Abstract
A low metabolic flexibility to lipid (MetF-lip) in skeletal muscle may promote ectopic lipid accumulation, thus inducing metabolic disturbances. We aimed to determine the association between MetF-lip in skeletal muscle and metabolic health outcomes in individuals without obesity. We also explored the association between MetF-lip and the inflammatory signaling pathway in skeletal muscle. This was a cross-sectional study in 17 individuals aged (median [IQR]) 55.4 [48.6, 58.5] years, with a BMI of 24.4 [22.6, 26.0] kg/m2. MetF-lip was assessed as the increase in relative lipid oxidation during a single exercise session (~ 50% VO2max, 2 hours), quantified as the drop in whole-body respiratory exchange ratio (ΔRER = RER at 2 hours - maximum RER attained). HOMA-IR, metabolic syndrome z-score, fat percentage, trunk-to-appendicular fat, and VO2max were included as metabolic health outcomes. The abundance of proteins of the inflammatory pathway was analyzed in resting muscle. Acute exercise progressively increased relative lipid oxidation (ΔRER = -0.04 [-0.08, -0.02]). MetF-lip was not associated with any metabolic health outcome but correlated inversely with p-p38Thr180/Tyr182 in muscle. A low MetF-lip in skeletal muscle does not seem a major determinant of metabolic disturbances but associates with a partial activation of the inflammatory signaling in individuals without obesity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79092-w.
Keywords: Mitogen-activated protein kinases, Cardiorespiratory Fitness, Body composition, Insulin resistance
Subject terms: Metabolism, Obesity
Introduction
Metabolic flexibility is the capacity to adapt fuel oxidation to fuel availability so ATP synthesis can match its demand1. Low metabolic flexibility has been linked to obesity and obesity-associated disturbances including insulin resistance, metabolic syndrome, and type 2 diabetes (for comprehensive reviews see1–3). In skeletal muscle, low metabolic flexibility to lipid (MetF-lip) may promote the accumulation of lipid metabolites and the activation of pro-inflammatory pathways that interfere with insulin signaling4,5. Impaired insulin sensitivity then increases the demand on the organism to maintain homeostasis, i.e. increases the allostatic load6. In the long term, this may trigger the accumulation of metabolic syndrome components as different organs/tissues become compromised7,8.
The metabolic flexibility of skeletal muscle has been measured by challenging the capacity to oxidize glucose with the hyperinsulinemic-euglycemic clamp9–12 and certain exercise protocols13,14. Nevertheless, in the context of lipid-induced insulin resistance, the measurement of MetF-lip appears more relevant. A protocol of incremental exercise intensity has been used to measure MetF-lip in adults15,16. This protocol challenges the capacity of skeletal muscle to sustain lipid oxidation as the metabolic rate increases. Maintaining a high absolute (in g/min) and relative (using the respiratory exchange ratio [RER = VCO2/VO2]) lipid oxidation as intensity increases would reflect high MetF-lip15,16. Using this protocol, adult athletes manifested higher metabolic flexibility than their counterparts with metabolic syndrome15 or excess body weight16. This protocol, however, does not consider lipid availability, a central component of the metabolic flexibility concept.
We previously measured MetF-lip in skeletal muscle using a moderate-intensity (50% VO2max) endurance exercise of long duration (∼80 min)17. The rationale is that continuous, moderate-intensity exercise (40–65% VO2max) progressively increases lipid availability in the form of circulating non-esterified fatty acids (NEFA)17–19. The increased lipid availability then leads to increased lipid oxidation in active skeletal muscle, manifested as a drop in RER. Thus, the protocol challenges the capacity of active skeletal muscle to adapt lipid oxidation (ΔRER) to lipid availability (ΔNEFA)17. This is the core idea of metabolic flexibility focused on lipid, i.e., the capacity to adapt fuel (lipid) oxidation to fuel (lipid) availability1. In our previous study, we split young healthy men into “high” and “low” insulin sensitivity groups based on the median of the homeostatic model assessment of insulin resistance (HOMA-IR). ΔRER and ΔNEFA correlated inversely only in participants with “high” insulin sensitivity. This better matching between lipid oxidation and availability suggested an enhanced MetF-lip in the group with “high” insulin sensitivity. But when individual MetF-lip was computed as the ΔRER adjusted by ΔNEFA, no differences between groups were detected. Interestingly, we noted an enhanced MetF-lip in individuals with high versus low insulin sensitivity at high ΔNEFA values (> 0.45 mM). Such a threshold, however, was achieved only by 7 out of 20 participants. The fact that participants were healthy and insulin sensitive (HOMA-IR from 0.39 to 1.65) may also explain the lack of difference in MetF-lip between groups. These findings suggested that larger increases in circulating NEFA and a wider spectrum of metabolic disturbances among individuals could help unmask a potential association between MetF-lip and metabolic health.
Herein, we measured MetF-lip using an exercise protocol of moderate intensity and longer duration (120 min) than our previous study to achieve ΔNEFA > 0.45 mM. We enrolled participants with metabolic disturbances but having normal weight or overweight to avoid other obesity-related comorbidities (e.g., lung dysfunction). As the prevalence of metabolic disturbances in individuals with normal weight and overweight increases with age20, we recruited middle-aged adults. Thus, we aimed to determine the association between MetF-lip in skeletal muscle and metabolic health outcomes in individuals without obesity. Additionally, for the first time, we explored the association between MetF-lip in skeletal muscle and the inflammatory signaling pathway in resting skeletal muscle.
Materials and methods
Participants
We included individuals that met the following eligibility criteria: [a] man or woman; [b] 40 to 64 years old; [c] normal weight or moderately overweight as indicated by a body mass index between 18.5 and 27.0 kg/m2; [d] self-reported stability of body weight (< 3 kg of change in the last three months); [e] free of diagnosed chronic diseases (metabolic risk factors such as impaired glycemia were allowed); [f] low-risk alcohol consumption as assessed by the Alcohol Use Disorders Identification Test21; [g] level of moderate-vigorous physical activity ≤1,200 MET×min/week as assessed by the Global Physical Activity Questionnaire22; [h] not taking medications; and [i] not pregnant (in women). All participants signed a written informed consent form before participating. The Scientific Ethics Committee at Universidad Finis Terrae approved the study (#76-18-2021). All procedures were performed in accordance with the Declaration of Helsinki.
Design
This was an observational, cross-sectional, analytical study conducted between 2019 and 2022. Supplementary Table 1 shows the STROBE checklist for observational studies. Potential participants were recruited via flyers, social media, and word of mouth. Interested individuals completed an online survey to pre-assess the eligibility criteria. Pre-selected individuals were invited to a screening visit. Individuals arrived after 10–14 h of fasting, and the necessary questionnaires along with standard clinical measurements were conducted (anthropometry, arterial blood pressure, electrocardiogram, temperature, and others). A venous blood sample was obtained to measure biochemical and lipid profiles, hemogram, circulating concentrations of thyroid stimulating hormone and free thyroxine, prothrombin time, and plasma electrolytes. Selected participants were invited to participate in four additional visits to: [a] determine the VO2max and daily time spent on physical behaviors; [b] assess MetF-lip; [c] determine body composition; and [d] obtain a skeletal muscle biopsy. Body weight was measured in each visit to ensure weight stability throughout the study (±2 kg from the screening).
VO2max and daily time spent on physical behaviors
VO2max was determined with an exercise test of incremental intensity in a cyclo-ergometer. The test began at 0 W for 2 min, followed by 50 W for 2 min, and then increments of 25 W every 2 min until exhaustion. Participants had to maintain a cadence of 55–65 rpm. Breath-by-breath VO2 and VCO2 (Ergocard, Medisoft), and heart rate (H10, Polar) were measured throughout the test. Participants were considered to reach exhaustion if were unable to continue pedaling even after verbal encouragement, or if were unable to maintain the minimum cadence. The last 30 s of gas exchange at each workload, along with the heart rate at the end of each workload, were used to determine whether the participant achieved the VO2max. Following the criteria described by Howley et al.23, participants were considered to achieve their VO2max if attaining at least one of these: [a] a plateau in VO2 as indicated by an increase < 150 mL/min in successive, incremental workloads; [b] RER > 1.10; or [c] heart rate within 10 bpm of the maximal heart rate predicted as 202 − 0.72 × age in years24. After confirming that the VO2max was reached, we computed a linear regression between VO2 and workload to calculate the workload (in W) that required 50% VO2max. Such a workload was then used in the exercise challenge to measure MetF-lip.
After the test, participants received an accelerometer to be worn on the hip for at least one week (90 Hz sampling frequency; wGT3X-BT, Actigraph). They were instructed to wear it while awake, except while showering or swimming. ActiLife version 6.13.4 (Actigraph) was used to process the raw data in 60-s epochs using the normal filter. Wear time was estimated according to Choi et al.25. A day was considered valid if had ≥10 h of recording. Thus, our participants wore the accelerometer a median [25th percentile, 75th percentile] of 13.8 [13.0, 14.6] hours/day for a minimum of 6 days. Time spent on physical behaviors was estimated as previously described26. In brief, sedentary behavior according to Kozey-Keadle et al.27, moderate-vigorous physical activity according to Sasaki et al.28, and light-intensity physical activity as the remaining wear time. Time spent on each physical behavior was weighted by weekdays and weekend days as described before29.
MetF-lip
MetF-lip was measured during an exercise challenge in cyclo-ergometer adapted from our previous protocol17. Participants were requested to avoid medication, supplements, alcohol, cigarettes, energy drinks, coffee, tea, and vigorous physical activity the day before this visit. They had to be free of injuries or other conditions that could impair exercise performance. Participants received a meal to be eaten the evening before the visit. The meal contained 30% of daily energy requirements estimated using sex-specific equations that consider age, weight, height, waist circumference, and resting metabolic rate30 (resting metabolic rate estimated from age and height31). 60% of the energy was provided as carbohydrates in an attempt to match glycogen stores in skeletal muscle among participants. Then, participants had to sleep at least seven hours and arrive at the laboratory after 10–14 h of overnight fasting. A venous blood sample was drawn while resting before exercise. Participants then warmed up at 0 W for 2 min in the cycle-ergometer. The exercise challenge began at the power (W) calculated to require 50% VO2max. After 8 min of exercise, power was adjusted to approach the expected VO2. Then, participants maintained the power until completing 120 min of exercise. The cadence was maintained at 55–65 rpm throughout the exercise. VO2 and VCO2 (Ergocard, Medisoft), along with heart rate (H10, Polar) were measured at 8, 15, 30, 60, 90, and 120 min of exercise (average of 3 min in each time point). RER was calculated from VO2 and VCO2 measurements. Venous blood samples were obtained at 15, 30, 60, 90, and 120 min of exercise.
MetF is the capacity to adapt fuel oxidation to fuel availability during metabolic challenges1. The adaptation in fuel oxidation was measured as the change in RER during exercise. This was computed as the difference between the RER at the final of the exercise and the maximum RER measured (ΔRER = RERfinal - RERmax). Negative ΔRER values indicate a drop in RER, which represents an increase in lipid oxidation relative to carbohydrate oxidation32. The adaptation in fuel availability was measured as the change in the circulating concentrations of NEFA. This was computed as the difference between the NEFA at the final of the exercise and the minimum NEFA measured (ΔNEFA = NEFAfinal - NEFAmin). Positive ΔNEFA values indicate an increase in the circulating availability of lipids. Thus, MetF was calculated as the ΔRER during exercise, adjusted for ΔNEFA if required (see Results). As the drop in RER during exercise is mostly driven by muscle activity, our method is deemed to measure MetF-lip in skeletal muscle.
Response of energy metabolism and circulating markers to the exercise challenge
Energy expenditure (in kcal/min) and the contribution of lipids and carbohydrates at each time point were calculated according to Elia and Livesey32. Null contribution of proteins was assumed. Total energy expenditure during exercise was computed as the AUC of energy expenditure assuming a constant expenditure during the first 8 min. Lipid and carbohydrate oxidation (in g/min) was calculated by assuming 9.41 kcal/g of lipid and 4.18 kcal/g of carbohydrates32. Circulating markers included plasma glucose (by dry chemistry), plasma lactate (by enzymatic/colorimetric analysis), serum insulin (by chemiluminescence), serum NEFA (NEFA-HR[2], Wako Diagnostics, Richmond, VA, USA), serum glycerol (MAK117, Sigma- Aldrich, St. Louis, MO, USA), serum triglycerides (by dry chemistry), and serum β-hydroxybutyrate (βOHB; #700190, Cayman Chemical, Ann Arbor, MI, USA).
Metabolic health outcomes
HOMA-IR was calculated from the blood sample withdrawn before beginning the exercise challenge as: plasma glucose [mM] × serum insulin [mIU/L] / 22.5. The components of the metabolic syndrome were obtained during the screening visit. These components included: waist circumference (midpoint between lower rib and iliac crest), serum HDL-C, serum triglycerides, systolic and diastolic blood pressure (by mercury sphygmomanometer), and plasma glucose concentration. Mean arterial pressure was computed as: diastolic [mmHg] + (systolic [mmHg] - diastolic [mmHg]) / 3. A metabolic syndrome z-score specific for Chilean adults was computed by integrating all metabolic syndrome components, as described before8. Fat mass, fat-free mass, fat percentage, trunk fat, arms fat, and gluteofemoral fat were determined by dual-energy X-ray absorptiometry (Lunar iDXA, GE Healthcare). Trunk-to-appendicular fat was computed as: trunk fat [kg] / (arms fat [kg] + gluteofemoral fat [kg]). VO2max was also considered a metabolic health outcome.
Skeletal muscle biopsy and inflammatory signaling pathway
Participants arrived at the laboratory after 10–14 h of overnight fasting. A muscle biopsy was taken at rest from the Vastus lateralis muscle using the Bergstrom technique with suction under local anesthesia (2% lidocaine). The sample was immediately frozen in liquid nitrogen and then stored at -80 °C. For the protein extraction, muscle biopsies were homogenized in a lysis buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 2 mM EDTA, 20 mM NaF, 1 mM Na2PO7, 10% glycerol, 150 mM NaCl, 10 mM Na3VO4, 1 mM PMSF, and a protease inhibitor cocktail (Complete TM, Roche Applied Science). Protein concentration was measured using the Micro BCA™ Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
For western blotting, 40–50 mg of proteins were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Thermo Fisher Scientific, Waltham, MA, USA). Two gels were processed in parallel to accommodate all the samples each time. The immunoblotting was developed with these primary antibodies from Santa Cruz (Dallas, TX, USA): mouse anti-TLR4 (1:1000, SC-293072), mouse anti-GAPDH (1:2000, SC-47724), and mouse anti-phospho-NFkBSer536 (1:1000, SC-136548); or with these primary antibodies from Cell Signaling (Danvers, MA, USA): rabbit anti-Myd88 (1:1000, 4283 S), rabbit anti-phospho-TAK1Ser412 (1:1000, 9339 S), rabbit anti-TAK1 (1:500, 4505 S), rabbit anti-phospho-p38Thr180/Tyr182 (1:1000, 9215 S), rabbit anti-p38 (1:1000, 9212 S), rabbit anti-phospho-ERK1/2Thr202/Tyr204 (1:1000, 9101 S), rabbit anti-ERK1/2 (1:1000, 4377 S), rabbit anti-phospho-JNKThr183/Tyr185 (1:1000, 9251 S), rabbit anti-JNK (1:1000, 9252 S), and rabbit anti-NFkB (1:1000, 8242 S). The membranes were then incubated with an appropriate secondary antibody (Santa Cruz, Dallas, TX, USA): goat anti-mouse IgGHRP (1:3000) or mouse anti-rabbit IgG-HRP (1:3000). The immunoreaction was visualized by enhanced chemiluminescence (Thermo Scientific, Waltham, MA, USA).
Images were acquired using the Fotodyne FOTO/Analyst Luminary Workstation Systems (Fisher Scientific, St. Waltham, MA, USA). Bands density was obtained with the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The same two control samples were charged in the extremes of each gel, and the ratio of their density was used to normalize the other samples within the gel: sample density / (control 1 density / control 2 density). Protein abundance was then expressed relative to a control protein and expressed in arbitrary units.
Statistics
Analyses were conducted in R version 4.3.3. A P-value lower than 0.05 was considered statistically significant. As normality tests have low power with sample sizes as the one in our current study33, we used non-parametric tests because they do not have the assumption of normally distributed data. Data are presented as median with interquartile ranges (25th percentile, 75th percentile). Friedman test for repeated measurements was used to determine the changes in metabolic variables during exercise; in the case of statistical significance, the Sign post-hoc test (two-sided) was used to compare the first time point versus the other time points. The Bonferroni correction was applied to adjust for multiple comparisons (adjusted P-value = 0.05 / 5 comparisons = 0.01). Spearman rho was used to test the correlations between variables.
Results
Characteristics of the participants and their response to exercise
Table 1 summarizes the main characteristics of participants. Eight men and nine women were included. By design, all participants were classified as normal-weight or moderately overweight according to their BMI. Yet three men and one woman had fat percentages consistent with obesity (≥28% for men and ≥40% for women) according to values calculated from 4-compartment estimates of a large sample of individuals34. Three men and three women engaged in ≥9.5 h/day of sedentary behavior, which is associated with an increased risk of all-cause mortality35. One man and two women engaged in < 150 min/week of moderate-vigorous physical activity, thus classifying as physically inactive36,37. Four men and seven women had VO2max values (in mL/kg×min) considered as poor or very poor by age and sex38. We used the harmonized definition of metabolic syndrome and specific cutoffs for Chilean adults to identify metabolic disturbances20,39–41. Thus, four men and four women had elevated waist circumference; two men and five women had reduced circulating HDL-C concentration; three men and four women had elevated blood pressure; two men and two women had elevated circulating triglyceride concentration; and one man had elevated glycemia. In total, one man and three women were afflicted with metabolic syndrome. Also, two men and three women had elevated HOMA-IR according to a Chilean cutoff of > 2.6. The interindividual variability in these variables was wide by design to maximize the potential of detecting an association with MetF-lip.
Table 1.
General characteristics of the participants.
| All (n = 17) | Men (n = 8) | Women (n = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | p25 | p75 | Median | p25 | p75 | Median | p25 | p75 | |
| Age, years | 55.4 | 48.6 | 58.5 | 55.6 | 50.2 | 59.4 | 52.2 | 46.2 | 58.5 |
| Weight, kg | 62.1 | 58.6 | 73.9 | 73.9 | 60.5 | 77.6 | 59.7 | 55.6 | 63.0 |
| Height, m | 1.58 | 1.56 | 1.71 | 1.71 | 1.68 | 1.75 | 1.57 | 1.55 | 1.58 |
| Body mass index, kg/m2 | 24.4 | 22.6 | 26.0 | 25.3 | 22.0 | 26.0 | 24.2 | 22.6 | 25.3 |
| Body composition and distribution | |||||||||
| Fat mass, kg | 21.5 | 16.9 | 23.1 | 20.8 | 16.4 | 22.9 | 21.5 | 18.6 | 23.3 |
| Fat-free mass, kg | 43.8 | 36.4 | 53.7 | 53.7 | 46.4 | 54.7 | 36.5 | 36.1 | 39.7 |
| Fat mass, % | 30.9 | 27.1 | 37.5 | 27.1 | 26.8 | 29.5 | 37.4 | 31.3 | 39.0 |
| Trunk-to-appendicular fat | 1.38 | 1.20 | 1.73 | 1.47 | 1.27 | 1.75 | 1.28 | 1.15 | 1.59 |
| Physical behaviors | |||||||||
| Accelerometer wear time, min/day | 827 | 781 | 875 | 833 | 785 | 955 | 816 | 779 | 846 |
| Sedentary behavior, min/day | 517 | 489 | 675 | 549 | 507 | 697 | 500 | 447 | 625 |
| Ligh-intensity physical activity, min/day | 230 | 183 | 264 | 227 | 207 | 249 | 248 | 167 | 317 |
| Moderate-vigorous physical activity, min/day | 39 | 30 | 60 | 41 | 29 | 61 | 38 | 28 | 60 |
| Metabolic health outcomes | |||||||||
| VO2max, L/min | 1.42 | 1.30 | 2.20 | 2.17 | 1.77 | 2.55 | 1.34 | 1.08 | 1.40 |
| VO2max, mL/kg body mass×min | 23.6 | 21.2 | 30.0 | 29.6 | 24.1 | 34.0 | 21.2 | 18.3 | 24.2 |
| VO2max, mL/kg fat-free mass×min | 37.1 | 30.8 | 41.8 | 40.6 | 35.5 | 46.8 | 31.6 | 29.6 | 39.3 |
| Waist circumference, cm | 83.2 | 77.5 | 91.0 | 91.0 | 82.0 | 92.8 | 81.6 | 76.3 | 83.6 |
| Systolic blood pressure, mmHg | 121 | 119 | 126 | 122 | 120 | 127 | 120 | 110 | 123 |
| Diastolic blood pressure, mmHg | 81 | 76 | 87 | 80 | 78 | 89 | 82 | 74 | 85 |
| Mean arterial pressure, mmHg | 94.3 | 89.3 | 99.7 | 94.3 | 92.3 | 100.8 | 95.3 | 85.8 | 98.2 |
| Plasma glucose, mg/dL | 90.0 | 86.0 | 91.5 | 90.0 | 88.5 | 97.0 | 88.0 | 83.5 | 91.5 |
| Serum triglycerides, mg/dL | 85.0 | 76.5 | 139.5 | 103.0 | 79.0 | 194.0 | 83.0 | 73.0 | 133.0 |
| Serum HDL-C, mg/dL | 51.0 | 40.5 | 63.0 | 53.5 | 38.5 | 60.0 | 49.0 | 40.5 | 65.0 |
| Metabolic syndrome, z-score | -1.40 | -4.30 | -0.21 | -1.54 | -4.39 | -0.08 | -1.40 | -4.30 | -0.32 |
| HOMA-IR | 1.33 | 0.71 | 2.71 | 1.08 | 0.37 | 2.49 | 1.36 | 0.91 | 3.40 |
HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; p25, percentile 25th; p75, percentile 75th; VO2max, maximum oxygen consumption.
Fifteen participants completed the 120-min exercise challenge. One man withdrew at 90 min, and one woman at 60 min. The final RER and NEFA values used in subsequent calculations correspond with those exercise durations (i.e., values obtained at 120 min for 15 participants, 90 min for one participant, and 60 min for one participant). The exercise challenge proceeded at 48.2% [45.6, 49.2] of VO2max and 67.9% [65.0, 75.8] of maximum heart rate.
Response of circulating metabolites and insulin to exercise
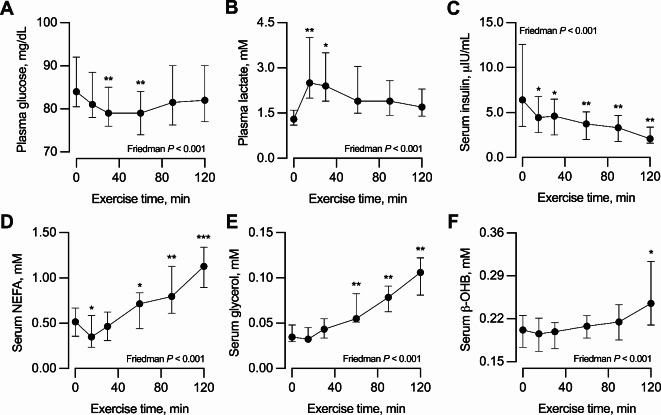
The exercise modified the circulating concentrations of all circulating metabolites and insulin. Glucose decreased slightly at 30 and 60 min of exercise but then recovered the pre-exercise concentrations (Fig. 1A). Lactate increased at 15 and 30 min of exercise and then progressively returned to pre-exercise concentrations (Fig. 1B). Insulin decreased progressively during exercise (Fig. 1C). As expected, NEFA decreased at 15 min but then increased progressively throughout the remaining exercise (Fig. 1D). ΔNEFA (i.e., NEFAfinal - NEFAmin) was 0.67 [0.46, 0.88] mM, with 14 participants achieving ΔNEFA values above the 0.45 mM threshold. Glycerol increased progressively during exercise (Fig. 1E). Finally, beta-hydroxybutyrate increased slightly but progressively during exercise (Fig. 1F).
Fig. 1.
Response to exercise of circulating (A) glucose, (B) lactate, (C) insulin, (D) non-esterified fatty acids [NEFA], (E) glycerol, and (F) beta-hydroxybutyrate [ß-OHB]. Data are median with interquartile range. Measurements were conducted before exercise (time 0 min) and at 15, 30, 60, 90, and 120 min of exercise. n = 17, except in time points 90 min [one missing value] and 120 min [two missing values]. The effect of exercise was tested with the Friedman test for repeated measurements; therefore, only the 15 participants with data in all time points were included in the analyses. *P < 0.05, **P < 0.01, ***P < 0.001 vs. time point 0 min (before exercise) for the Sign post-hoc test with Bonferroni adjustment.
Energy expenditure and fuel utilization during exercise
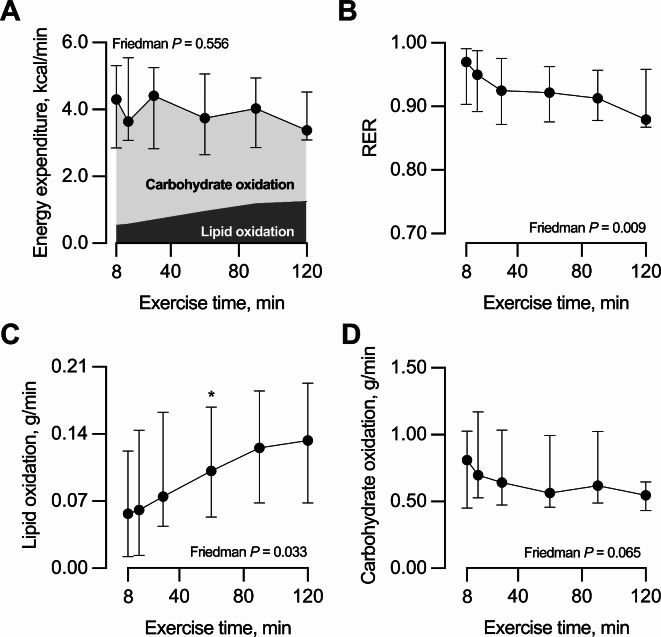
Energy expenditure (in kcal/min) remained constant during exercise (Fig. 2A). Total energy expenditure during exercise was 452 [330, 570] kcal. As expected, the RER decreased progressively during exercise, with a ΔRER value (i.e., RERfinal - RERmax) of -0.04 [-0.08, -0.02] (Fig. 2B). This ΔRER reflected a progressive increase in lipid oxidation relative to carbohydrate oxidation (Fig. 2A). Thus, lipid oxidation (in g/min) increased progressively during exercise (Fig. 2C); whereas carbohydrate oxidation (in g/min) showed a decreasing trend that did not reach statistical significance (P = 0.065; Fig. 2D). The median lipid oxidation (in g/min) correlated inversely with the median lactate concentration during exercise (Spearman’s rho = -0.60, P = 0.010, n = 17).
Fig. 2.
Response to exercise of (A) energy expenditure along with the proportions derived from carbohydrate and lipid oxidation, (B) respiratory exchange ratio [RER = VCO2/VO2], (C) lipid oxidation, and (D) carbohydrate oxidation. Data are median with interquartile range. Measurements were conducted at 8, 15, 30, 60, 90, and 120 min of exercise. n = 17, except in time points 90 min [one missing value] and 120 min [two missing values]. The effect of exercise was tested with the Friedman test for repeated measurements; therefore, only the 15 participants with data in all time points were included in the analyses. *P < 0.05 vs. time point 8 min for the Sign post-hoc test with Bonferroni adjustment.
Association between MetF-lip and metabolic health outcomes
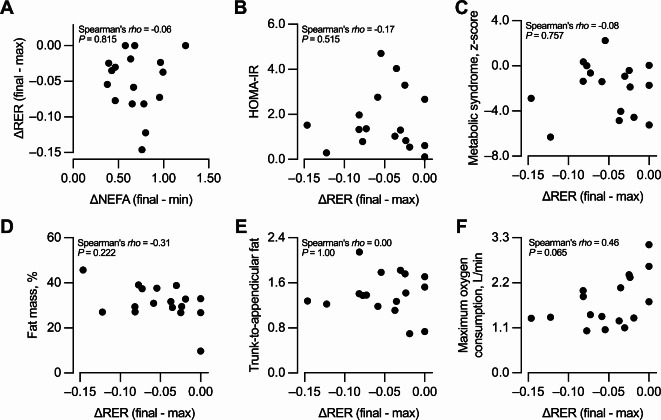
ΔRER was not correlated with ΔNEFA (Fig. 3A), the AUC of NEFA circulating concentrations (Spearman’s rho = -0.05, P = 0.836, n = 17), or the NEFAfinal (Spearman’s rho = -0.08, P = 0.742, n = 17). Therefore, ΔRER by itself (i.e., unadjusted) was considered as the marker of MetF-lip. The more negative the ΔRER, the higher the MetF-lip as it represents a higher switch from carbohydrate to fat oxidation. ΔRER was not correlated with HOMA-IR (Fig. 3B), the metabolic syndrome z-score (Fig. 3C), fat mass percentage (Fig. 3D), trunk-to-appendicular fat (Fig. 3E), or VO2max expressed in L/min (Fig. 3F), mLO2/min per kg of body weight (Spearman’s rho = 0.37, P = 0.143, n = 17), or mLO2/min per kg of fat-free mass (Spearman’s rho = 0.35, P = 0.167, n = 17). Similar results were obtained when considering only the 15 participants that completed the 120 min of the exercise challenge (Supplementary Fig. 1). Similar results were also obtained when ΔRER was calculated excluding the 8-min time point, in which exercise workload was adjusted (by 5.0 [-5.0, 12.5] W) to approach 50% VO2max (not shown).
Fig. 3.
Association between metabolic flexibility to lipid and metabolic health outcomes. Correlation between the change in respiratory exchange ration [ΔRER], the marker of metabolic flexibility to lipid, and (A) the change in the circulating concentrations of non-esterified fatty acids [ΔNEFA], (B) homeostatic model assessment of insulin resistance [HOMA-IR], (C) metabolic syndrome z-score, (D) fat mass percentage, (E) trunk-to-appendicular fat, and (F) maximum oxygen consumption. n = 17.
Association between MetF-lip and inflammatory signaling pathway in skeletal muscle
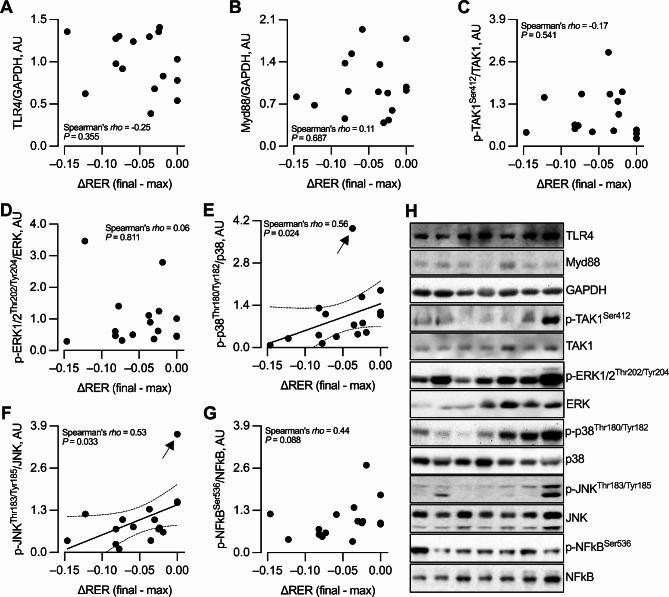
Skeletal muscle biopsies were obtained from 16 participants (8 men and 8 women). ΔRER was not correlated with the protein abundance of TLR4, Myd88, p-TAK1Ser412, or p-ERK1/2Thr202/Tyr204 (Fig. 4A-D). Nevertheless, ΔRER correlated directly with p-p38Thr180/Tyr182 (Fig. 4E), and such association became stronger after removing one extreme value (Spearman’s rho = 0.63, P = 0.011, n = 15). These findings indicate that the higher the MetF-lip, the lower the p-p38Thr180/Tyr182. ΔRER also correlated directly with p-JNKThr183/Tyr185 (Fig. 4F), but such association became non-significant after removing one extreme value (Spearman’s rho = 0.44, P = 0.102, n = 15). Finally, ΔRER was not correlated with p-NFkBSer536 (Fig. 4G). Representative blots of 7 participants are shown in Fig. 4H, the summary of all blots is shown in Supplementary Fig. 2, and the original blots and membranes per gel are shown in Supplementary Fig. 3.
Fig. 4.
Association between metabolic flexibility to lipid and the inflammatory signaling pathway in resting skeletal muscle. Correlation between the change in respiratory exchange ratio [ΔRER], the marker of metabolic flexibility to lipid, and (A) TLR4, (B) Myod88, (C) p-TAKSer412, (D) p-ERK1/2Thr202/Tyr204, (E) p-p38Thr180/Tyr182, (F) p-JNKThr183/Tyr185, and (G) p-NFkBSer536. n = 16. (H) Representative blots of seven participants. A summary of all the blots analyzed is presented in Supplementary Fig. 2, and the original blots and membranes are presented in Supplementary Fig. 3. In E and F, the arrows indicate extreme values that were included (in this Figure) or excluded (see text) in the analyses. AU, arbitrary units.
Discussion
Exercise of moderate intensity and long duration progressively increases circulating lipid availability and lipid oxidation in active skeletal muscle. Therefore, this type of exercise appears suitable to measure the capacity of skeletal muscle to adapt lipid oxidation to lipid availability, i.e. its MetF-lip. We reasoned that a low MetF-lip in skeletal muscle potentially leads to metabolic disturbances even in the absence of obesity. Nevertheless, we found that MetF-lip was not associated with metabolic health outcomes in participants without obesity. MetF-lip in skeletal muscle does not seem to be a major driver of metabolic disturbances in this population.
Our exercise challenge produced the expected responses in circulating markers and energy metabolism17–19,42–44. Glucose was maintained relatively constant while insulin progressively decreased. Lactate transiently increased denoting the involvement of anaerobic glycolysis at the beginning of exercise. Also, lactate concentration was inversely associated with lipid oxidation, as observed in another study15. NEFA and glycerol progressively increased due to the stimulation of adipose tissue lipolysis. The energy expenditure rate remained constant along the exercise, consistent with the constant power required. RER decreased, thus demonstrating progressive increases in the contribution of lipid oxidation to total energy expenditure. The 120-min exercise was long enough to produce ΔNEFA values over 0.45 mM in 14 out of 17 participants. This was the ΔNEFA cutoff over which MetF-lip seemed to differentiate individuals with contrasting HOMA-IR in our previous study17. Also, ΔRER showed a wider range in our current study versus our previous one (-0.15 to 0.00 versus -0.12 to 0.00, respectively)17. These observations, along with the wide interindividual variability in the metabolic health of our participants, indicate that our current study was well-designed to test the association between MetF-lip and metabolic health.
We found that ΔRER was not correlated with ΔNEFA during exercise. Previously, we showed that ΔRER and ΔNEFA were only correlated in individuals with very low HOMA-IR (from 0.30 to 0.59)17. The HOMA-IR values in our current participants ranged from 0.12 to 4.70, with only three participants having HOMA-IR ≤0.59. Therefore, the lack of matching between lipid oxidation and lipid availability suggests that our participants had a low MetF-lip as a group. In these participants with seemingly low MetF-lip, we did not detect a correlation between the degree of MetF-lip and metabolic health outcomes including HOMA-IR. This suggests that MetF-lip during contractile activity is not implicated in the development of metabolic disturbances in individuals without obesity.
Perhaps, MetF-lip measured during exercise is irrelevant as a trigger for metabolic disturbances. Exercise challenges the capacity, mostly of skeletal muscle, to oxidize lipids in a context of high energy expenditure45. Instead, MetF-lip measured throughout a day in non-exercise conditions may be more relevant for health, as it integrates the capacity of several organs and tissues to oxidize lipids over the entire day1,45. A recent study measured MetF-lip as the difference between the RER in a 24-hour energy balance condition and a 24-hour fasting46. A lower MetF-lip was associated with a higher ad-libitum energy intake the day after the 24-hour fasting. Another study measured MetF-lip as the difference in the 24-hour RER between a high-fat overfeeding (60% from fat) and an energy balance condition with a mixed diet (30% from fat)47. A lower MetF-lip was associated with a higher weight gain after six and twelve months. These studies suggest that low MetF-lip measured throughout a day in non-exercise conditions is associated with weight gain. Importantly, recent evidence showed that individuals with obesity have an elevated rate of NEFA clearance, probably driven by skeletal muscle48. And the NEFA clearance rate was correlated inversely with insulin sensitivity in skeletal muscle48. Together, this evidence suggests that a low MetF-lip measured at rest could promote the development of obesity and thus increase the NEFA clearance in skeletal muscle. In that context, an impaired MetF-lip in skeletal muscle may enhance the deposition of intramyocellular lipid metabolites that trigger insulin resistance and metabolic disturbances, accelerating the transition from a metabolically healthy to unhealthy phenotype. This agrees with our previous data suggesting that the accumulation of metabolic disturbances most often begins with an increase in waist circumference (obesity)8. Future studies should test the association between MetF-lip during exercise and metabolic health outcomes in individuals with metabolically healthy and unhealthy obesity.
A low MetF-lip in skeletal muscle could lead not only to the accumulation of intramyocellular lipid metabolites but also to the activation of inflammatory pathways. NEFA can activate TLR4 in macrophages, adipocytes, and skeletal muscle cells and thus trigger a pro-inflammatory response49,50. A low metabolic flexibility could also induce the differentiation of fibro-adipogenic progenitors into intramuscular adipocytes, which would exacerbate inflammation5,51,52. The chronic inflammatory state could lead to the development of insulin resistance in the long term5. To explore a potential association between MetF-lip in skeletal muscle and inflammation, we measured the protein content of proteins involved in the inflammatory pathway. We observed that the lower the MetF-lip (i.e., higher ΔRER values), the higher the content of p-JNKThr183/Tyr185 and p-p38Thr180/Tyr182. The correlation with p-JNKThr183/Tyr185 was, however, mostly driven by one individual with very a high p-JNKThr183/Tyr185 value. Thus, our results indicate that a low MetF-lip in skeletal muscle measured during exercise is associated with an elevated p-p38Thr180/Tyr182 in resting skeletal muscle. p38 belongs to the family of Mitogen-Activated Protein Kinases and is activated by phosphorylation in response to stressful stimuli53. For example, we have shown that p-p38Thr180/Tyr182 transiently increases in skeletal muscle after prolonged exercise potentially activating an anti-inflammatory program54. Notably, NEFA activate p38 through TLR4 in muscle cells55, and this mechanism partially explains the increase in p-p38Thr180/Tyr182 observed after prolonged exercise56. An elevated p-p38Thr180/Tyr182 in resting skeletal muscle, however, may indicate a chronic stress resulting from a low MetF-lip. That effect could be mediated directly through the activation of TLR4 in muscle cells55 and indirectly through an effect of NEFA on macrophages57.
Our study has limitations worth considering. First, we aimed to include individuals with large interindividual variability in metabolic health outcomes to increase the chances of observing associations with MetF-lip. Even so, only 4 out of 17 participants were afflicted with metabolic syndrome, consistent with the low prevalence of metabolic syndrome in individuals without obesity in Chile20. The interindividual variability in metabolic health outcomes, MetF-lip, or both may thus have been insufficient to reveal the associations. Yet we considered various metabolic health outcomes that have been associated with metabolic flexibility in previous studies, including body composition and fat distribution58, cardiorespiratory fitness15, and HOMA-IR17. Second, we did not measure intramyocellular lipid metabolites because of technical reasons. Although we speculate that lipid metabolites may link impaired MetF-lip to metabolic disturbances, this could not be tested. Third, the exercise challenge was conducted at a fixed 50% VO2max instead of using the individual intensity that elicits the maximum fat oxidation (i.e., the Fatmax). The Fatmax, however, is commonly attained between 47% and 52% of VO2max in untrained individuals59. Our participants were thus probably close to their Fatmax. Important, using 50% of VO2max allowed a direct comparison with our previous study conducted at the same intensity17. Also note that although we aimed for a 50% of VO2max (and even adjusted the power to approach that), exercise intensity was 48.2% [45.6–49.2] of VO2max. So even if we had aimed for the Fatmax, such intensity may not be accurately maintained for 2 h. Fourth, with 17 individuals, we were only powered to detect moderate to large associations. Note, however, that most of the observed correlation coefficients were very small and thus would require over 100 individuals to reach statistical significance. Moreover, if those were actual associations, the association between metabolic flexibility and metabolic health outcomes would be very small, and most probably, clinically irrelevant. Finally, changes in whole-body RER during exercise can be mostly, but not fully, attributed to skeletal muscle activity.
Conclusion
We found no association between MetF-lip in skeletal muscle measured during exercise and clinically relevant metabolic health outcomes. Consequently, MetF-lip in skeletal muscle does not seem to be a major determinant of metabolic disturbances in individuals without obesity. Having a low MetF-lip may, however, activate a stress response that potentially impairs insulin sensitivity in the long term. Whether metabolic flexibility and other similar physiological traits60 play a preponderant role in metabolic health remains to be demonstrated. Studies including various methods to assess metabolic flexibility to lipid (e.g., fasting, high-fat diet), different populations (e.g., individuals with metabolically healthy and unhealthy obesity), and longitudinal designs (to test the long-term effects of different levels of metabolic flexibility) will be essential to accomplish that purpose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Alejandra Díaz for her assistance with the DXA measurements. The study was funded by: ANID/CONICYT FONDECYT Iniciación 11180361 to RFV; ANID/CONICYT FONDECYT Regular 1220551 to JEG; FONDECYT 1241947 to CCV; Agencia Nacional de Investigación y Desarrollo (ANID) - Millennium Science Initiative Program - ICN09_016 / ICN 2021_045: Millennium Institute on Immunology and Immunotherapy (ICN09_016 / ICN 2021_045; former P09/016-F) to CCV; and Proyecto Núcleo UNAB (DI-03-23/NUC) to CCV. Research reported in this publication was also supported by the National Institute of Diabetes and Digestive And Kidney Diseases of the National Institutes of Health under Award Number U24 DK132740. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- MetF-lip
metabolic flexibility to lipid
- NEFA
non-esterified fatty acids
- RER
respiratory exchange ratio
- VCO2
rate of carbon dioxide production
- VO2
rate of oxygen consumption
- VO2max
maximum oxygen consumption
Author contributions
RFV: Conceptualization, Methodology, Formal Analysis, Writing - Original Draft, Visualization, Supervision, Funding Acquisition. JGP, THO, MVB, MTS, and RVF: Methodology, Investigation, Writing - Review & Editing. HZF and CCV: Methodology, Resources, Supervision, Funding Acquisition, Writing - Review & Editing. JEG: Conceptualization, Methodology, Funding Acquisition, Writing - Review & Editing.
Data availability
The data that support the findings of this study are available from the corresponding author, RFV, upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rodrigo Fernández-Verdejo, Email: rodrigo.fernandez@pbrc.edu.
Jose E. Galgani, Email: jgalgani@uc.cl
References
- 1.Galgani, J. E. & Fernández-Verdejo, R. Pathophysiological role of metabolic flexibility on metabolic health. Obes. Rev.22, e13131 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster, B. H. & Sparks, L. M. Metabolic flexibility in health and disease. Cell. Metab.25, 1027–1036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsilingiris, D., Tzeravini, E., Koliaki, C., Dalamaga, M. & Kokkinos, A. The role of mitochondrial adaptation and metabolic flexibility in the pathophysiology of obesity and insulin resistance: An updated overview. Curr. Obes. Rep.10, 191–213 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Petersen, M. C. & Shulman, G. I. Mechanisms of insulin action and insulin resistance. Physiol. Rev.98, 2133–2223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu, H. & Ballantyne, C. M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Invest.127, 43–54 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal-Puig, A. The metabolic syndrome and its complex pathophysiology. In A systems biology approach to study metabolic syndrome (eds Oresic, M. & Vidal-Puig, A.) 3–16 (Springer, doi:10.1007/978-3-319-01008-3. (2014). [Google Scholar]
- 7.DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: The missing links. Claude Bernard Lecture 2009 Diabetologia. 53, 1270–1287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández-Verdejo, R. & Galgani, J. E. Exploring the sequential accumulation of metabolic syndrome components in adults. Sci. Rep.12, 15925 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley, D. E., Goodpaster, B., Wing, R. R. & Simoneau, J. A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol.277, E1130–E1141 (1999). [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Verdejo, R. et al. Direct relationship between metabolic flexibility measured during glucose clamp and prolonged fast in men. Obes. (Silver Spring). 28, 1110–1116 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Galgani, J. E. et al. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes. 57, 841–845 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolopoulou, M. et al. Metabolic flexibility and oxidative capacity independently associate with insulin sensitivity in individuals with newly diagnosed type 2 diabetes. Diabetologia. 59, 2203–2207 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Chu, L., Morrison, K. M., Riddell, M. C., Raha, S. & Timmons, B. W. No difference in exogenous carbohydrate oxidation during exercise in children with and without impaired glucose tolerance. J. Appl. Physiol. (1985). 121, 724–729 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Chu, L., Morrison, K. M., Riddell, M. C., Raha, S. & Timmons, B. W. Metabolic flexibility during Exercise in children with obesity and matched controls. Med. Sci. Sports Exerc.53, 159–164 (2021). [DOI] [PubMed] [Google Scholar]
- 15.San-Millán, I. & Brooks, G. A. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med.48, 467–479 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Waldman, H. S. et al. Assessment of metabolic flexibility by substrate oxidation responses and blood lactate in women expressing varying levels of Aerobic Fitness and Body Fat. J. Strength. Cond Res.37, 581–588 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Fernández-Verdejo, R., Bajpeyi, S., Ravussin, E. & Galgani, J. E. Metabolic flexibility to lipid availability during exercise is enhanced in individuals with high insulin sensitivity. Am. J. Physiol. Endocrinol. Metab.315, E715–E722 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfe, R. R., Klein, S., Carraro, F. & Weber, J. M. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am. J. Physiology-Endocrinology Metabolism. 258, E382–E389 (1990). [DOI] [PubMed] [Google Scholar]
- 19.Romijn, J. A. et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Endocrinol. Metab.265, 380–391 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Verdejo, R., Moya-Osorio, J. L., Fuentes-López, E. & Galgani, J. E. Metabolic health and its association with lifestyle habits according to nutritional status in Chile: A cross-sectional study from the National Health Survey 2016–2017. PLoS One. 15, e0236451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monteiro, M. G. & Organización Panamericana de la Salud. Alcohol y Atencion Primaria de La Salud: Informaciones Clínicas Básicas Para La Identificación y El Manejo de Riesgos y Problemas (2008).
- 22.World Health Organization. Global Physical Activity Questionnaire (GPAQ). Analysis Guide. (2021). https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/physical-activity-surveillance (Accessed on October 2024).
- 23.Howley, E. T., Bassett, D. R. & Welch, H. G. Criteria for maximal oxygen uptake: Review and commentary. Med. Sci. Sports Exerc.27, 1292–1301 (1995). [PubMed] [Google Scholar]
- 24.Jones, N. L., Makrides, L., Hitchcock, C. Chypchar, T. & McCartney, N. Normal standards for an incremental progressive cycle ergometer test. Am. Rev. Respir Dis.131, 700–708 (1985). [DOI] [PubMed]
- 25.Choi, L., Liu, Z., Matthews, C. E. & Buchowski, M. S. Validation of accelerometer wear and nonwear time classification algorithm. Med. Sci. Sports Exerc.43, 357–364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes-Ortiz, T., Suárez-Reyes, M., Galgani, J. E., Zbinden-Foncea, H. & Fernández-Verdejo, R. Time reallocation of physical behaviours induced by endurance exercise in physically active individuals. Eur. J. Sport Sci.23, 1810–1820 (2023). [DOI] [PubMed] [Google Scholar]
- 27.Kozey-Keadle, S., Libertine, A., Lyden, K., Staudenmayer, J. & Freedson, P. S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc.43, 1561–1567 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Sasaki, J. E., John, D. & Freedson, P. S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport. 14, 411–416 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Verdejo, R. et al. Deciphering the constrained total energy expenditure model in humans by associating accelerometer-measured physical activity from wrist and hip. Sci. Rep.11, 12302 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plucker, A. et al. Adult energy requirements predicted from doubly labeled water. Int. J. Obes.42, 1515–1523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry, C. Basal metabolic rate studies in humans: Measurement and development of new equations. Public. Health Nutr.8, 1133–1152 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Elia, M. & Livesey, G. Energy expenditure and fuel selection in biological systems: The theory and practice of calculations based on indirect calorimetry and tracer methods. World Rev. Nutr. Diet.70, 68–131 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Ghasemi, A. & Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab.10, 486–489 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher, D. et al. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr.72, 694–701 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Ekelund, U. et al. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ. 366, l4570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suárez-Reyes, M. & Fernández-Verdejo, R. Work/household, transport, and leisure domains account for the sex gap in physical activity in Chile. Front. Public. Health. 10, 1011790 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-Verdejo, R. & Suárez-Reyes, M. [Physical inactivity versus sedentariness: analysis of the Chilean national health survey 2016–2017]. Rev. Med. Chil.149, 103–109 (2021). [DOI] [PubMed] [Google Scholar]
- 38.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Pescription (Wolters Kluwer, 2018). [Google Scholar]
- 39.Villanueva, B., Arteaga, A., Maiz, A. & Cortés, V. A. Abdominal obesity is a common finding in normal and overweight subjects of Chile and is associated with increased frequency of cardiometabolic risk factors. PLoS One. 13, e0194644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pollak, F. et al. [Second Consensus of the Chilean Society of Endocrinology and Diabetes about insulin resistance]. Rev. Med. Chil.143, 627–636 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Alberti, K. G. M. M. et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120, 1640–1645 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Goodpaster, B. H., Wolfe, R. R. & Kelley, D. E. Effects of obesity on substrate utilization during exercise. Obes. Res.10, 575–584 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Egan, B. & Zierath, J. R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell. Metab.17, 162–184 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Hargreaves, M. & Spriet, L. L. Skeletal muscle energy metabolism during exercise. Nat. Metab.2, 817–828 (2020). [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Verdejo, R., Sanchez-Delgado, G. & Ravussin, E. Energy expenditure in humans: Principles, methods, and changes throughout the Life Course. Annu. Rev. Nutr.44, 51–76 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Unlu, Y. et al. Impaired metabolic flexibility to fasting is associated with increased ad libitum energy intake in healthy adults. Obes. (Silver Spring). 32, 949–958 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Begaye, B. et al. Impaired metabolic flexibility to high-fat overfeeding predicts future weight gain in healthy adults. Diabetes. 69, 181–192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao, C. et al. Increased plasma fatty acid clearance, not fatty acid concentration, is associated with muscle insulin resistance in people with obesity. Metabolism. 132, 155216 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest.116, 3015–3025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyna, S. M. et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 57, 2595–2602 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan, I. M. et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. (Lond). 39, 1607–1618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores-Opazo, M. et al. Fibro-adipogenic progenitors in physiological adipogenesis and intermuscular adipose tissue remodeling. Mol. Aspects Med.97, 101277 (2024). [DOI] [PubMed] [Google Scholar]
- 53.Kramer, H. F., Goodyear, L. J. & Exercise MAPK, and NF-kappaB signaling in skeletal muscle. J. Appl. Physiol. (1985). 103, 388–395 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Fernández-Verdejo, R. et al. Activating transcription factor 3 attenuates chemokine and cytokine expression in mouse skeletal muscle after exercise and facilitates molecular adaptation to endurance training. FASEB J.31, 840–851 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Zbinden-Foncea, H., Deldicque, L., Pierre, N., Francaux, M. & Raymackers, J. TLR2 and TLR4 activation induces p38 MAPK‐dependent phosphorylation of S6 kinase 1 in C2C12 myotubes. Cell. Biol. Int.36, 1107–1113 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Zbinden-Foncea, H., Van Loon, L. J. C., Raymackers, J. M., Francaux, M. & Deldicque, L. Contribution of nonesterified fatty acids to mitogen-activated protein kinase activation in human skeletal muscle during endurance exercise. Int. J. Sport Nutr. Exerc. Metab.23, 201–209 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Talbot, N. A., Wheeler-Jones, C. P. & Cleasby, M. E. Palmitoleic acid prevents palmitic acid-induced macrophage activation and consequent p38 MAPK-mediated skeletal muscle insulin resistance. Mol. Cell. Endocrinol.393, 129–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaves, A. et al. Association between Adipose Tissue Characteristics and metabolic flexibility in humans: A systematic review. Front. Nutr.8, 744187 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Achten, J. & Jeukendrup, A. E. Optimizing fat oxidation through exercise and diet. Nutrition. 20, 716–727 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Fernández-Verdejo, R. & Galgani, J. E. Metabolic elasticity - a new trait associated with health? Nat. Rev. Endocrinol.19, 689–690 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, RFV, upon reasonable request.