Abstract
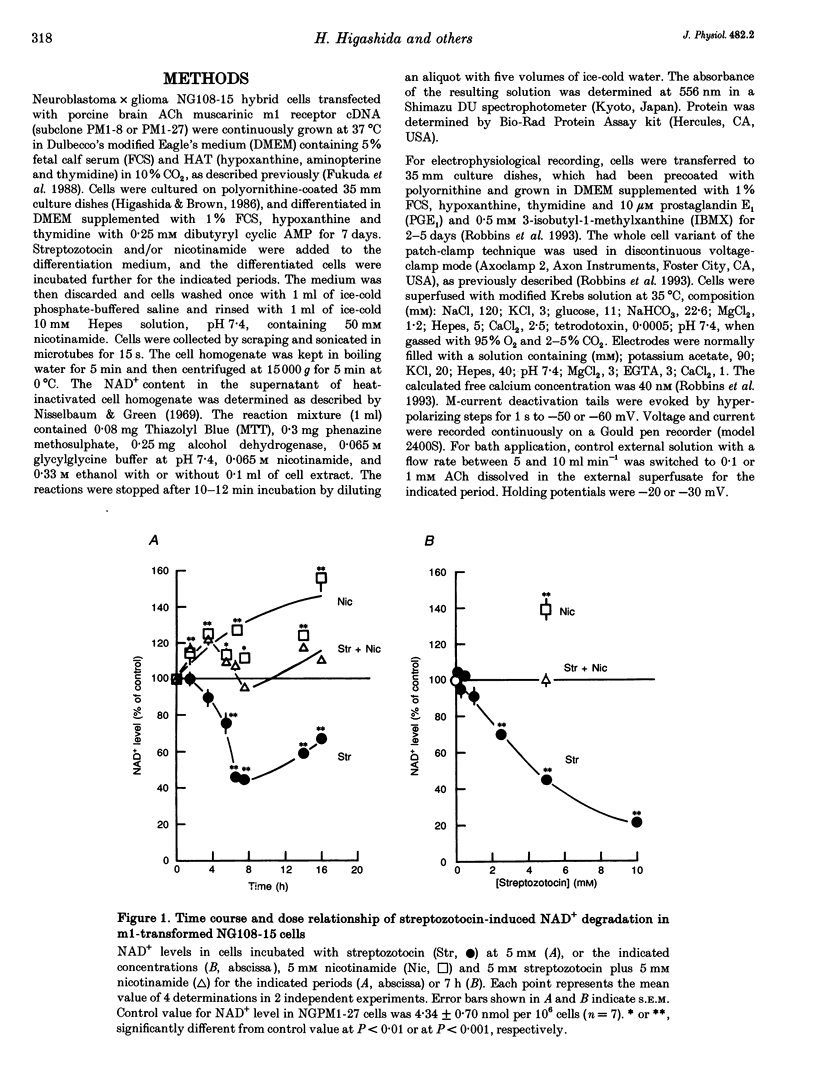
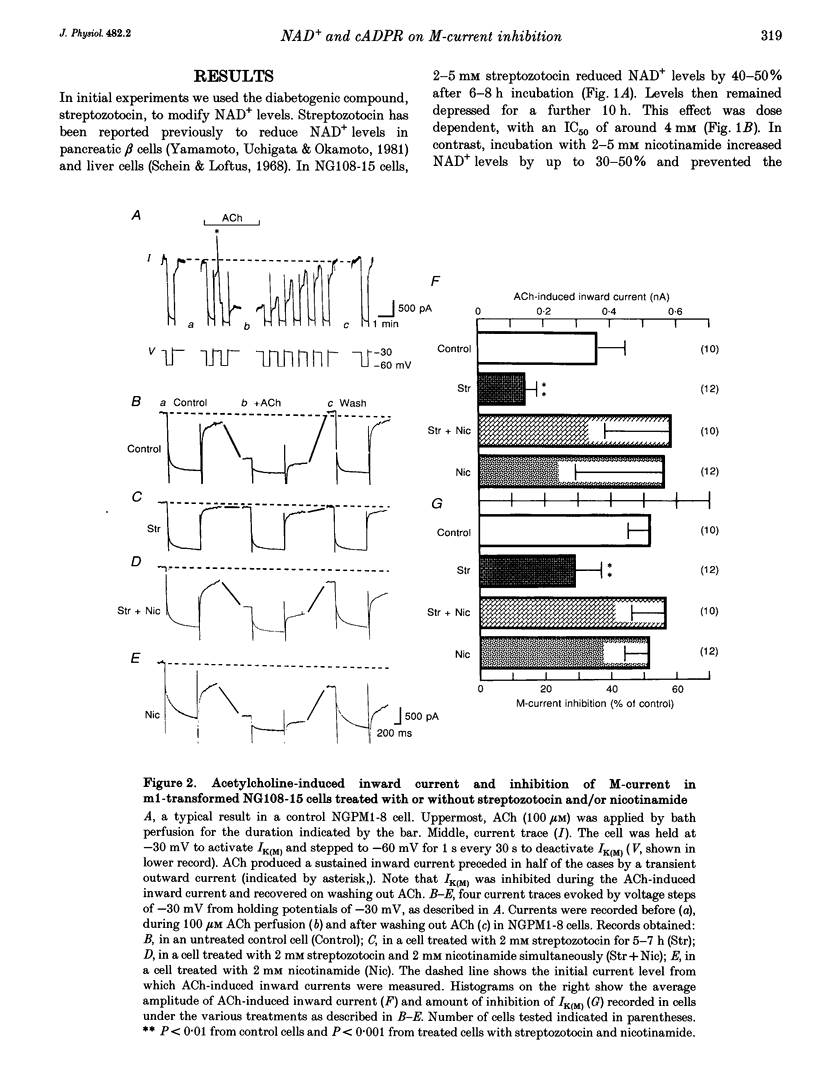
1. The possible role of nicotinamide-adenine dinucleotide (NAD+) and cyclic adenosine diphosphate ribose (cADPR) as regulators of M-type K+ currents (IK(M)) has been studied in whole-cell patch-clamped NG108-15 mouse neuroblastoma x rat glioma cells that had been transformed to express m1 muscarinic acetylcholine receptors (mAChRs). 2. Pre-incubation of NG108-15 cells for 6-8 h with streptozotocin (2-5 mM) reduced NAD+ levels by 40-50%. Nicotinamide (2-5 mM) increased NAD+ levels and prevented depletion by streptozotocin. 3. Streptozotocin pretreatment reduced the inhibition of IK(M) produced by 100 microM acetylcholine (ACh) from 51.6 +/- 7.0 to 29.1 +/- 7.5%. This was prevented by simultaneous pre-incubation with 2 mM nicotinamide or by adding 2 mM NAD+ to the pipette solution. Neither procedure significantly affected the initial amplitude of IK(M). 4. Inclusion of 2 microM cADPR in the pipette solution induced a slow loss of IK(M) with a time constant of about 20 min. 5. It is concluded that mAChR-induced inhibition of IK(M) requires intracellular NAD+. This might be needed for the formation of cADPR as a regulator or messenger for IK(M) inhibition.
Full text
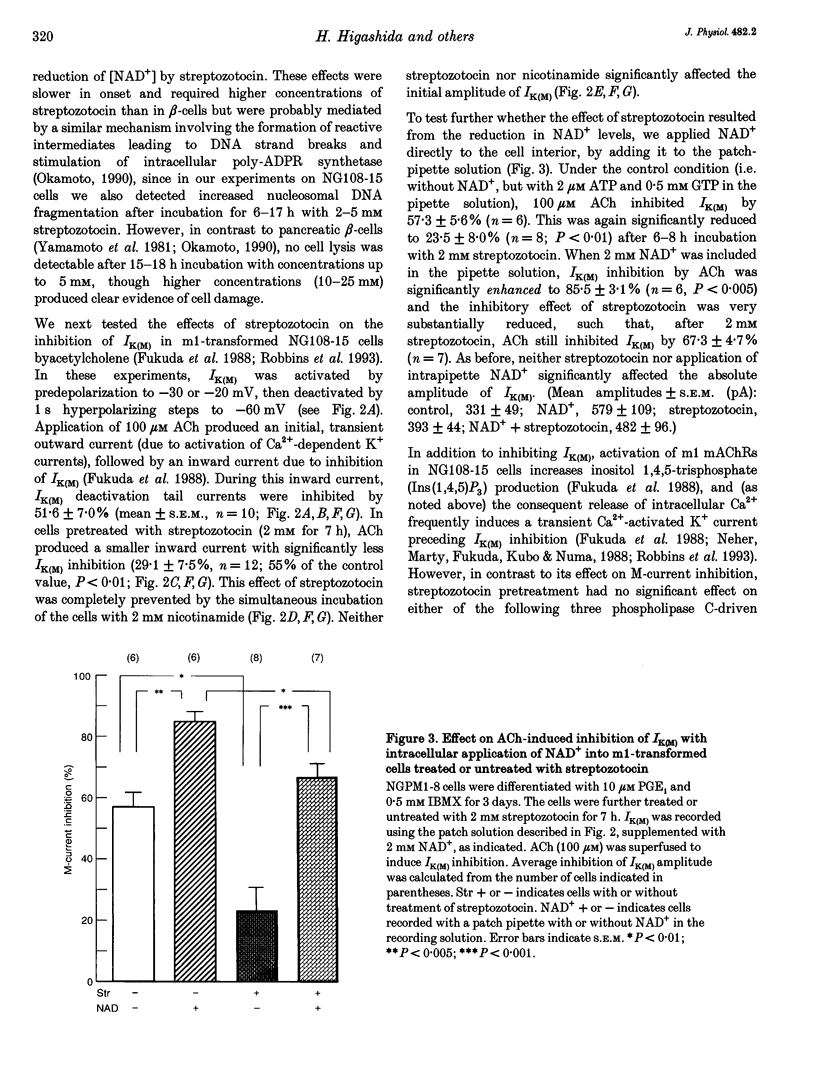
PDF
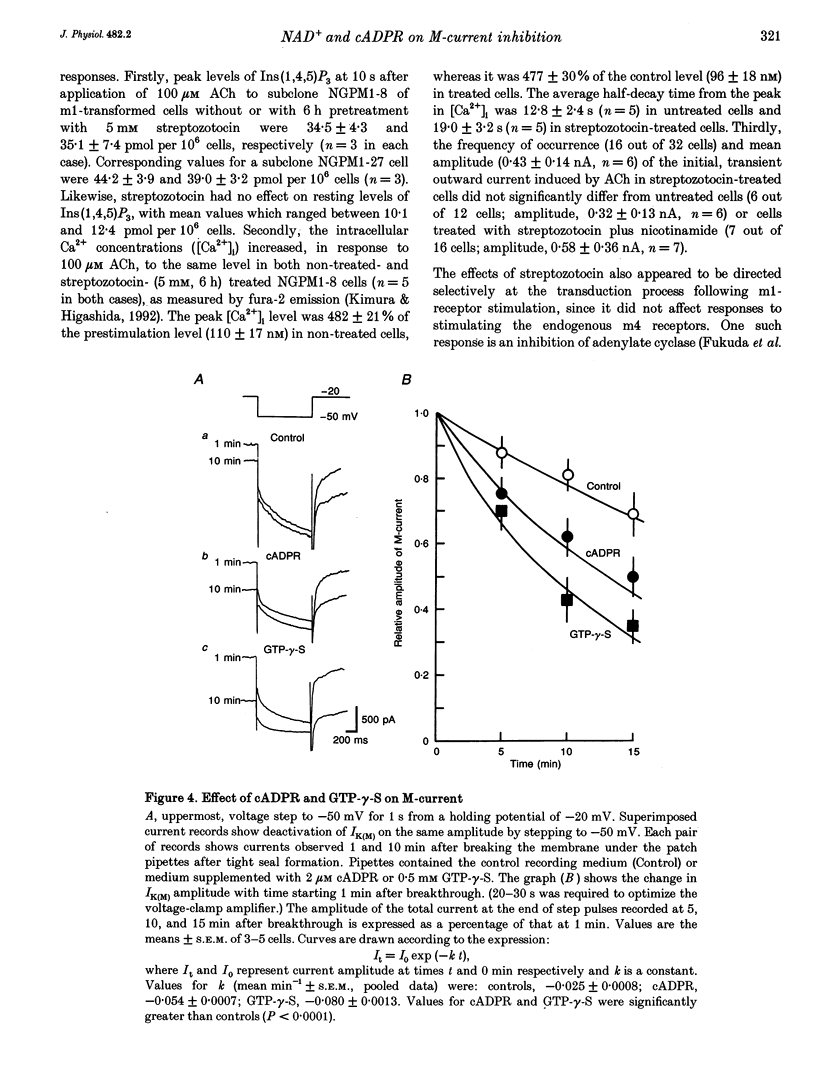


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caulfield M. P., Jones S., Vallis Y., Buckley N. J., Kim G. D., Milligan G., Brown D. A. Muscarinic M-current inhibition via G alpha q/11 and alpha-adrenoceptor inhibition of Ca2+ current via G alpha o in rat sympathetic neurones. J Physiol. 1994 Jun 15;477(Pt 3):415–422. doi: 10.1113/jphysiol.1994.sp020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly L. E., Boyd R. S., MacDermot J. Gs alpha is a substrate for mono(ADP-ribosyl)transferase of NG108-15 cells. ADP-ribosylation regulates Gs alpha activity and abundance. Biochem J. 1992 Nov 15;288(Pt 1):331–336. doi: 10.1042/bj2880331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Higashida H., Kubo T., Maeda A., Akiba I., Bujo H., Mishina M., Numa S. Selective coupling with K+ currents of muscarinic acetylcholine receptor subtypes in NG108-15 cells. Nature. 1988 Sep 22;335(6188):355–358. doi: 10.1038/335355a0. [DOI] [PubMed] [Google Scholar]
- Galione A., Lee H. C., Busa W. B. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991 Sep 6;253(5024):1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Galione A., White A., Willmott N., Turner M., Potter B. V., Watson S. P. cGMP mobilizes intracellular Ca2+ in sea urchin eggs by stimulating cyclic ADP-ribose synthesis. Nature. 1993 Sep 30;365(6445):456–459. doi: 10.1038/365456a0. [DOI] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. 1986 Sep 25-Oct 1Nature. 323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- Hua S. Y., Tokimasa T., Takasawa S., Furuya Y., Nohmi M., Okamoto H., Kuba K. Cyclic ADP-ribose modulates Ca2+ release channels for activation by physiological Ca2+ entry in bullfrog sympathetic neurons. Neuron. 1994 May;12(5):1073–1079. doi: 10.1016/0896-6273(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Kim H., Jacobson E. L., Jacobson M. K. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993 Sep 3;261(5126):1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Higashida H. Dissection of bradykinin-evoked responses by buffering intracellular Ca2+ in neuroblastoma x glioma hybrid NG108-15 cells. Neurosci Res. 1992 Nov;15(3):213–220. doi: 10.1016/0168-0102(92)90007-y. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A., Fukuda K., Kubo T., Numa S. Intracellular calcium release mediated by two muscarinic receptor subtypes. FEBS Lett. 1988 Nov 21;240(1-2):88–94. doi: 10.1016/0014-5793(88)80345-4. [DOI] [PubMed] [Google Scholar]
- Nisselbaum J. S., Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem. 1969 Feb;27(2):212–217. doi: 10.1016/0003-2697(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Robbins J., Marsh S. J., Brown D. A. On the mechanism of M-current inhibition by muscarinic m1 receptors in DNA-transfected rodent neuroblastoma x glioma cells. J Physiol. 1993 Sep;469:153–178. doi: 10.1113/jphysiol.1993.sp019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein P. S., Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res. 1968 Aug;28(8):1501–1506. [PubMed] [Google Scholar]
- Selyanko A. A., Stansfeld C. E., Brown D. A. Closure of potassium M-channels by muscarinic acetylcholine-receptor stimulants requires a diffusible messenger. Proc Biol Sci. 1992 Nov 23;250(1328):119–125. doi: 10.1098/rspb.1992.0139. [DOI] [PubMed] [Google Scholar]
- Stansfeld C. E., Marsh S. J., Gibb A. J., Brown D. A. Identification of M-channels in outside-out patches excised from sympathetic ganglion cells. Neuron. 1993 Apr;10(4):639–654. doi: 10.1016/0896-6273(93)90166-o. [DOI] [PubMed] [Google Scholar]
- Takasawa S., Nata K., Yonekura H., Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic beta cells. Science. 1993 Jan 15;259(5093):370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- White A. M., Watson S. P., Galione A. Cyclic ADP-ribose-induced Ca2+ release from rat brain microsomes. FEBS Lett. 1993 Mar 8;318(3):259–263. doi: 10.1016/0014-5793(93)80524-x. [DOI] [PubMed] [Google Scholar]
- Wilk-Blaszczak M. A., Gutowski S., Sternweis P. C., Belardetti F. Bradykinin modulates potassium and calcium currents in neuroblastoma hybrid cells via different pertussis toxin-insensitive pathways. Neuron. 1994 Jan;12(1):109–116. doi: 10.1016/0896-6273(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Uchigata Y., Okamoto H. Streptozotocin and alloxan induce DNA strand breaks and poly(ADP-ribose) synthetase in pancreatic islets. Nature. 1981 Nov 19;294(5838):284–286. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]


