Abstract
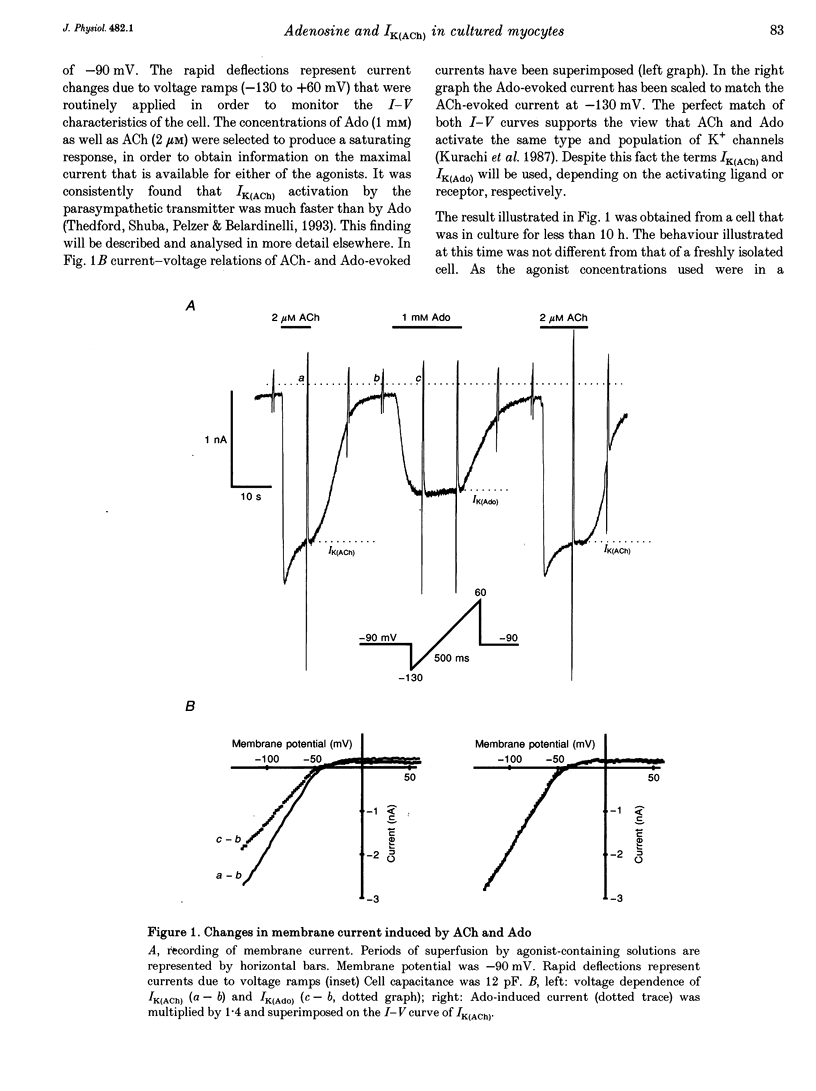
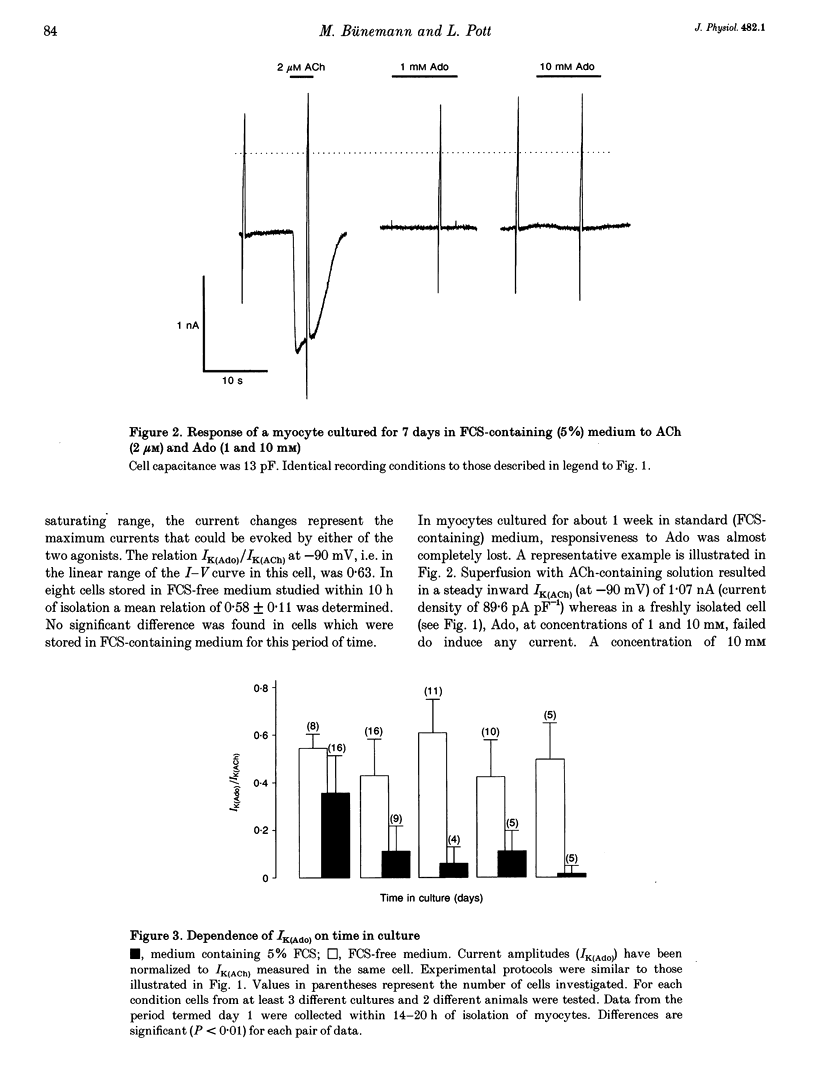
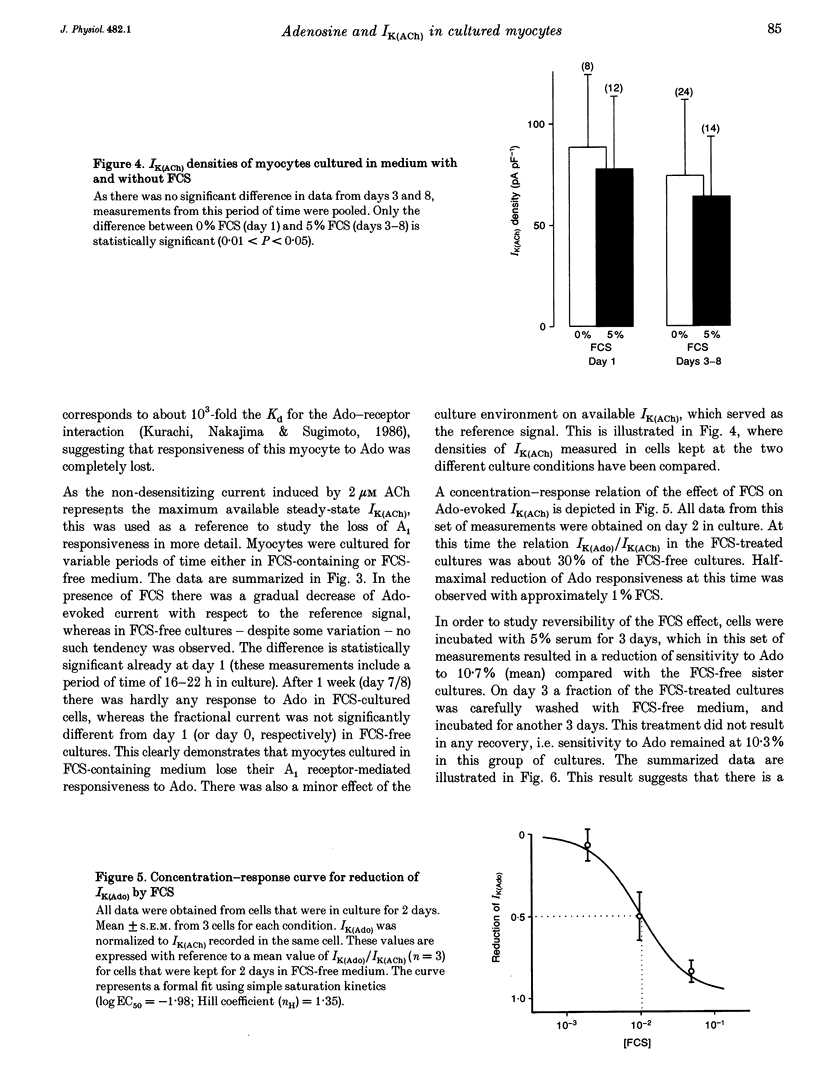
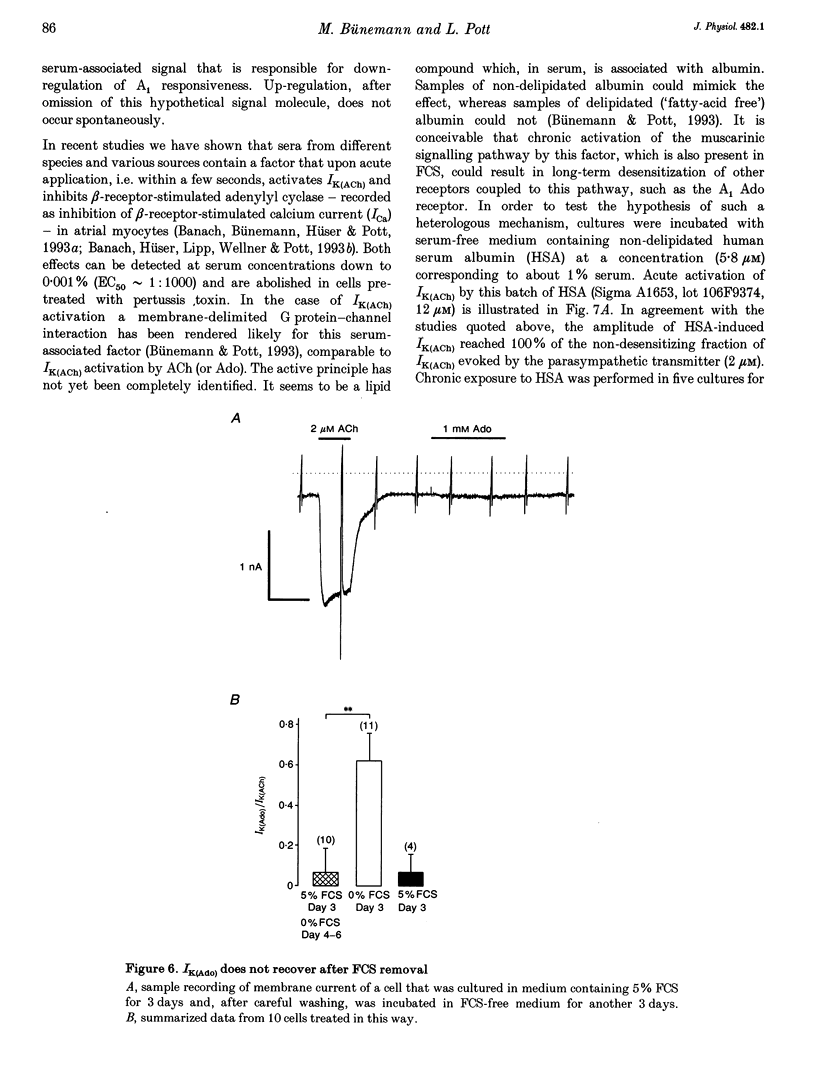
1. Muscarinic K+ current (IK(ACh)) was measured in cultured atrial myocytes from hearts of adult guinea-pigs using whole-cell voltage clamp. IK(ACh) was activated by superfusion with solutions containing either acetylcholine (ACh) or adenosine (Ado), in saturating concentrations of 2 microM (ACh) and 1 mM (Ado), respectively. 2. In freshly isolated cells the amplitude of the current activated by Ado (IK(Ado)) was 58% (mean) of the current that was induced by ACh. In serum-free culture this relation, but also the absolute density of IK(ACh), remained fairly constant for up to 8 days. 3. If the culture medium was supplemented with fetal calf serum (FCS, 5%) the relation IK(Ado)/IK(ACh) gradually decayed, reaching a value of less than 0.1 on days 7-8, whereas the response to ACh remained stable over this period of time. 4. After treatment of cells with FCS-containing medium, no recovery was observed upon FCS withdrawal for up to 4 days. 5. The effect of FCS on responsiveness to Ado was half-maximal at about 1% (v/v). The active principle can be dialysed (mol. mass exclusion: 10 kDa). It is not identical with an albumin-associated factor that has been shown to be a potent activator of atrial IK(ACh) upon acute superfusion. Loss of responsiveness to Ado was paralleled by a reduction of binding sites to the A1 adenosine receptor-specific radioligand 8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX). 6. It is concluded that FCS contains a factor that causes down-regulation of A1 Ado receptors. The signalling pathway that leads to an increased opening activity of IK(ACh) channels and other receptors, such as the M2 muscarinic receptor, linked to this signalling pathway are not affected by this factor.
Full text
PDF

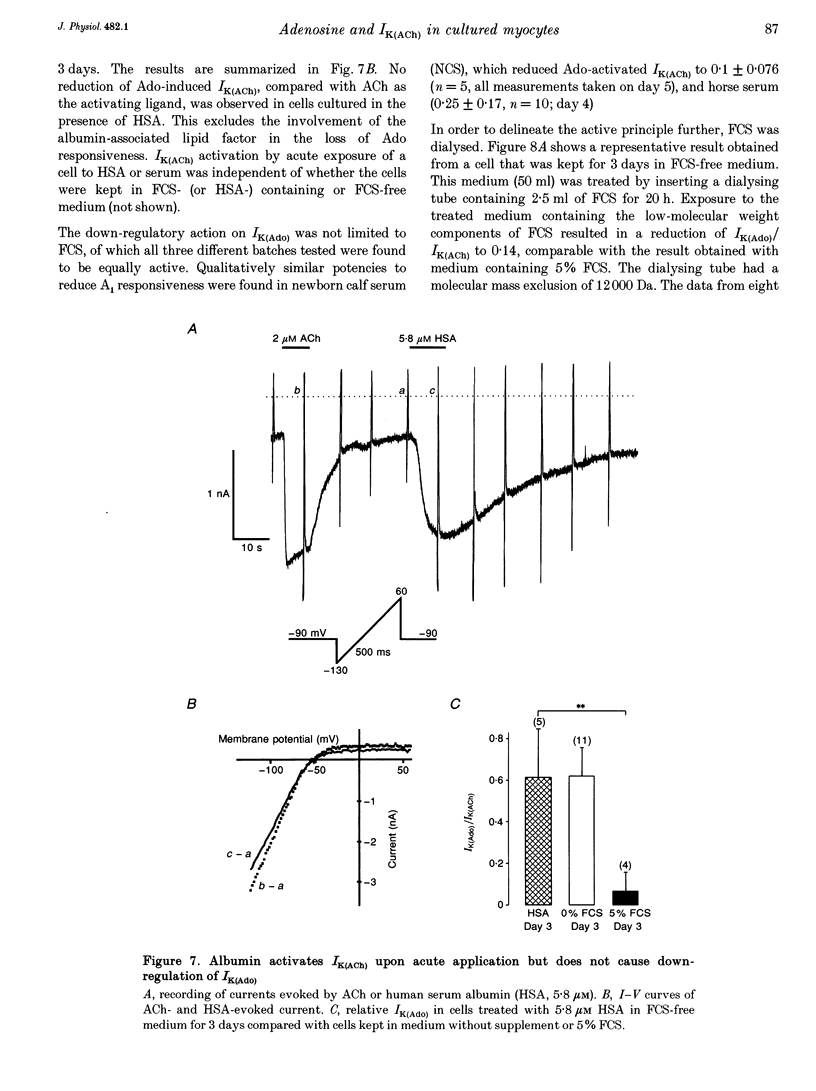
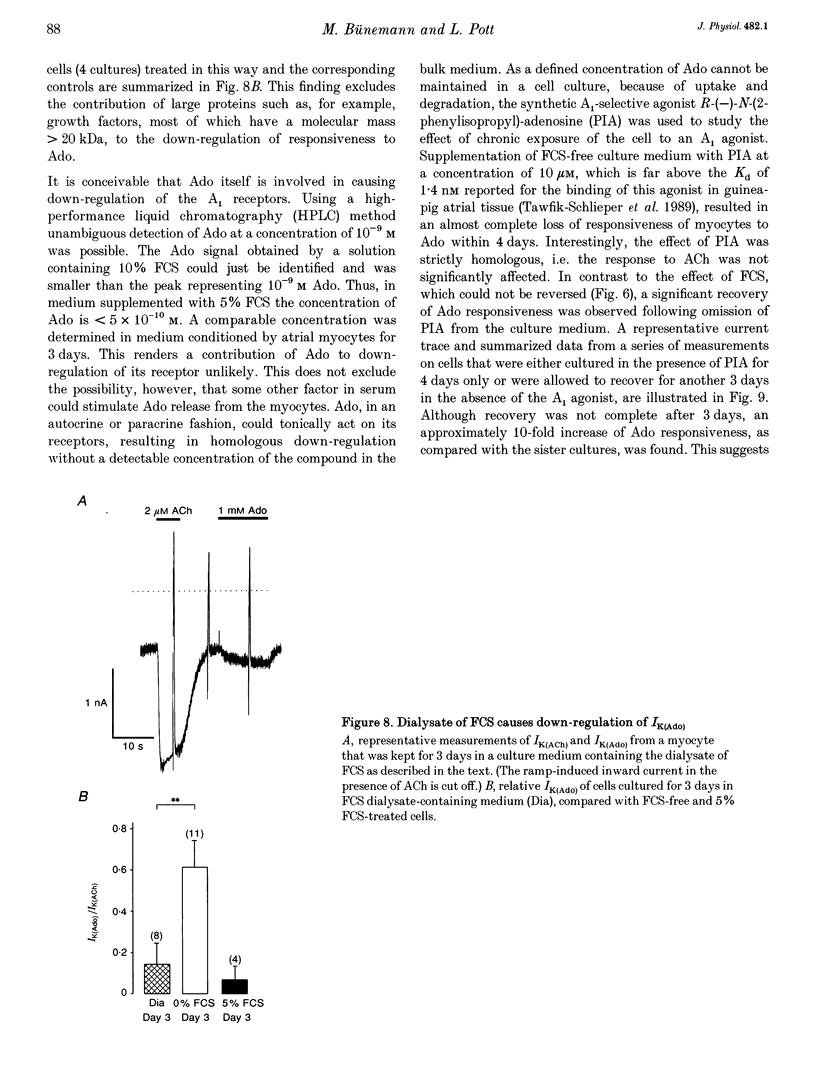
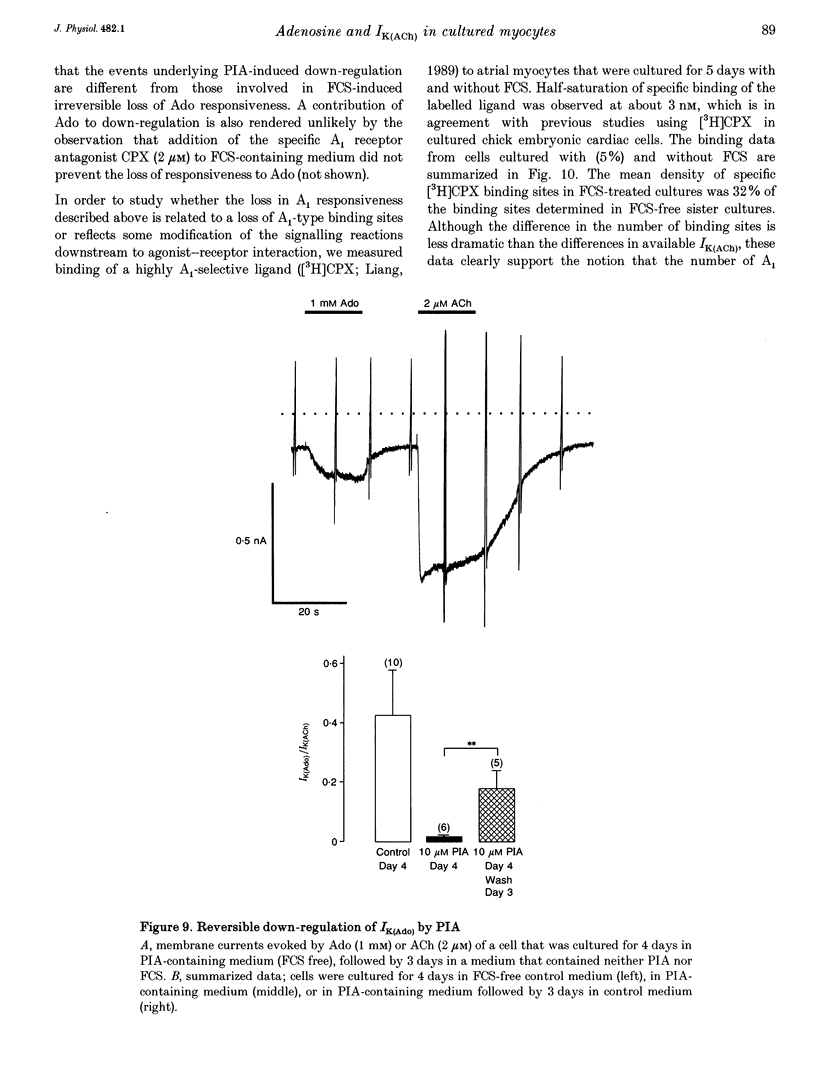
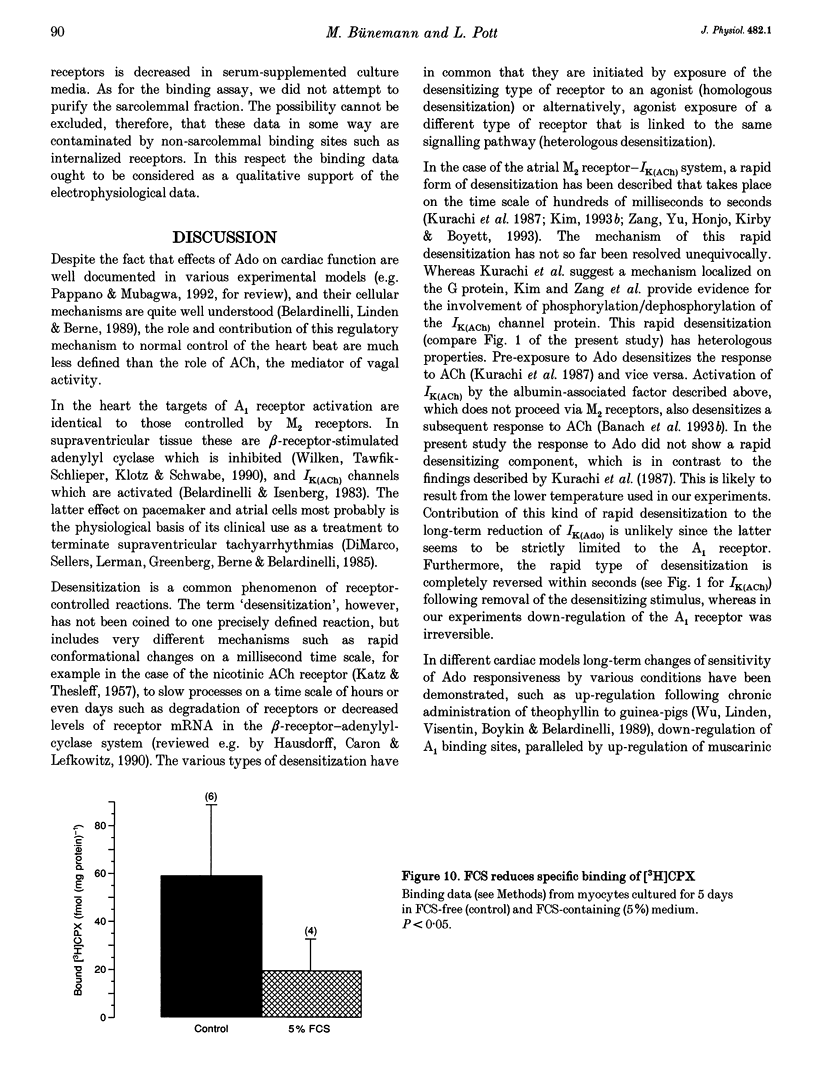


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banach K., Hüser J., Lipp P., Wellner M. C., Pott L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by a serum factor. J Physiol. 1993 Feb;461:263–281. doi: 10.1113/jphysiol.1993.sp019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Pott L. Removal of Ca current inactivation in dialysed guinea-pig atrial cardioballs by Ca chelators. Pflugers Arch. 1985 May;404(1):10–20. doi: 10.1007/BF00581485. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Giles W. R., West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988 Nov;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Linden J., Berne R. M. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989 Jul-Aug;32(1):73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- Bünemann M., Pott L. Membrane-delimited activation of muscarinic K current by an albumin-associated factor in guinea-pig atrial myocytes. Pflugers Arch. 1993 Nov;425(3-4):329–334. doi: 10.1007/BF00374183. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Caron M. G., Lefkowitz R. J. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–2889. [PubMed] [Google Scholar]
- Horie M., Irisawa H. Rectification of muscarinic K+ current by magnesium ion in guinea pig atrial cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H210–H214. doi: 10.1152/ajpheart.1987.253.1.H210. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacimi R., Richalet J. P., Crozatier B. Hypoxia-induced differential modulation of adenosinergic and muscarinic receptors in rat heart. J Appl Physiol (1985) 1993 Sep;75(3):1123–1128. doi: 10.1152/jappl.1993.75.3.1123. [DOI] [PubMed] [Google Scholar]
- Kim D. Novel cation-selective mechanosensitive ion channel in the atrial cell membrane. Circ Res. 1993 Jan;72(1):225–231. doi: 10.1161/01.res.72.1.225. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. Short-term desensitization of muscarinic K+ channel current in isolated atrial myocytes and possible role of GTP-binding proteins. Pflugers Arch. 1987 Oct;410(3):227–233. doi: 10.1007/BF00580270. [DOI] [PubMed] [Google Scholar]
- Liang B. T. Characterization of the adenosine receptor in cultured embryonic chick atrial myocytes: coupling to modulation of contractility and adenylate cyclase activity and identification by direct radioligand binding. J Pharmacol Exp Ther. 1989 Jun;249(3):775–784. [PubMed] [Google Scholar]
- Lipp P., Pott L., Callewaert G., Carmeliet E. Calcium transients caused by calcium entry are influenced by the sarcoplasmic reticulum in guinea-pig atrial myocytes. J Physiol. 1992 Aug;454:321–338. doi: 10.1113/jphysiol.1992.sp019266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechmann S., Pott L. Identification of Na-Ca exchange current in single cardiac myocytes. Nature. 1986 Feb 13;319(6054):597–599. doi: 10.1038/319597a0. [DOI] [PubMed] [Google Scholar]
- Olah M. E., Stiles G. L. Adenosine receptors. Annu Rev Physiol. 1992;54:211–225. doi: 10.1146/annurev.ph.54.030192.001235. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Noma A., Trautwein W. Acetylcholine activation of single muscarinic K+ channels in isolated pacemaker cells of the mammalian heart. Nature. 1983 May 19;303(5914):250–253. doi: 10.1038/303250a0. [DOI] [PubMed] [Google Scholar]
- Tawfik-Schlieper H., Klotz K. N., Kreye V. A., Schwabe U. Characterization of the K(+)-channel-coupled adenosine receptor in guinea pig atria. Naunyn Schmiedebergs Arch Pharmacol. 1989 Dec;340(6):684–688. doi: 10.1007/BF00717745. [DOI] [PubMed] [Google Scholar]
- Wilken A., Tawfik-Schlieper H., Klotz K. N., Schwabe U. Pharmacological characterization of the adenylate cyclase-coupled adenosine receptor in isolated guinea pig atrial myocytes. Mol Pharmacol. 1990 Jun;37(6):916–920. [PubMed] [Google Scholar]
- Wu S. N., Linden J., Visentin S., Boykin M., Belardinelli L. Enhanced sensitivity of heart cells to adenosine and up-regulation of receptor number after treatment of guinea pigs with theophylline. Circ Res. 1989 Oct;65(4):1066–1077. doi: 10.1161/01.res.65.4.1066. [DOI] [PubMed] [Google Scholar]
- Zang W. J., Yu X. J., Honjo H., Kirby M. S., Boyett M. R. On the role of G protein activation and phosphorylation in desensitization to acetylcholine in guinea-pig atrial cells. J Physiol. 1993 May;464:649–679. doi: 10.1113/jphysiol.1993.sp019656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- diMarco J. P., Sellers T. D., Lerman B. B., Greenberg M. L., Berne R. M., Belardinelli L. Diagnostic and therapeutic use of adenosine in patients with supraventricular tachyarrhythmias. J Am Coll Cardiol. 1985 Aug;6(2):417–425. doi: 10.1016/s0735-1097(85)80181-9. [DOI] [PubMed] [Google Scholar]


