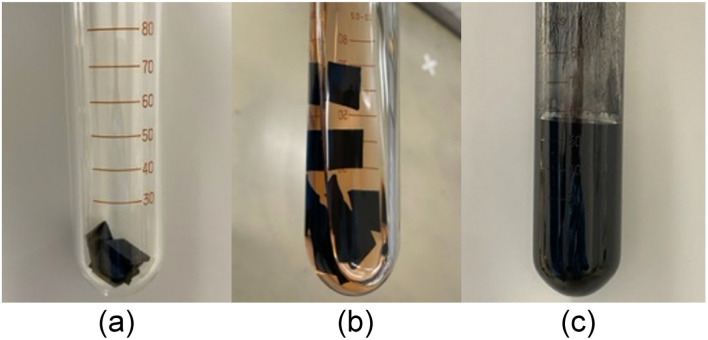
Abstract
Herein, we report a study on the preparation, properties, and depolymerization of pristine and vulcanized poly(cyclopentene) (poly(CP)), poly(norbornene-ran-cyclopentene) (poly(NB-ran-CP)) and poly(endo-dicyclopentadiene-ran-cyclopentene) (poly (DCP-ran-CP)). First, poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) were prepared with high molecular weight control (Mw = 200 000–500 000) using dichloro(3-phenyl-1H-inden-1-ylidene)bis(tricyclohexylphosphine)ruthenium(ii). Next, carbon black, zinc oxide and other additives were blended into the pristine polymers using a mixer and twin roll rubber mills at 50 °C, followed by vulcanization in metal molds at 160 °C for 10 min, resulting in molded black rubber specimens. Crosslinking of the vulcanized rubbers was confirmed by solvent swelling test. Ring-closing metathesis depolymerization (RCMD) of the pristine and vulcanized polymers was conducted. Pristine poly(CP) was smoothly degraded into cyclopentene monomers with only 0.001 mol% of [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphino)ruthenium (H2IMes)(PCy3)Cl2Ru CHPh (H2IMes = 1,3-dimesityl-4,5-dihydroimidazolylidene) in toluene at 25 °C for 1 h ([poly(CP) unit] = 0.50 M). In the case of the copolymers, degradation of pristine poly(NB-ran-CP) and poly(DCP-ran-CP) via RCMD also delivered a cyclopentene monomer and residual polynorbornene and poly(endo-dicyclopentadiene), respectively, demonstrating the feasibility of cyclopentene recycling from copolymers. Complete depolymerization of vulcanized poly(CP) rubber was also efficiently achieved using 1 mol% of (H2IMes)(PCy3)Cl2Ru CHPh, affording black inorganic precipitate and separable volatile cyclopentene monomer (in toluene at 60 °C for 24 h, [poly(CP)] = 0.50 M). Similarly, vulcanized poly(NB-ran-CP) (or poly(DCP-ran-CP)) rubber was successfully depolymerized under the same conditions, resulting in black inorganic precipitate, polynorbornene (or poly(endo-dicyclopentadiene)) and a cyclopentene monomer. This study provides a new strategy for monomer recycling of rubber wastes made from cyclopentene-based rubber under relatively mild conditions, contributing to a circular economy and resource efficiency.
Vulcanized, crosslinked cyclopentene-based rubbers were depolymerized to give a cyclopentene monomer with 80–90% yields.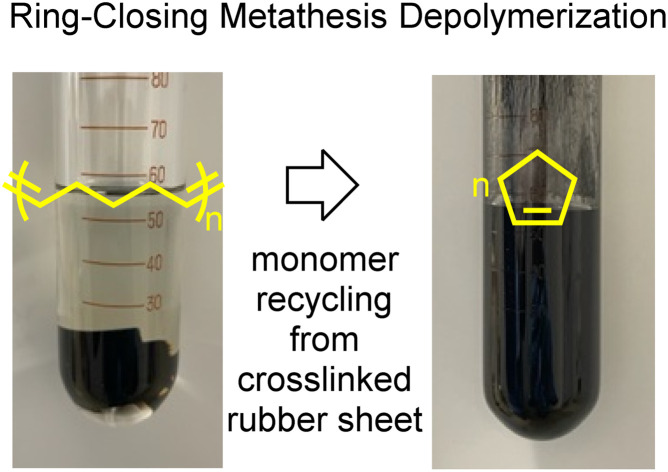
Introduction
Benefitting from their interchain network structures, vulcanized rubber materials have excellent mechanical properties, morphological stability, and oil resistance. Hence, rubber materials are now pervasive in diverse fields and have become indispensable to our modern society. However, while permanent strong cross-linkage significantly enhances mechanical performance, it also makes chemical recycling of rubber materials extremely challenging.1 Currently, most recycled commercial rubber products are simply subjected to fragmentation or combustion, e.g., most waste tires are directly crushed and burned as fuel. The low sustainability of rubber materials is a serious problem in today's society that must be resolved.
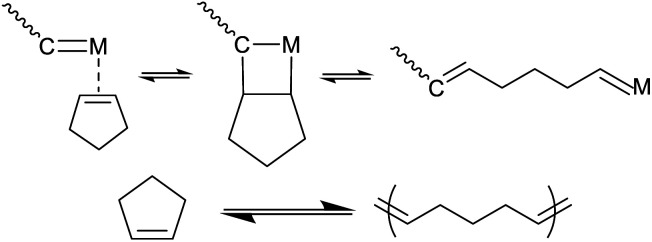
Cyclopentene (CP) is well known for its unique thermodynamic equilibrium nature during ring-opening metathesis polymerization (ROMP) (Scheme 1). Equilibrium ROMP of CP is controlled by reaction temperature and concentration of monomers in the presence of an olefin metathesis catalyst. For instance, the critical concentration of CP at 30 °C is reported to be [C]eq = 1.17 M.2
Scheme 1. Reversible ring-opening and ring-closing of cyclopentene in the presence of a metathesis catalyst.
Therefore, taking advantage of such reversible ring-opening and ring-closing behaviour, it is theoretically possible to chemically degrade poly(CP) by converting the polymer back to the CP monomer in a dilute solution under relatively mild conditions and achieve recycling of waste poly(CP) materials.
Since the discovery of the ROMP of cyclopentene in the 1950s, for about two decades, both industry and academia have paid considerable attention to the synthesis and properties of crystalline trans-poly(cyclopentene) (trans-pentenamer).3–6 There were two scientific and industrial reasons for this. First, cyclopentene could be potentially produced on an industrial scale from the C5 stream of petroleum refineries. Second, the resulting trans-pentenamer was an entirely new material. Vulcanized trans-pentenamer was identified as a new synthetic rubber, with low-temperature characteristics, tensile properties, and stretch-induced crystallization. Green (handling) strength of trans-pentenamer was also excellent because of its high crystallinity. However, industrialization and commercialization of trans-pentenamer have not been fruitful so far: the rubber performance of vulcanized trans-pentenamer was comparable to that of conventional diene-based rubbers such as natural rubber and butadiene rubber; therefore, at that time, there was no industrial advantage to start a trans-pentenamer rubber business. Here, we mention that several unsolved/unachieved scientific issues still remained in the field of cyclopentene ROMP in 1970s, including development of well-defined high performance metathesis catalysts, elucidation of the equilibrium mechanism of ring-opening/closing of cyclopentene, development of various cyclopentene copolymers, vulcanization of the CP-incorporated copolymers, and depolymerization of pristine cyclopentene copolymers and crosslinked CP-based rubbers.
Since the discovery of metal alkylidene catalysts in the 1980s–1990s, equilibrium ROMP of CP and its derivatives has again received great attention from the academia. Grubbs and his coworkers studied ruthenium catalysed ROMP of CP in detail with focus on the equilibrium nature of it.7–9 Register's group well investigated the equilibrium behaviour of metathesis copolymerization of cyclopentene and norbornene above and below critical concentration using Mo-based Schrock-catalyst.10 Statistical multiblock copolymers were produced via cross metathesis of poly(norbornene) and poly(CP).11 Research on the properties of vulcanized trans-pentenamer and its copolymers has also recently received renewed attention.12–14
Depolymerization of modified CP copolymers has been intensively studied in this decade. Tuba et al. reported equilibrium copolymerization of CP with functional CP and successive depolymerization of the copolymers.15 Kennemur's group figured out the depolymerization behaviour of functionalized poly(CP) containing various polymer architectures, such as bottlebrush polypentenamer.16–18 Equilibrium ROMP of CP-dimers and trimers was applied for a reversible transformation of covalent networks.19 Degradable substrates such as 2,3-dihydrofuran are employed by many researchers to develop degradable ROMPed copolymers.20–22 Among unsaturated elastomers, only poly(CP) can be readily depolymerized to give CP monomer. For instance, poly(butadiene) can be degraded by metathesis catalyst to form low molecular weight poly(butadiene) and various fragments, but 1,3-butadiene monomer cannot be recovered.23,24 Thus, the depolymerization of poly(CP) has been studied in various ways. To the best of our knowledge, the depolymerization of vulcanized poly(CP) and copolymers has not yet been studied.
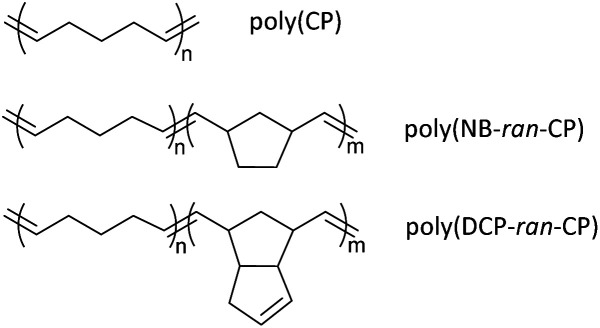
Based on the findings above, this study focuses on the preparation, properties, and monomer recycling of vulcanized poly(CP), poly(norbornene-ran-cyclopentene) (poly(NB-ran-CP)) and poly(endo-dicyclopentadiene-ran-cyclopentene) (poly(DCP-ran-CP)) rubbers (Scheme 2). Initially, poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) were synthesized via ROMP. Subsequently, blending of the pristine polymers with inorganic fillers, sulfur, and additives, followed by thermally induced crosslinking, afforded the desired vulcanized rubber specimens. Finally, depolymerization of the obtained rubber sample sheets was undertaken successfully in a diluted solution of (H2IMes)(PCy3)Cl2Ru CHPh (G2), converting the CP repeating unit into cyclopentene monomer.
Scheme 2. Poly(cyclopentene)(poly(cp)), poly(norbornene-ran-cyclopentene)poly(NB-ran-CP) and poly(endo-dicyclopentadiene-ran-cyclopentene)poly(DCP-ran-CP).
Experimental
General remarks
Polymerization and depolymerization were carried out under argon atmosphere by using standard Schlenk techniques. Dichloro(3-phenyl-1H-inden-1-ylidene)bis(tricyclohexylphosphine)ruthenium(ii) (M1) (Sigma-Aldrich) and [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphino)ruthenium (H2IMes)(PCy3)Cl2Ru CHPh (H2IMes = 1,3-dimesityl-4,5-dihydroimidazolylidene) (G2) (Sigma-Aldrich) were used as received. Anhydrous toluene (Kanto Chemicals) was used without further purification. Cyclohexane (Wako Chemicals) was degassed and stored over molecular sieves (3 Å). Norbornene (NB) (Zeon Corporation) was distilled and stored as a toluene solution over molecular sieves (3 Å). endo-Dicyclopentadiene (DCP) (Zeon Corporation) was distilled and stored as a cyclohexane solution over molecular sieves (3 Å) and silica gel. Cyclopentene (CP) (Zeon Corporation) was distilled and stored over molecular sieves (3 Å). 1-Hexene (Wako Chemicals) was distilled over calcium hydride. n-Butyl vinyl ether (Wako Chemicals) was dried and stored over molecular sieves (3 Å). Carbon black (Seast 9H, TOKAI CARBON), zinc oxide (Sakai Chemical Industry), stearic acid (NOF CORPORATION), N1-(4-methylpentan-2-yl)-N4-phenylbenzene-1,4-diamine (Ouchi Shinko Chemical Industrial), sulfur (sulfur#325 Hosoi Chemical Industry), and N-cyclohexyl-2-benzothiazole sulfenamide (Ouchi Shinko Chemical Industrial) were used as received.
Analyses of polymer and monomer
Polymer yields were determined by gravimetry. Cyclopentene recovery was first determined by gas chromatography (GC) with reference to the calibration curve illustrated in Fig. S16.† GC was performed on a Shimadzu GC-2025 gas chromatograph with capillary column Inert Cap 1 (0.25 mm I.D. × 60 m, df = 1.50 μm; GL Sciences Inc.) using nitrogen as a carrier gas. Column temperature was initially 35 °C, and, after a 3 min hold, it was increased by 5 °C min−1 up to 150 °C and then by 10 °C min−1 up to 280 °C. Retention time was as follows: cyclopentene; 11.3–11.4 min, toluene; 20.5–20.6 min. As an example, a PDF file of a GC measurement report of the depolymerization mixture of poly(NB-ran-CP) described in Table 4 is copied and shown in Fig. S17.†1H (500 MHz) and 13C NMR (125 MHz) spectra were recorded on a Bruker Avance III 500 MHz spectrometer at 27 °C. 1H NMR spectral data were reported as δ values in ppm relative to TMS (δ 0.00 ppm) or chloroform (δ 7.26 ppm) if collected in CDCl3. 13C NMR spectral data were reported as δ values in ppm relative to chloroform (δ 77.00 ppm) if collected in CDCl3. The molecular weight distribution (MWD) of the polymers was determined by gel-permeation chromatography (GPC) at 40 °C in tetrahydrofuran (THF) on a Tosoh HLC-8320 GPC instrument equipped with TSKgel SuperMultiporeHZ-H that covers the MW range 107–102. The relative number- and weight-average molecular weights (Mn and Mw, respectively) were determined by calibration against polystyrene standards. Differential scanning calorimetry (DSC) was performed on a SII NanoTechnology X-DSC7000 under nitrogen at a 10 °C min−1 heating rate using hermetically sealed aluminium T-zero pans.
Depolymerization of pristine poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP)a.
| poly(CP) | poly(NB-ran-CP)b | poly(DCP-ran-CP)c | ||
|---|---|---|---|---|
| Initial | Polymer | 3.01 g | 3.00 g | 3.02 g |
| CP unit | 3.01 g | 1.41 g | 1.84 g | |
| NB (or DCP) unit | — | 1.59 g | 1.18 g | |
| CP/NB (or CP/DCP) | 100/0 in mol | 55/45 in mol | 76/24 in mol | |
| — | 47/53 in wt | 61/39 in wt | ||
| M w (g mol−1) | 453 000 | 238 000 | 250 000 | |
| M w/Mn | 2.01 | 1.98 | 1.98 | |
| G2 | 0.38 g | 0.31 g | 0.28 g | |
| After RCMD | Non-volatile | 0.47 g | 1.91 g | 1.57 g |
| Polymerd | 0.09 g | 1.60 g | 1.29 g | |
| CP unit | — | 0.03 g, (2%) | 0.13 g, (7%) | |
| NB (or DCP) unit | — | 1.57 g, (99%) | 1.16 g, (99%) | |
| CP/NB (or CP/DCP) | 100/0 in mol | 3/97 in mol | 18/82 in mol | |
| — | 2/98 in wt | 10/90 in wt | ||
| M w(g mol−1) | — | 4700 | 6700 | |
| M w/Mn | — | 4.0 | 5.1 | |
| Volatile part CP recovery | 2.92 g | 1.32 g | 1.69 g | |
| 97% | 94% | 92% | ||
Depolymerized in toluene at 60 °C for 24 h; [total polymer units] = 0.5 M, [(H2IMes)(PCy3)Cl2Ru CHPh] = 5 mM, [(H2IMes)(PCy3)Cl2Ru CHPh]:[total polymer units] = 1 : 100.
[Poly(CP) units] = 0.28 M.
[Poly(CP) units] = 0.38 M.
Weight of polymer = weight of non-volatile − weight of G2.
Polymerization
A general procedure for producing the polymers in this study is described as follows. Polymerization of cyclopentene was carried out in a 20 L stainless steel reactor at 25 °C. The reactor was charged with cyclohexane (6.77 kg), cyclopentene (2.90 kg, 42.6 mol) and 1-hexene (2.03 g, 0.0241 mol), followed by the addition of dichloro(3-phenyl-1H-inden-1-ylidene)bis(tricyclohexylphosphine)ruthenium(ii) (M1) (0.393 g, 0.426 mmol) solution in toluene (7.47 g, 8.6 mL, 0.05 M) at the prescribed temperature. The reaction mixture was stirred for 3 h and the reaction was terminated by adding 2.13 g (21.3 mmol, 50 eq. vs. Ru) of butyl vinyl ether. The obtained poly(CP) (Mw = 453 000, Mw/Mn = 2.01) was precipitated from ethanol and dried in vacuo (at 1 mbar) at 40 °C for 24 h in a vacuum drying oven. The polymer yield was determined to be 2.12 kg (73%) by gravimetry. The polymer composition was determined by 1H NMR measurement.
Depolymerization of unvulcanized polymer
To a toluene solution of poly(CP) (3.01 g, 0.0442 mol unit, Mw = 453 000, Mw/Mn = 2.01), a toluene solution of (H2IMes)(PCy3)Cl2Ru CHPh (G2) (0.380 g, 0.442 mmol) ([Ru]/[C C] = 1/100) was added at 60 °C in a glass ampoule equipped with a rubber septum. The mixture ([poly(CP) unit]o = 0.50 M in 76.6 g of toluene) was stirred at 60 °C for 24 h. The viscosity of the polymer solution decreased dramatically as soon as the catalyst solution was added. The reaction was quenched with 2.21 g (22.1 mmol, 50 eq. vs. Ru) of butyl vinyl ether and cooled down to −20 °C. Very small aliquots were taken from the crude reaction mixture and diluted with THF at −20 °C to suppress loss of cyclopentene vapor by handling. The diluted THF mixture was analysed by GC. After removal of volatiles, the crude product was analysed by GPC. The rest of the crude reaction mixture was subjected to vacuum drying (1 mbar) in a vacuum drying oven at 40 °C for 24 h. The yield of the wax-like non-volatile remaining was determined by gravimetric measurement.
Composition mixing and vulcanization
A general procedure for the composition mixing and vulcanization process in this study is described as follows: 100.0 g of poly(CP), 50.0 g of carbon black, 3.0 g of zinc oxide, 2.0 g of stearic acid, 2.0 g of N1-(4-methylpentan-2-yl)-N4-phenylbenzene-1,4-diamine as an antioxidant, 1.75 g of sulfur, and 1 g of N-cyclohexyl-2-benzothiazole sulfenamide as vulcanization accelerator were blended in a mixing Banbury Kneader (Labo Plastomill Mixers for elastomers, electric heating model No.655-B250, Toyoseiki) at 80–150 °C for 8 min, then mixed on a twin roll rubber mill (6 inch, 25 rpm, IKEDA KIKAI KOGYO) at 50 °C to give a black rubber composition. The black rubber composition was transferred to a metal mold and vulcanized at 160 °C for 10 min to afford a crosslinked rubber specimen.
Depolymerization of vulcanized rubber sheet
Several pieces of vulcanized poly(CP) rubber sheet (20 mm × 20 mm × 1 mm), 2.81 g in total, that contained 1.76 g (0.0258 mol) of poly(cyclopentene), were added to a solution of (H2IMes)(PCy3)Cl2Ru CHPh (G2) (0.220 g, 0.258 mmol) ([Ru]/[C C] = 1/100) in toluene at 60 °C in a glass ampoule equipped with a rubber septum ([poly(CP) unit]0 = 0.50 M in 49.8 g of toluene). The reaction mixture was stirred at the same temperature. After 1 h, the pieces of vulcanized poly(CP) rubber were severely deformed, resulting in a notable loss of their original shape; after 24 h, the pieces of rubber sheet completely disappeared, giving a black opaque suspension. The depolymerization reaction was quenched with 1.29 g (12.9 mmol, 50 eq. vs. Ru) of butyl vinyl ether and cooled to −20 °C. Very small aliquots were taken from the crude reaction mixture and diluted by THF at −20 °C to analyse by GC to measure cyclopentene recovery and then by GPC to determine MWD after removal of volatiles. Insoluble inorganic black fillers were separated from the crude reaction mixture by centrifugation (3000 rpm, 10 min × 3) and extraction by toluene at r.t. Insoluble inorganic black fillers were dried in vacuo (1 mbar) in the vacuum drying oven at 40 °C for 24 h, and the weight was determined by gravimetry. The separated transparent solution was concentrated and dried in vacuo by evaporator (5 mbar) followed by vacuum drying oven (1 mbar) at 40 °C for 24 h. After drying, the non-volatile soluble remaining was obtained as a wax-like compound. The yield of it was determined by gravimetric measurement.
Results and discussion
Syntheses and properties of poly(cyclopentene) (poly(CP)), poly(norbornene-ran-cyclopentene) (poly(NB-ran-CP) and poly(endo-dicyclopentadiene-ran-cyclopentene) (poly(DCP-ran-CP)
Control of cyclopentene ROMP has been thoroughly studied by academia and industry in the past. For instance, cis/trans ratio control of the C C double bonds in the main chain of poly(CP) can be achieved by the selection of catalysts (metal centre and ligands), additives, temperature, etc. Generally, Ru-based catalysts tend to deliver trans-rich poly(CP), while Mo and W catalysts can yield cis-rich poly(CP) depending on catalyst design. The molecular weight of the resulting poly(CP) can be easily controlled by a chain-transfer agent, commonly α-olefins. Similarly, copolymerization of cyclopentene and norbornene has been also extensively investigated, with a focus on the control of copolymerization ratio between the two monomers, which can be fine-tuned through catalyst design.25–27 In addition to those factors, control of polymerization temperature is also an important practical factor. In this study, polymerization must be performed on a large scale to synthesize the amount of polymer needed to produce crosslinked rubber in an industrial laboratory scale. For large scale synthesis, polymerization heat removal is crucial for controlling the reaction temperature. On the other hand, polymerization of cyclic olefins is generally very fast. If the reaction proceeds instantaneously, the polymerization heat will not be removed in time and the polymerization will become uncontrollable. For this reason, we had to carefully choose a catalyst with mild reaction activity in order to slow down the rate of polymerization heat generation. Therefore, in this part, we utilized dichloro(3-phenyl-1H-inden-1-ylidene)bis(tricyclohexylphosphine)ruthenium(ii) (M1), with moderate activity, as a polymerization catalyst.28
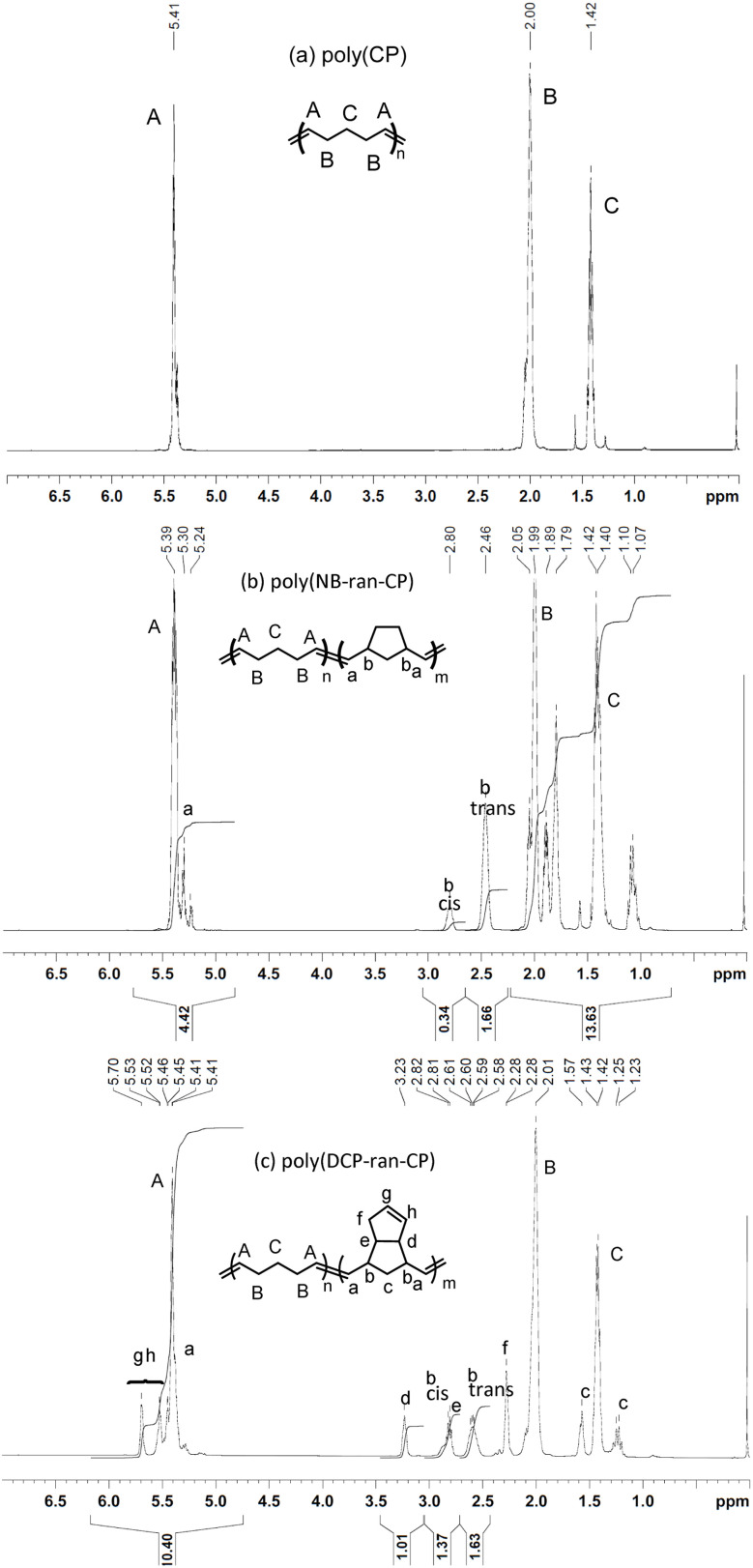
Polymerization results are summarized in Table 1. 1H NMR spectra of the obtained poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) are shown in Fig. 1. We measured the cis/trans ratio of poly(CP) by 13C NMR (Fig. S1†). In poly(NB-ran-CP), the mole ratios of NB and CP and the cis/trans ratios of C C double bonds in poly(NB) units were determined by 1H NMR (Fig. 1b). The cis/trans ratio of CP repeating units was confirmed by 13C NMR (Fig. S2†). Similarly, in poly(DCP-ran-CP) copolymer, the mole ratio of DCP and CP and the cis/trans ratio of C C double bonds in DCP repeating units were determined by 1H NMR (Fig. 1c). The cis/trans ratio of C C double bonds in CP units was confirmed by 13C NMR measurement (Fig. S3†). As expected, ROMP using M1 catalyst afforded the desired poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) with a predominance of trans-C C bonds. The weight-average molecular weights of the polymers were controlled within the range of Mw = 200 000–500 000 using a chain-transfer agent to facilitate subsequent rubber mill treatments for preparation of vulcanizing compositions.
Syntheses and properties of poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) by M1.
| Polymers | Repeating units CP/NB (or CP/DCP) | M w (Da, g mol−1) | M w/Mn | cis/trans ratio (in mol%) CP NB (or DCP) | Glass transition point (Tg) | |
|---|---|---|---|---|---|---|
| poly(CP)a | 100/0 in mol% | 453 000 | 2.01 | 19/81 | −99 °C | |
| T m: 9 °C | ||||||
| ΔH: 45 J g−1 | ||||||
| poly(NB-ran-CP)b | 55/45 in mol% | 238 000 | 1.98 | 19/81 | 17/83 | −48 °C |
| 47/53 in wt% | ||||||
| poly(DCP-ran-CP)c | 76/24 in mol% | 250 000 | 1.98 | 19/81 | 19/81 | −46 °C |
| 61/39 in wt% | ||||||
Polymerized in cyclohexane at 25 °C for 3 h; [CP] = 30 wt% (3.38 M), [1-hexene] = 0.956 mM, [M1] = 0.0338 mM, polymer yield was 73%.
Polymerized in cyclohexane at 25 °C for 3 h; [CP] = 20.6 wt% (2.57 M), [NB] = 9.3 wt% (0.83 M), [1-hexene] = 1.37 mM, [M1] = 0.0338 mM, polymer yield was 60%.
Polymerized in cyclohexane at 25 °C for 3 h; [CP] = 18.1 wt% (2.12 M), [DCP] = 6.9 wt% (0.42 M), [1-hexene] = 1.54 mM, [M1] = 0.0507 mM, polymer yield was 68%.
Fig. 1. 1H NMR spectra of (a) poly(CP), (b) poly(NB-ran-CP) and (c) poly(DCP-ran-CP).
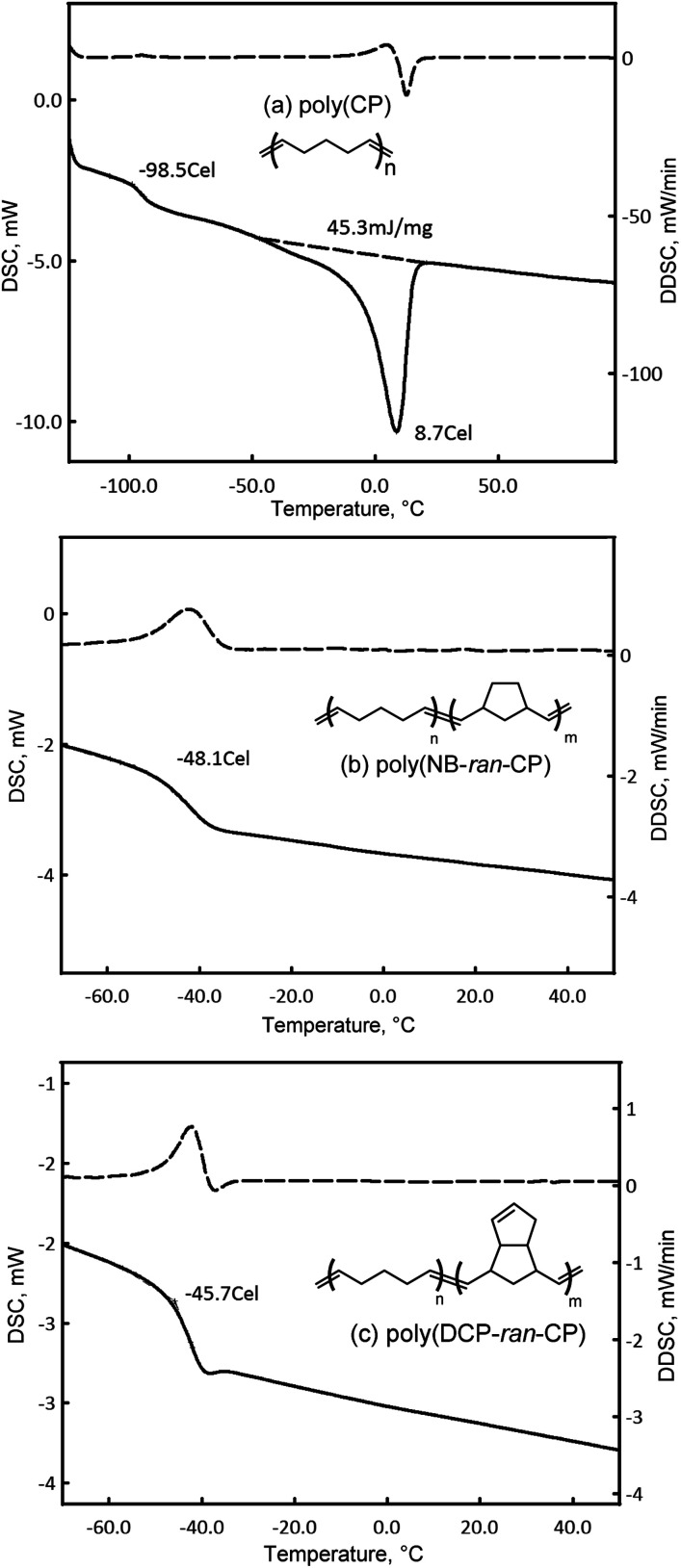
The thermal properties of the polymers were studied by DSC (Fig. 2). It revealed that the glass transition point (Tg) of poly(CP) was at −99 °C and its melting point (Tm) and melting enthalpy (ΔH) were 9 °C and 45 J g−1, respectively, suggesting that the present poly(CP) is a crystalline elastomer. The glass transition points of poly(NB-ran-CP) and poly(DCP-ran-CP) were detected only at −48 °C and −46 °C, respectively. There was no endothermic peak in these copolymers. DSC results clearly support that poly(NB-ran-CP) and poly(DCP-ran-CP) were random copolymers.
Fig. 2. DSC charts of (a) poly(CP), (b) poly(NB-ran-CP) and (c) poly(DCP-ran-CP) (determined in the second scan under N2 for 10 °C min−1 after full annealing; solid line is DSC; dashed line is DDSC).
Vulcanization of poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP): preparation and properties of cyclopentene-based crosslinked rubbers
Rubber products in commercial use are typically fully crosslinked. To tackle real-world challenges in polymer recycling, we also investigated the depolymerization of vulcanized poly(CP) and copolymers. Our goal is to enable the recycling of cyclopentene monomer for subsequent applications.
First, we conducted vulcanization of poly(CP), poly(NB-ran-CP), and poly(DCP-ran-CP). Vulcanization of poly(CP) was carried out as follows: 100 g of poly(CP), 50 g of carbon black, 3 g of zinc oxide, and other components were blended using a Banbury mixer followed by twin roll rubber mills to give a black rubber composition (Table 2).
Mixing composition of poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP).
| poly(CP) rubber | poly(NB-ran-CP) rubber | poly(DCP-ran-CP) rubber | |
|---|---|---|---|
| Poly(CP) | 100 g | ||
| Poly(NB-ran-CP) | 100 g | ||
| Poly(DCP-ran-CP) | 100 g | ||
| Carbon black | 50 g | 50 g | 50 g |
| Zinc oxide | 3 g | 3 g | 3 g |
| Stearic acid | 2 g | 2 g | 2 g |
| Antioxidanta | 2 g | 2 g | 2 g |
| Sulfur | 1.75 g | 1.75 g | 1.75 g |
| Vulcanization acceleratorb | 1 g | 1 g | 1 g |
N 1-(4-methylpentan-2-yl)-N4-phenylbenzene-1,4-diamine.
N-cyclohexyl-2-benzothiazole sulfenamide.
After the blending, the obtained black vulcanizing composition was transferred to a metal mold and vulcanized at 160 °C for 10 min to make a crosslinked rubber specimen. Crosslinked rubbers of poly(NB-ran-CP) and poly(DCP-ran-CP) were prepared in the same manner.
In order to confirm the crosslinking of the three rubbers, we performed swelling tests on them. First, the rubber sheets were cut into small pieces (approximately 20 mm × 20 mm × 1 mm), and their weights were measured by gravimetry. The resulting small rubber pieces were situated in round-bottom flask, then 50 mL of toluene was added and stirred by magnetic stirrer at 60 °C for 24 h. After 24 h immersion, the rubber pieces were taken from flask, their surfaces were washed with toluene and wiped, and they were immediately weighed to determine swelling.
After the measurement of swelling, the rubber pieces were dried in vacuo to measure weight loss due to immersion. The volatiles were removed from the toluene used for immersion to confirm absence of the polymeric compounds.
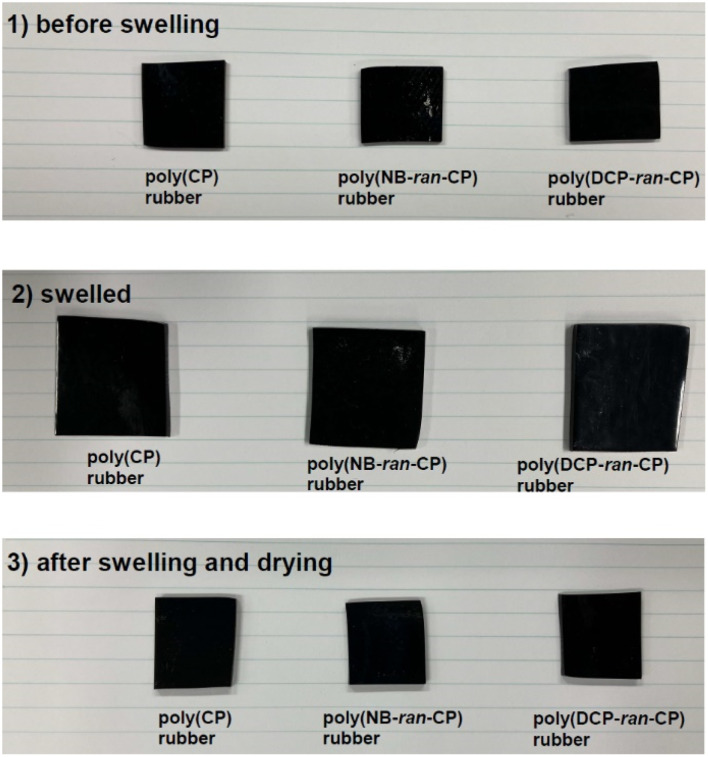
The results of the swelling tests are summarized in Table 3. Photos of the rubbers (1) before swelling, (2) swelled, and (3) after swelling and drying are shown in Fig. 3. All the vulcanized rubbers increased their weight up to approximately 150% by toluene swelling, and they expanded while keeping their shape. After drying, the swelled rubbers shrank to become their original size. Weight losses from immersion were around just 3–4% and quite low. The toluene soluble part contained only a very low MW fraction (Fig. S4–S6†). To conclude, all three vulcanized rubbers are well crosslinked.
Swelling test of vulcanized poly(CP) rubber, poly(NB-ran-CP) rubber and poly(DCP-ran-CP) rubber.
| poly(CP) rubber | poly(NB-ran-CP) rubber | poly(DCP-ran-CP) rubber | |
|---|---|---|---|
| Rubber weight | 0.98 g | 0.97 g | 1.03 g |
| Weight after 24 h swelling at 60 °C | 2.51 g | 2.54 g | 2.43 g |
| Swelling ratio in % | 156% | 162% | 136% |
| Weight after swelling/drying | 0.95 g | 0.94 g | 0.99g |
| Weight loss in % | 3% | 3% | 4% |
Fig. 3. Macroscopic views of vulcanized poly(CP) rubber, poly(NB-ran-CP) rubber and poly(DCP-ran-CP) rubber (1) before swelling, (2) swelled, (3) after swelling and drying.
Depolymerization of pristine poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP)
It is recognized that a dynamic equilibrium between monomers and polymers governs the ROMP process of cyclopentene. The critical concentration of CP is reported around [C]eq = 1.17 M at 30 °C,2 and the ΔG of ring-opening is −0.3 to −2.6 kJ mol−1, varying with the cis/trans ratio in poly(CP).29 Therefore, depolymerization of poly(CP) in the presence of a metathesis catalyst is mainly subject to reaction temperature and concentration of monomers and polymers. To the best of our knowledge, depolymerization of pristine poly(NB-ran-CP) and poly(DCP-ran-CP) has not been well reported yet.
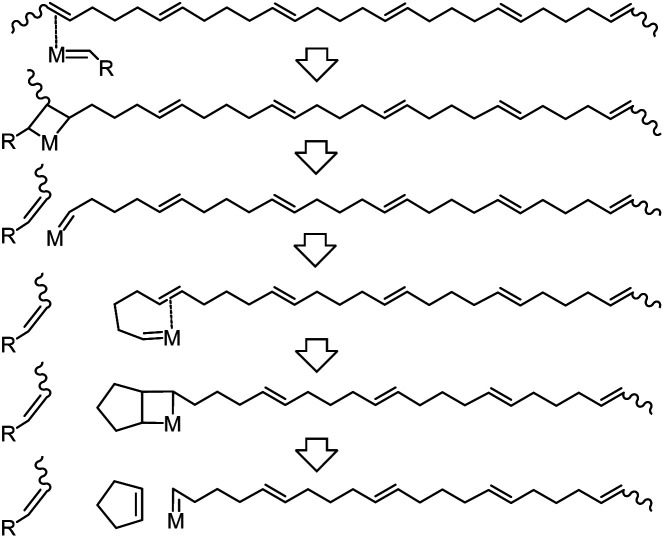
During the depolymerization process of poly(CP), the catalyst is able to depolymerize the polymer chain via chain-end backbiting mechanism (Scheme 3). Initially, the catalyst interacts with one of the double bonds in the poly(CP) chain, followed by the olefin metathesis process to cleave the polymer chain and transfer the reactive metal carbene moiety to the end of the remaining polymer chain. Subsequently, the adjacent double bond coordinates to the vacant site of the metal carbene moiety, enabling the depropagation of the ring-closing metathesis depolymerization (RCMD) process. This chain depolymerization may proceed quickly until the concentration of CP reaches a thermodynamic equilibrium. If the polymer concentration is low enough, all of the poly(CP) chain would convert to CP monomer.
Scheme 3. .Elementary reaction of ring-closing metathesis depolymerization of poly(CP) via the chain-end backbiting mechanism.
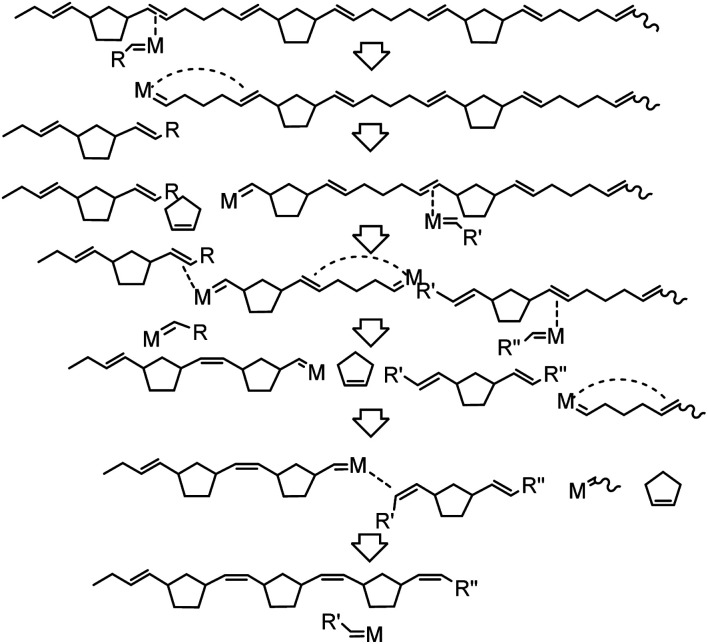
In contrast, depolymerization of poly(NB-ran-CP) and poly(DCP-ran-CP) may proceed in a complicated manner. Scheme 4 illustrates a simplified possible RCMD process of poly(NB-ran-CP). The ΔG of ring-opening of CP is almost zero, whereas the ring-opening of norbornene is highly exergonic. Hence, metathesis catalysts can depolymerize ring-opened CP units efficiently but fail to induce RCMD of NB units at ambient temperature. Therefore, the depolymerization of the NB-CP copolymer chain stops when the carbene moiety reaches the NB unit, and the reactive chain end must be transferred to another polymer chain by cross-metathesis to initiate the next depolymerization process.
Scheme 4. Possible ring-closing metathesis depolymerization process of poly(NB-ran-CP).
Taken together, only CP units can be cut out as a CP monomer from poly(NB-ran-CP), resulting in molecular weight decrease of the polymer. At the end of the depolymerization reaction, only poly(NB) homopolymer with low molecular weight would remain.
Results of RCMD of pristine poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) are summarized in Table 4. In this part, we employed highly active (H2IMes)(PCy3)Cl2Ru CHPh as a depolymerization catalyst.
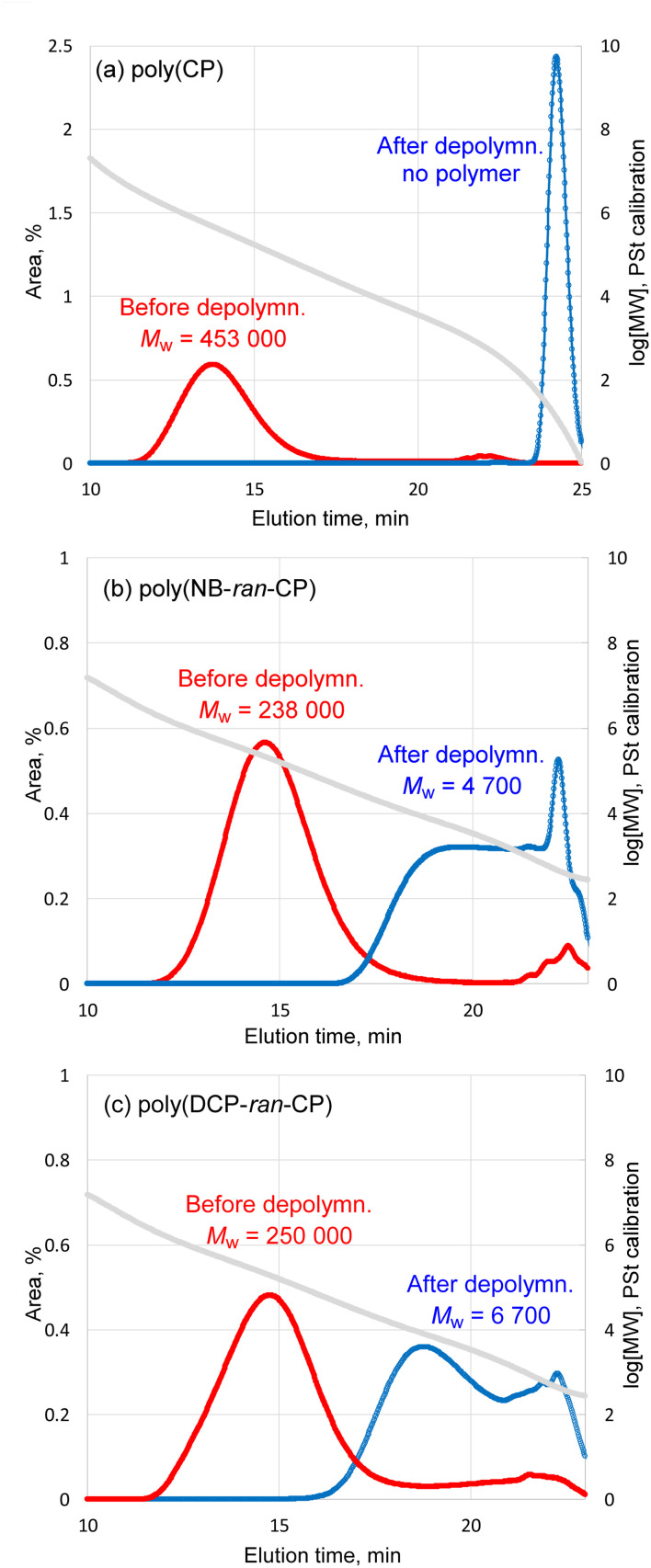
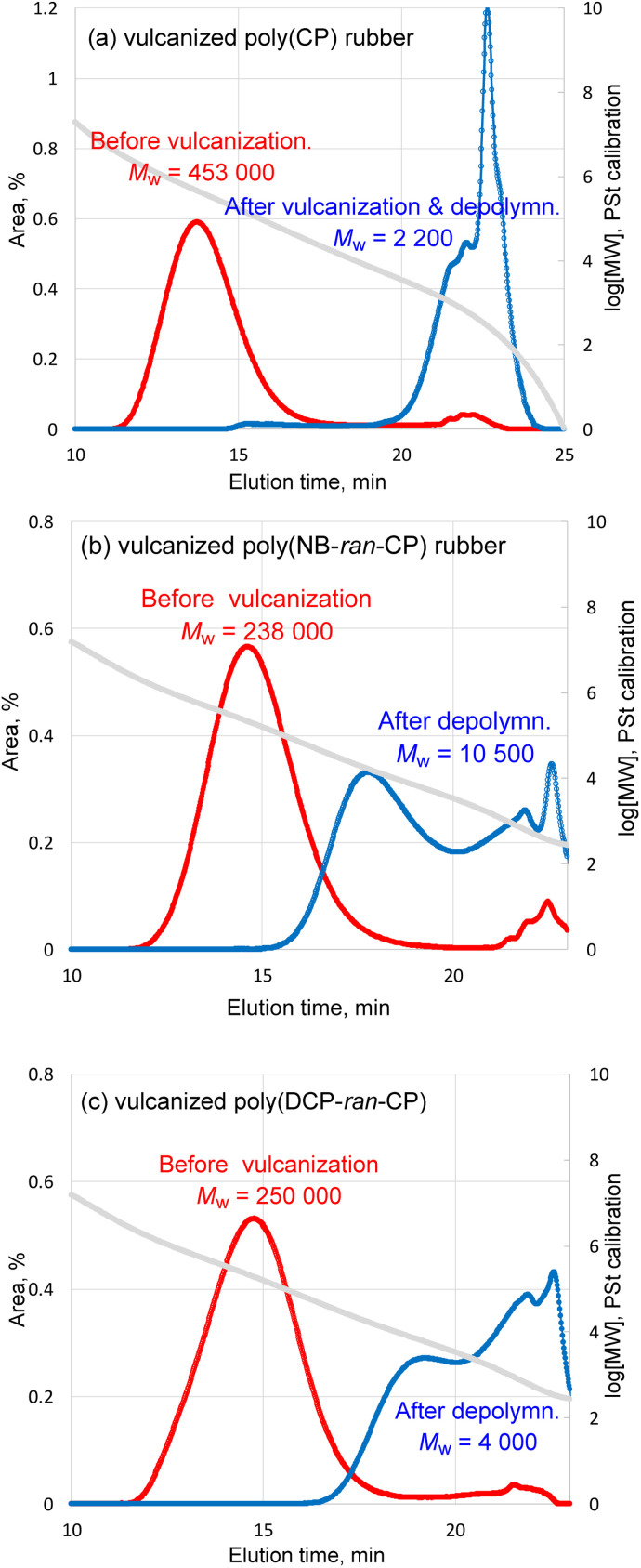
First, 3.01 g of poly(CP) was subjected to depolymerization by 1 mol% ([Ru]/[C C] = 1/100) of (H2IMes)(PCy3)Cl2Ru CHPh at 60 °C for 24 h. In the case of depolymerization of poly(CP), only 3% of non-volatile remained after the reaction. GC measurement identified 97% of CP monomer relative to theoretical yield. GPC trace of non-volatile remaining shows that the Mw of the polymer decreased from 453 000 to almost zero (Fig. 4a). The 1H NMR spectrum implied that the non-volatile remaining contained small amount of poly(CP) (Fig. S7†). Indeed, the depolymerization of poly(CP) proceeded almost instantly, suggesting that the used catalyst amount was largely in excess and the reaction temperature was high enough. Therefore, we lowered the reaction temperature to 25 °C and lowered catalyst loading from 1 mol% to 0.05 mol% (and 0.001 mol%). Time-Mw plots of each depolymerization mixture are illustrated in Fig. S10.† In each case, the Mw of the polymers decreased to around 40 000–60 000 after just 10 min reaction. Even 0.001 mol% of (H2IMes)(PCy3)Cl2Ru CHPh was able to completely depolymerize the poly(CP) at 25 °C within 1 h.
Fig. 4. GPC traces of (a) pristine poly(CP), (b) pristine poly(NB-ran-CP) and (c) pristine poly(DCP-ran-CP) before and after the RCMD reaction.
Likewise, poly(NB-ran-CP) was subjected to RCMD reaction. 3.00 g of the poly(NB-ran-CP) contains 1.41 g of CP unit and 1.59 g of NB unit. The depolymerization reaction was confirmed by GC analysis to give 1.32 g (94% of theoretical value) of cyclopentene monomer in 24 h at 60 °C. 1.60 g of non-volatile soluble polymer was left after depolymerization. The 1H NMR spectrum demonstrates that only 0.03 g of cyclopentene unit remained in the remaining copolymer (Fig. S8†). GPC results showed that the Mw of the copolymer decreased from 238 000 to 4700 (Fig. 4b). Based on the results above, the olefin metathesis reaction succeeded in cutting out cyclopentene monomer from copolymer chains, leaving “polynorbornene” with low MW.
The RCMD of poly(DCP-ran-CP) was also conducted at 60 °C for 24 h. 3.02 g of the poly(DCP-ran-CP) comprises 1.84 g of CP unit and 1.18 g of DCP unit. GC analysis of the reaction mixture confirmed that we afforded 1.69 g (92% of theoretical value) of cyclopentene monomer. The 1H NMR spectrum of the non-volatile soluble part suggests that only 0.13 g of cyclopentene unit remained in the copolymer (Fig. S9†). GPC results (Fig. 4c) showed that the Mw of the copolymer decreased from 250 000 to 6700.
The time dependence of the depolymerization of poly(NB-ran-CP) and poly(DCP-ran-CP) was investigated with varied reaction temperature with 0.05 mol% catalyst loading. Time-Mw plots of the depolymerization of poly(NB-ran-CP) and poly(DCP-ran-CP) are shown in Fig. S11 and S12,† respectively. The depolymerization rates of the copolymers were much lower than that of poly(CP). At 60 °C, depolymerization proceeded quickly; in contrast, at 25 °C, decrease of Mw of the copolymers did not level off within 60 min.
Based on the depolymerization rate of poly(CP), we presume that the chain-end backbiting process of CP segment would proceed very rapidly. In contrast, depolymerization of the two copolymers was slow compared to that of poly(CP). The slow depolymerization rate of copolymers seems reasonable if the reaction mechanism is inferred. Ring-closing depolymerization of CP unit of copolymers must undergo cross metathesis between polymer chains. That is, the reactive chain end has to transfer to another polymer chain to initiate the next depolymerization process. Comparing the two copolymers, the depolymerization rate of poly(NB-ran-CP) was faster than that of poly(DCP-ran-CP), even taking into account the difference in the amount of CP units and of catalyst introduction, probably due to steric hindrance of the bulky DCP unit.
Depolymerization and cyclopentene recovery of vulcanized rubber of Poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP)
As described above, it has been revealed that CP monomer can be recovered by depolymerization of pristine poly(CP) and its copolymers. However, the rubbers in actual uses are crosslinked, making them insoluble; they only swell in organic solvents. When depolymerization is performed with metathesis catalyst, the reaction is expected to be a heterogeneous reaction, which is extremely unfavourable to promote. Furthermore, vulcanized rubber contains polar additives that can terminate or retard the metathesis reaction. To achieve social implementation of this technology, it is essential to realize depolymerization and monomer recycling under such unfavourable conditions.
We prepared the black rubber composition first (Table 2), then conducted successive vulcanization to produce a rubber specimen. 100 mm × 100 mm × 1 mm rubber sheets were made from poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP). The rubber sheets were manually cut into small pieces using scissors, sized around 20 mm × 20 mm × 1 mm, in preparation for depolymerization in a glass ampoule.
Fig. 5 shows photos of the depolymerization reaction procedure of the aforementioned vulcanized poly(CP) rubber. At first, small pieces of poly(CP) rubber sheet were placed into a glass ampoule (Fig. 5a). After that, toluene solution of (H2IMes)(PCy3)Cl2Ru CHPh (G2) (1 mol%; [Ru]/[C C] = 1/100) was added (Fig. 5b). The resulting mixture was heated to 60 °C and continuously stirred ([CP unit]o = 0.50 M). After 1 h, the pieces of crosslinked rubber became thin and small, and black precipitates appeared in the solution. 24 h later, the pieces of rubber sheet completely disappeared, and a black opaque dispersion was obtained (Fig. 5c).
Fig. 5. Photos of crosslinked poly(CP) rubber in an ampoule tube (a), just after adding G2 solution (b) and 24 h after the depolymerization reaction at 60 °C (c).
The resulting depolymerization mixture was quenched with a small amount of butyl vinyl ether. After taking small aliquots from the crude liquid for GC and GPC analyses, insoluble inorganic fillers were separated from the organic solution through repeated extraction with toluene and centrifugation (3000 rpm, 10 min × 3). All the volatiles were evaporated from the transparent organic solution in vacuo at 40 °C for 24 h to obtain residual non-volatiles. Inorganic fillers were dried in vacuo at 40 °C for 24 h. Yields of both residuals were determined by gravimetric measurement.
The RCMD results of vulcanized poly(CP) rubber, poly(NB-ran-CP) rubber and poly(DCP-ran-CP) rubber are summarized in Table 5.
Depolymerization of crosslinked poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP)a.
| Poly(CP) rubber | poly(NB-ran-CP) rubberb | poly(DCP-ran-CP) rubberc | ||
|---|---|---|---|---|
| Initial rubbers | Vulcanized rubber | 2.81 g | 3.00 g | 3.00 g |
| Filler | 0.93 g | 1.00 g | 1.00 g | |
| Additives | 0.12 g | 0.12 g | 0.12 g | |
| Polymer | 1.76 g | 1.88 g | 1.88 g | |
| CP unit | 1.76 g | 0.85 g | 1.15 g | |
| NB (or DCP) unit | — | 1.03 g | 0.73 g | |
| CP/NB (or CP/DCP) | 100/0 in mol | 55/45 in mol | 76/24 in mol | |
| M w(initial) (g mol−1) | 453 000 | 238 000 | 250 000 | |
| M W/Mn | 2.01 | 1.98 | 1.98 | |
| G2 | 0.22 g | 0.19 g | 0.17 g | |
| After depolymerization | Total non-volatile part | 1.48 g | 2.42 g | 2.24 g |
| Insoluble black filler | 1.14 g | 1.15 g | 1.13 g | |
| Soluble part | 0.34 g | 1.27 g | 1.11 g | |
| CP unit | Tracee | 0.05 g, 6% | 0.18 g, 16% | |
| NB (or DCP) unitd | — | 1.03 g, 100% | 0.73 g, 100% | |
| CP/NB (or CP/DCP) | 100/0 in mol | 4/96 in mol | 33/67 in mol | |
| M w (g mol−1) | 2200 | 10 500 | 4000 | |
| M W/Mn | 5.0 | 8.4 | 4.5 | |
| Volatile part | 1.54 g | 0.77 g | 0.94 g | |
| CP recovery in % | 88% | 91% | 82% | |
Depolymerized in toluene at 60 °C for 24 h; [total polymer units] = 0.5 M, [(H2IMes)(PCy3)Cl2Ru CHPh] = 5 mM, [(H2IMes)(PCy3)Cl2Ru CHPh]:[total polymer units] = 1 : 100.
[Poly(CP) units] = 0.28 M.
[Poly(CP) units] = 0.38 M.
It is believed that metathesis catalyst cannot close NB ring at ambient temperature. Therefore, NB repeating unit and DCP repeating unit would never be depolymerized and, therefore, weight of NB unit and DCP unit would be intact during depolymerization. Based on this hypothesis and integration of 1H NMR, ratios of CP unit in the residual copolymers were calculated.
Weight of remaining CP unit can be roughly calculated using the following equation: (CP unit) = (total insoluble part) – (filler) – (additives) – (G2); however, poly(CP) was not a majority of the obtained mixture.
In the case of the depolymerization of poly(CP) rubber, it is noteworthy that 88% of cyclopentene monomer was identified by GC relative to the theoretical yield. Namely, this demonstrates that monomer recycling of vulcanized poly(CP) could be realized. In the case of depolymerization of pristine poly(CP), 97% of cyclopentene unit was depolymerized and identified as a monomer. The cyclopentene recovery efficiency of vulcanized poly(CP) rubber appeared to be 9% lower than that of pristine poly(CP). Regarding the material balance of non-volatile part, 2.81 g of vulcanized poly(CP) rubber consisted of 1.76 g of poly(CP) and 1.05 g of other components; in addition, we employed 0.22 g of G2 as a depolymerization catalyst. Therefore, in total, 3.03 g of starting mixture was subjected to the depolymerization reaction. After the depolymerization of poly(CP) rubber sheet, 1.14 g of insoluble, black powdery filler part was obtained by centrifugation and vacuum drying, and 0.34 g of wax-like non-volatile soluble part was collected after evaporation of the organic solution. GC analysis revealed that there was 1.54 g of cyclopentene monomer in the volatile part. The retrieved mass of all components from the depolymerization reaction was 3.02 g, aligning well with the total mass of starting materials. According to the combination of 1H NMR (Fig. S13†) and GPC results (Fig. 6a), the non-volatile soluble part comprised a small amount of poly(CP) and unidentified residues. The GPC chromatogram indicated that the Mw of remaining polymer fraction in the non-volatile soluble part was 2 200, and almost the entire fraction consisted of very low MW compounds, probably representing unidentified residues. 1H NMR implied that there was poly(CP) in the non-volatile soluble part, although considering the combination of GPC chromatogram and integration of 1H NMR, it is not a major ingredient of the mixture.
Fig. 6. GPC traces of (a) the vulcanized poly(CP) rubber, (b) vulcanized poly(NB-ran-CP) rubber and (c) vulcanized poly(DCP-ran-CP) rubber before vulcanization and after RCMD reaction.
Likewise, recovery of cyclopentene monomer from vulcanized poly(NB-ran-CP) and poly(DCP-ran-CP) rubbers could be achieved. Following depolymerization of poly(NB-ran-CP) rubber and poly(DCP-ran-CP) rubber, 91% and 82% of cyclopentene monomers were identified by GC, respectively, compared to the theoretical yields. GC-based monomer recovery efficiencies of vulcanized poly(CP) rubber, poly(NB-ran-CP) rubber and poly(DCP-ran-CP) rubber were lower than those of pristine ones. It is plausible that the crosslinked moiety could not be depolymerized as CP monomer. In other words, the decrease in monomer recycling efficiency and the quantity of unidentified residues may be correlated with the degree of crosslinking of the present rubbers. 3.00 g of the vulcanized poly(NB-ran-CP) rubber consisted of 1.88 g of poly(NB-ran-CP) and 1.12 g of other ingredients. 0.19 g of G2 catalyst was employed for the depolymerization; therefore, the total mass of the non-volatile starting mixture was 3.19 g. After the RCMD reaction and work-up, 1.27 g of non-volatile soluble part and 1.15 g of insoluble, black powdery filler residues remained. GC analysis of depolymerization mixture reflected that 0.77 g of CP monomer was produced.
The 1H NMR study of the non-volatile soluble part implied that the majority of it was the copolymer of NB and CP, with a small quantity of unidentified residues. The 1H NMR study also revealed that 6% of cyclopentene unit remained in the copolymer and 94% became cyclopentene monomer, which was in good agreement with the GC results (cyclopentene recovery = 91%) (Fig. S14†). The Mw of the non-volatile soluble part was 10 500 and a bimodal MWD was identified (Fig. 6b). To conclude, olefin metathesis reaction can selectively cut out cyclopentene monomer from vulcanized poly(NB-ran-CP) rubber, leaving soluble norbornene-rich “homopolymer”.
In the case of poly(DCP-ran-CP) rubber, 3.00 g of the vulcanized rubber consisted of 1.88 g of poly(DCP-ran-CP) and 1.12 g of other ingredients. We employed 0.19 g of G2 for depolymerization; therefore, the total mass of the starting mixture was 3.19 g. After RCMD reaction and work-up, 1.11 g of non-volatile soluble part and 1.13 g of insoluble, black powdery filler residues remained. As expected from the GC analysis data of cyclopentene recovery (82%), the remaining copolymer contained a lot of undepolymerized cyclopentene unit: the 1H NMR study suggested that the remaining copolymer comprised 33 mol% of CP unit and 67 mol% of DCP unit (Fig. S15†). The CP unit in the remaining copolymer was calculated to be 0.18 g and 16% of initial CP unit. The Mw of the non-volatile soluble part was 4000 (Fig. 6c). To summarize, crosslinked poly(DCP-ran-CP) rubber was depolymerized to give cyclopentene monomer; however, the cyclopentene recovery was lower than those of poly(CP) rubber and poly(NB-ran-CP) rubber.
In this section, a large amount of (H2IMes)(PCy3)Cl2Ru CHPh (G2) (1 mol%; [Ru]/[C C] = 1/100) was employed for the RCMD reaction of vulcanized rubbers of poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP). In fact, the vulcanized rubbers contain many additives that retard the metathesis depolymerization. Particularly, antioxidant and vulcanization accelerator are harmful for the present reaction, and, as a result, a large amount of G2 was necessary for this study. The influence of additives and catalysts are under intensive research and will be reported in near future.
Conclusions
In this study, we successfully demonstrated the depolymerization of vulcanized poly(CP), poly(NB-ran-CP) and poly(DCP-ran-CP) rubbers, uncovering the potential recyclability of cyclopentene monomer. The recovery efficiency of cyclopentene monomer was consistently around 90%, regardless of whether the crosslinked rubber was a homopolymer or copolymer. This groundbreaking discovery is noteworthy, as monomer recycling has been historically challenging with conventional rubber materials.
As we move toward sustainable industrialization and social implementation, we will continue our strategic research focus on key areas such as minimization of catalyst loading in the depolymerization, enhancement of the depolymerization efficiency, investigation into the reuse of recovered inorganic fillers, and exploration of potential applications of residual NB- and DCP-rich polymers. Development of engineering technology is also important to the success of this study. Process investigation such as recovery technologies for cyclopentene monomers, solvents, polymers, and inorganic fillers, reduction of their energy consumption, and new technologies for the recovery of transition metal catalysts, will also be important in real manufacturing plants to achieve broader social implementation with sustainability.
We are currently researching these issues and will report detailed findings in near future.
Data availability
We would like to declare that the data supporting this manuscript have been included as part of the ESI.†
Author contributions
S. H. proposed the concept of the present research. K. I. and T. S. synthesized all the polymers and S. H. analysed all of them. K. I. and T. S. prepared vulcanization composites. K. I., T. S. and S. H. carried out vulcanization of the composites, and measurement of properties of the crosslinked rubbers. K. K., S. W. and S. H. conducted ring-closing metathesis depolymerization of all the pristine polymers and vulcanized rubbers. K. K., S. W. and S. H. performed all the work-up procedures and the analyses of the depolymerization reaction. S. H. and S. W. wrote the first version of the manuscript with contribution of all the author members. All the authors contributed to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We are thankful to Dr Tsukuru Yagihara (Chemicals Evaluation and Research Institute; CERI) for his contribution of operating vulcanization of polymer composites. The authors are grateful to Prof. Hiroaki Kouzai (Kanto Gakuin University, College of Science and Engineering) for his fruitful discussions and kind support in the course of this work.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra06914e
Notes and references
- Fukumori K. Matsushita M. Material Recycling Technology of Crosslinked Rubber Waste. R&D Rev. Toyota CRDL. 2003;38:39–47. [Google Scholar]
- Neary W. J. Kennemur J. G. Polypentenamer Renaissance: Challenges and Opportunities. ACS Macro Lett. 2019;8:46–56. doi: 10.1021/acsmacrolett.8b00885. [DOI] [PubMed] [Google Scholar]
- Flisi U. Zamboni V. Novajra G. Peveri G. Stress-induced crystallization and physical properties of trans-polypentenamer. Eur. Polym. J. 1973;9:1187–1203. [Google Scholar]
- Porri L. Rossi R. Diversi P. Lucherini A. Ring-opening polymerization of cycloolefins with catalysts derived from ruthenium and iridium. Makromol. Chem. 1974;175:3097–3115. [Google Scholar]
- Braun H.-G. Rehage G. Compatibility and Molecular Structure of Rubbers. Angew. Makromol. Chem. 1985;131:107–115. [Google Scholar]
- Rahrig D. MacKnight W. J. Lenz R. W. Sulfonation of a Polypentenamer and Preparation of Its Hydrogenated Derivatives. Macromolecules. 1979;12:195–203. [Google Scholar]
- Hejl A. Scherman O. A. Grubbs R. H. Ring-Opening Metathesis Polymerization of Functionalized Low-Strain Monomers with Ruthenium-Based Catalysts. Macromolecules. 2005;38:7214–7218. [Google Scholar]
- Bielawski C. W. Grubbs R. H. Highly Efficient Ring-Opening Metathesis Polymerization (ROMP) Using New Ruthenium Catalysts Containing N-Heterocyclic Carbene Ligands. Angew. Chem., Int. Ed. 2000;39:2903–2906. doi: 10.1002/1521-3773(20000818)39:16<2903::aid-anie2903>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tuba R. Grubbs R. H. Ruthenium catalyzed equilibrium ring-opening metathesis polymerization of cyclopentene. Polym. Chem. 2013;4:3959–3962. [Google Scholar]
- Torre III M. Mulhearn W. D. Register R. A. Ring-Opening Metathesis Copolymerization of Cyclopentene Above and Below Its Equilibrium Monomer Concentration. Macromol. Chem. Phys. 2018;219:1800030. [Google Scholar]
- Denisova Y. I. Zhigarev V. A. Gringolts M. L. Shandryuk G. A. Peregudov A. S. Finkelshtein E. S. Kudryavtsev Y. V. Olefin cross-metathesis of polynorbornene with polypentenamer: New norbornene–cyclopentene multiblock copolymers. Eur. Polym. J. 2022;173:111264. [Google Scholar]
- López-Barrón C. R. Rohde B. Zabula A. V. Schaefer J. J. Throckmorton J. A. Molecular Orientation and Strain-Induced Crystallization in trans-Polypentenamer. Macromolecules. 2020;53:1356–1367. [Google Scholar]
- Weller D. W. Halbach R. Rohde B. Kang S. Dwivedi S. Mehringer K. D. Shankar R. Storey R. F. Morgan S. E. Zabula A. V. Gu X. López-Barrón C. R. Long-Chain Branched Polypentenamer Rubber: Topological Impact on Tensile Properties, Chain Dynamics, and Strain-Induced Crystallization. ACS Appl. Polym. Mater. 2021;3:2498–2506. [Google Scholar]
- Weller D. W. Halbach R. Zabula A. V. Mattler S. J. Gu X. López-Barrón C. R. Polypentenamer thermoplastic elastomers via copolymerization of cyclopentene and dicyclopentadiene. RSC Appl. Polym. 2023;1:281–291. [Google Scholar]
- Tuba R. Balogh J. Hlil A. Barłóg M. Al-Hashimi M. Bazzi H. S. Synthesis of Recyclable Tire Additives via Equilibrium Ring Opening Metathesis Polymerization. ACS Sustain. Chem. Eng. 2016;4:6090–6094. [Google Scholar]
- Guillory G. A. Kennemur J. G. Investigating the effects of bulky allylic substituents on the regioregularity and thermodynamics of ROMP on cyclopentene. Eur. Polym. J. 2019;120:109251. [Google Scholar]
- Neary W. J. Isais T. A. Kennemur J. G. Depolymerization of Bottlebrush Polypentenamers and Their Macromolecular Metamorphosis. J. Am. Chem. Soc. 2019;141:14220–14229. doi: 10.1021/jacs.9b05560. [DOI] [PubMed] [Google Scholar]
- Coia B. M. Werner S. E. Kennemur J. G. Conformational bias in density functional theory ring strain energy calculations of cyclopentene derivatives: Towards predictive design of chemically recyclable elastomers. J. Polym. Sci. 2022;60:3391–3403. [Google Scholar]
- Liu H. Nelson A. Z. Ren Y. Yang K. Ewoldt R. H. Moore J. S. Dynamic Remodelling of Covalent Networks via Ring-Opening Metathesis Polymerization. ACS Macro Lett. 2018;7:933–937. doi: 10.1021/acsmacrolett.8b00422. [DOI] [PubMed] [Google Scholar]
- Feist J. D. Lee D. C. Xi Y. A versatile approach for the synthesis of degradable polymers via controlled ring-opening metathesis copolymerization. Nat. Chem. 2022;14:53–58. doi: 10.1038/s41557-021-00810-2. [DOI] [PubMed] [Google Scholar]
- An T. Ryu H. Choi T.-L. Living Alternating Ring-Opening Metathesis Copolymerization of 2,3-Dihydrofuran to Provide Completely Degradable Polymers. Angew. Chem., Int. Ed. 2023;62:e202309632. doi: 10.1002/anie.202309632. [DOI] [PubMed] [Google Scholar]
- Si G. Chen C. Cyclic–acyclic monomers metathesis polymerization for the synthesis of degradable thermosets, thermoplastics and elastomers. Nat. Synth. 2022;1:956–966. [Google Scholar]
- Herman J. A. Seazzu M. E. Hughes L. G. Wheeler D. R. Washburn C. M. Jones B. H. Depolymerization of Cross-Linked Polybutadiene Networks in Situ Using Latent Alkene Metathesis. ACS Appl. Polym. Mater. 2019;1:2177–2188. [Google Scholar]
- Warner M. J. Lassa J. P. Narcross H. Commisso A. Ghosh K. Romero M. Schwartz J. M. Engler A. C. Kohl P. A. Leguizamon S. C. Jones B. H. Chemical Recycling of Polybutadiene Rubber with Tailored Depolymerization Enabled by Microencapsulated Metathesis Catalysts. ACS Sustainable Chem. Eng. 2023;11:14538–14548. [Google Scholar]
- Vehlow K. Wang D. Buchmeiser M. R. Blechert S. Alternating Copolymerizations Using a Grubbs-Type Initiator with an Unsymmetrical, Chiral N-Heterocyclic Carbene Ligand. Angew. Chem., Int. Ed. 2008;47:2615–2618. doi: 10.1002/anie.200704822. [DOI] [PubMed] [Google Scholar]
- Al Samak B. Carvill A. G. Hamilton J. G. Rooney J. J. Thompson J. M. Alternating ring-opening metathesis copolymerization of bicyclo[2.2.1]hept-2-ene and cyclopentene. Chem. Commun. 1997:2057–2058. [Google Scholar]
- Ivin K. J. O'Donnell J. H. Rooney J. J. Stewart C. D. The 13C NMR spectra of ring-opened copolymers of cyclopentene and norbornene; reactivity ratios. Makromol. Chem. 1979;180:1975–1988. [Google Scholar]
- Nikovia C. Maroudas A.-P. Goulis P. Tzimis D. Paraskevopoulou P. Pitsikalis M. Statistical Ring Opening Metathesis Copolymerization of Norbornene and Cyclopentene by Grubbs' 1st-Generation Catalyst. Molecules. 2015;20:15597–15615. doi: 10.3390/molecules200915597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivin K. J. and Mol J. C., Olefin Metathesis and Metathesis Polymerization, Academic Press, San Diego, 1997 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We would like to declare that the data supporting this manuscript have been included as part of the ESI.†