frommer WB. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr Opin Plant Biol. 2015;25:53–62. [DOI] [PubMed] [Google Scholar]
- 5.Chen LQ. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014;201(4):1150–5. [DOI] [PubMed] [Google Scholar]
- 6.Chan L. Research advances in SWEET gene family in plants. Plant Physiol. 2014;50(9):1367–73. [Google Scholar]
- 7.Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science (New York, NY). 2012;335(6065):207–11. [DOI] [PubMed] [Google Scholar]
- 8.Guo WJ, Nagy R, Chen HY, Pfrunder S, Yu YC, Santelia D, Frommer WB, Martinoia E. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiol. 2014;164(2):777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan M, Wang S. Rice MtN3/saliva/SWEET family genes and their homologs in cellular organisms. Mol Plant. 2013;6(3):665–74. [DOI] [PubMed] [Google Scholar]
- 10.Patil G, Valliyodan B, Deshmukh R, Prince S, Nicander B, Zhao M, Sonah H, Song L, Lin L, Chaudhary J, et al. Soybean (Glycine max) SWEET gene family: insights through comparative genomics, transcriptome profiling and whole genome re-sequence analysis. BMC Genomics. 2015;16:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manck-Götzenberger J, Requena N. Arbuscular mycorrhiza symbiosis induces a major transcriptional reprogramming of the Potato SWEET sugar transporter family. Front Plant Sci. 2016;7:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng L, Frommer WB. Structure and function of SemiSWEET and SWEET sugar transporters. Trends Biochem Sci. 2015;40(8):480–6. [DOI] [PubMed] [Google Scholar]
- 13.Mizuno H, Kasuga S, Kawahigashi H. The sorghum SWEET gene family: stem sucrose accumulation as revealed through transcriptome profiling. Biotechnol Biofuels. 2016;9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Liu L, Huang W, Yuan M, Zhou F, Li X, Lin Y. Overexpression of OsSWEET5 in rice causes growth retardation and precocious senescence. PLoS ONE. 2014;9(4):e94210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong J, Piron MC, Meyer S, Merdinoglu D, Bertsch C, Mestre P. The SWEET family of sugar transporters in grapevine: VvSWEET4 is involved in the interaction with Botrytis cinerea. J Exp Bot. 2014;65(22):6589–601. [DOI] [PubMed] [Google Scholar]
- 16.Sun MX, Huang XY, Yang J, Guan YF, Yang ZN. Arabidopsis RPG1 is important for primexine deposition and functions redundantly with RPG2 for plant fertility at the late reproductive stage. Plant Reprod. 2013;26(2):83–91. [DOI] [PubMed] [Google Scholar]
- 17.Seo PJ, Park J-M, Kang SK, Kim S-G, Park C-M. An Arabidopsis senescence-associated protein SAG29 regulates cell viability under high salinity. Planta. 2011;233(1):189–200. [DOI] [PubMed] [Google Scholar]
- 18.Gookin TE, Kim J, Assmann SM. Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: computational prediction and in-vivo protein coupling. Genome Biol. 2008;9(7):R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asai Y, Kobayashi Y. Increased Expression of the Tomato SISWEET15 Gene During Grey Mold Infection and the Possible Involvement of the Sugar Efflux to Apoplasm in the Disease Susceptibility. J Plant Pathol Microbiol. 2016;07(01):1000329. [Google Scholar]
- 20.Lin IW, Sosso D, Chen L-Q, Gase K, Kim S-G, Kessler D, Klinkenberg PM, Gorder MK, Hou B-H, Qu X-Q, et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature. 2014;508(7497):546–9. [DOI] [PubMed] [Google Scholar]
- 21.Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, Refay Y, Dindaroglu T, Abdul-Wajid HH, Battaglia ML. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants (Basel, Switzerland). 2021;10(2):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valifard M, Le Hir R, Müller J, Scheuring D, Neuhaus HE, Pommerrenig B. Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance. Plant Physiol. 2021;187(4):2716–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Q, Hu T, Li X, Song CP, Zhu JK, Chen L, Zhao Y. Phosphorylation of SWEET sucrose transporters regulates plant root:shoot ratio under drought. Nature plants. 2022;8(1):68–77. [DOI] [PubMed] [Google Scholar]
- 24.Miao L, Lv Y, Kong L, Chen Q, Chen C, Li J, Zeng F, Wang S, Li J, Huang L, et al. Genome-wide identification, phylogeny, evolution, and expression patterns of MtN3/saliva/SWEET genes and functional analysis of BcNS in Brassica rapa. BMC Genomics. 2018;19(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei Y, Xiao D, Zhang C, Hou X. The expanded SWEET gene family following whole genome triplication in Brassica rapa. Genes. 2019;10(9):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Wang S, Yu F, Tang J, Shan X, Bao K, Yu L, Wang H, Fei Z, Li J. Genome-wide characterization and expression profiling of SWEET genes in cabbage (Brassica oleracea var. capitata L.) reveal their roles in chilling and clubroot disease responses. BMC genomics. 2019;20(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jian H, Lu K, Yang B, Wang T, Zhang L, Zhang A, Wang J, Liu L, Qu C, Li J. Genome-wide analysis and expression profiling of the SUC and SWEET gene families of sucrose transporters in oilseed rape (Brassica napus L.). Front Plant Sci. 2016;7:1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nu: Genome analysis in Brassica with special reference to the experimental formation of B napus and peculiar mode of fertilization. Jour Bot. 1935;7:389-452.
- 29.Yang B, Sugio A, White FF. Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci U S A. 2006;103(27):10503–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony G, Zhou J, Huang S, Li T, Liu B, White F, Yang B. Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os-11N3. Plant Cell. 2010;22(11):3864–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Fan H, Ma Z. Comparison of SWEET gene family between maize and foxtail millet through genomic, transcriptomic, and proteomic analyses. Plant Genome. 2022;15(3):e20226. [DOI] [PubMed] [Google Scholar]
- 32.Xuan YH, Hu YB, Chen LQ, Sosso D, Ducat DC, Hou B-H, Frommer WB. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc Natl Acad Sci. 2013;110:E3685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Y, Tao Y, Cheung LS, Fan C, Chen L-Q, Xu S, Perry K, Frommer WB, Feng L. Structures of bacterial homologues of SWEET transporters in two distinct conformations. Nature. 2014;515(7527):448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei Y, Xiao D, Zhang C, Hou X. The expanded SWEET gene family following whole genome triplication in Brassica rapa. Genes. 2019;10(9):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W, Hua X, Zhang Q, Wang J, Shen Q, Zhang X, Wang K, Yu Q, Lin YR, Ming R, et al. New insights into the evolution and functional divergence of the SWEET family in Saccharum based on comparative genomics. BMC Plant Biol. 2018;18(1):270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang S, Wang H, Wang T, Zhang J, Liu W, Fang H, Zhang Z, Peng F, Chen X, Wang N. Abscisic acid and regulation of the sugar transporter gene MdSWEET9b promote apple sugar accumulation. Plant Physiol. 2023;192(3):2081–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao L, Wang J, Wang L, Liu H, Wu W, Hou F, Liu Y, Gao Y, Cheng X, Li S, et al. Genome-wide analysis of the SWEET gene family in Hemerocallis citrina and functional characterization of HcSWEET4a in response to salt stress. BMC Plant Biol. 2024;24(1):661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X, Han S, Zhu Y, Liu Y, Gao S, Yin J, Wang F, Yao M. Genome-wide identification and expression analysis of the SWEET gene family in capsicum annuum L. Int J Mol Sci. 2023;24(24):17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comas LH, Becker SR, Cruz VM, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front Plant Sci. 2013;4:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durand M, Porcheron B, Hennion N, Maurousset L, Lemoine R, Pourtau N. Water Deficit Enhances C Export to the Roots in Arabidopsis thaliana Plants with Contribution of Sucrose Transporters in Both Shoot and Roots. Plant Physiol. 2016;170(3):1460–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol Plant. 2020;13(8):1194–202. [DOI] [PubMed] [Google Scholar]
- 42.Rozewicki J, Li S, Amada KM, Standley DM, Katoh K. MAFFT-DASH: integrated protein sequence and structural alignment. Nucleic Acids Res. 2019;47(W1):W5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol Biol Evol. 2020;37(5):1530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heng S, Cui M, Li X, Zhang S, Mao G, Xing F, Wan Z, Wen J, Shen J, Fu T. High quality genome of potherb mustard XC (Brassica juncea var. multiceps) provides new insight into leaf shape variation. J Integrative Agri. 2024. 10.1016/j.jia.2024.04.031.
- 45.Jiao S, Zeng F, Huang Y, Zhang L, Mao J, Chen B. Physiological, biochemical and molecular responses associated with drought tolerance in grafted grapevine. BMC Plant Biol. 2023;23(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ge M, Tang Y, Guan Y, Lv M, Zhou C, Ma H, Lv J. TaWRKY31, a novel WRKY transcription factor in wheat, participates in regulation of plant drought stress tolerance. BMC Plant Biol. 2024;24(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wormit A, Trentmann O, Feifer I, Lohr C, Tjaden J, Meyer S, Schmidt U, Martinoia E, Neuhaus HE. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell. 2006;18(12):3476–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–8. [DOI] [PubMed] [Google Scholar]
- 49.Heng S, Gao J, Wei C, Chen F, Li X, Wen J, Yi B, Ma C, Tu J, Fu T, et al. Transcript levels of orf288 are associated with the hau cytoplasmic male sterility system and altered nuclear gene expression in Brassica juncea. J Exp Bot. 2018;69(3):455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Multiple sequence alignment of SWEET family proteins in Arabidopsis and B. juncea. The conserved domain of the BjSWEET proteins was localized at the N-terminus. The box showed that all 66 BjSWEET genes have a segment of conserved sequence constituting the conserved MtN3-slv or PQ-loop superfamily.
Additional file 2: Figure S2. Specific-conserved motifs of SWEET family proteins in B. juncea. The colored boxes represent different conserved motifs with different sequences and sizes.
Additional file 3: Figure S3. Gene duplication of BjSWEETs on chromosomes. Colored lines indicate duplicated SWEET gene pairs on the same chromosome.
Additional file 4: Table S1. Characteristics of the SWEET genes identified in B. juncea. Table S2. Conserved motifs and functional annotation of BjSWEETs. Table S3. The gene duplication pairs of SWEET genes in B. juncea. Table S4 Analysis of cis-acting elements in the SWEET gene family of B. juncea. Table S5 FPKM values of BjSWEET genes in 8 different tissues of B. juncea. Table S6 BjSWEET genes analyzed by qRT‒PCR. Table S7 SWEET genes in B. nigra and B. rapa. Table S8 Primers used for qRT‒PCR.
Data Availability Statement
The transcriptome data was deposited in National Genomics Data Center with accession number PRJCA015688. Other datasets used and analyzed during the current study are contained within the article or supplementary files.