Abstract
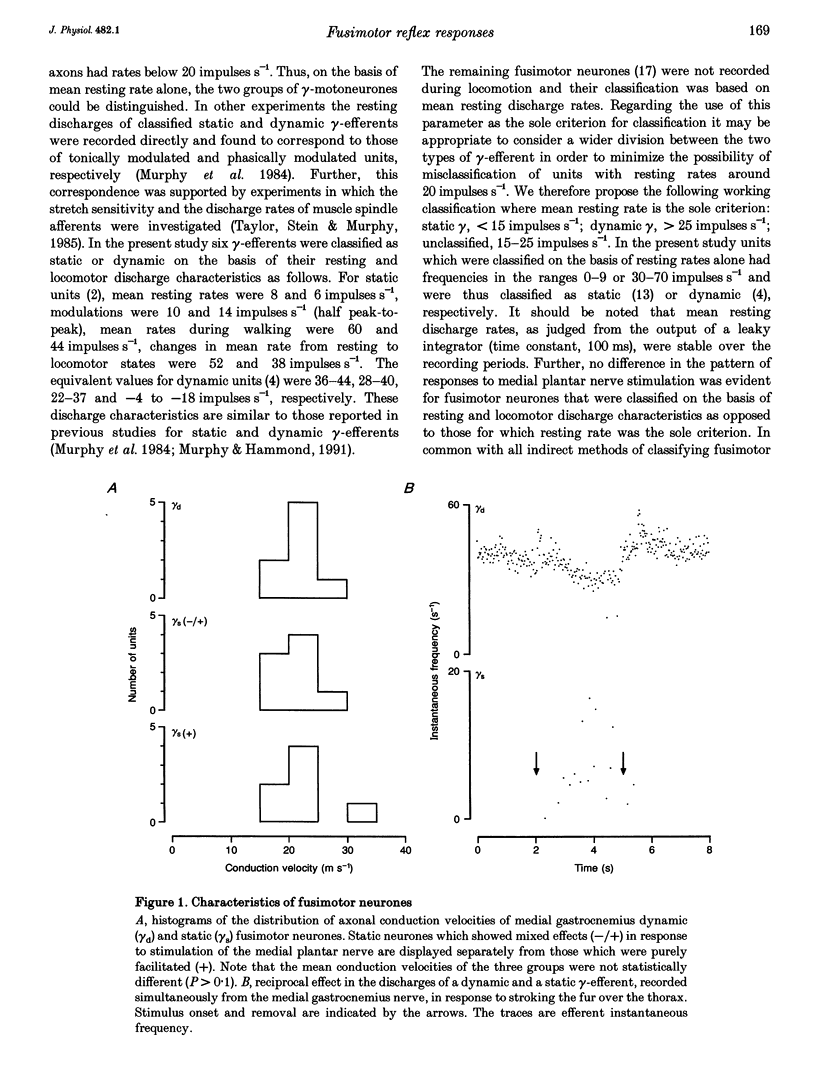
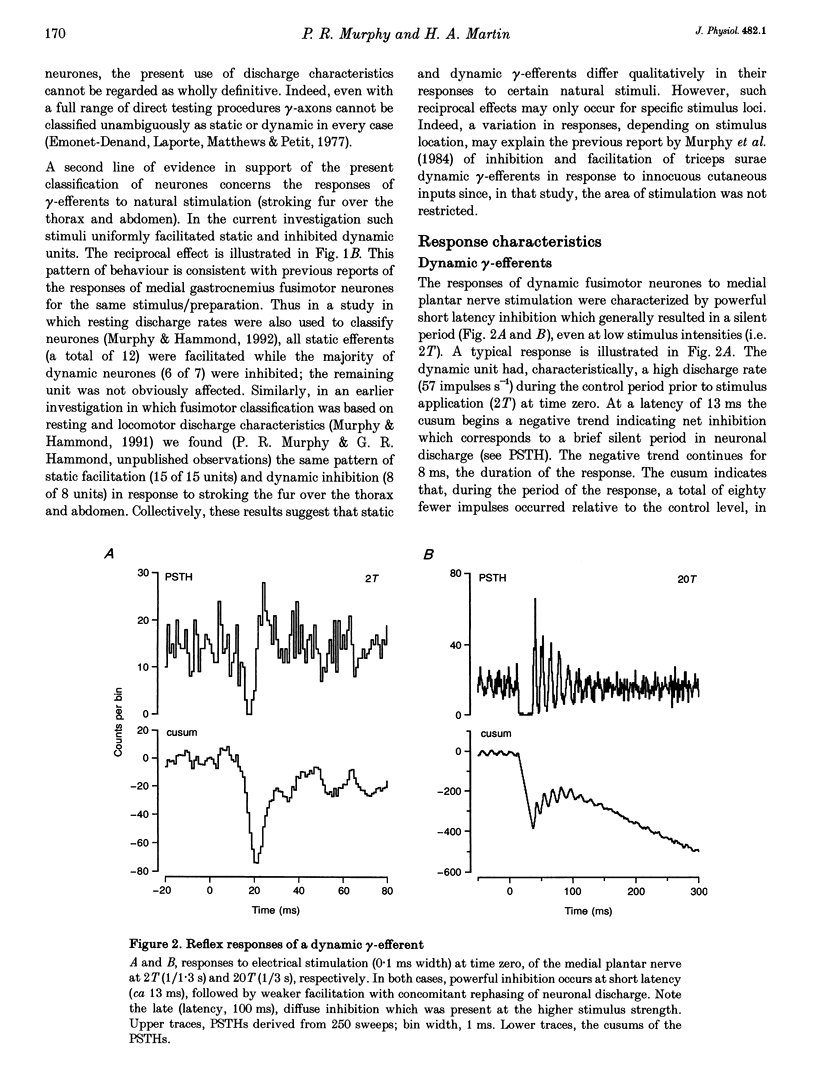
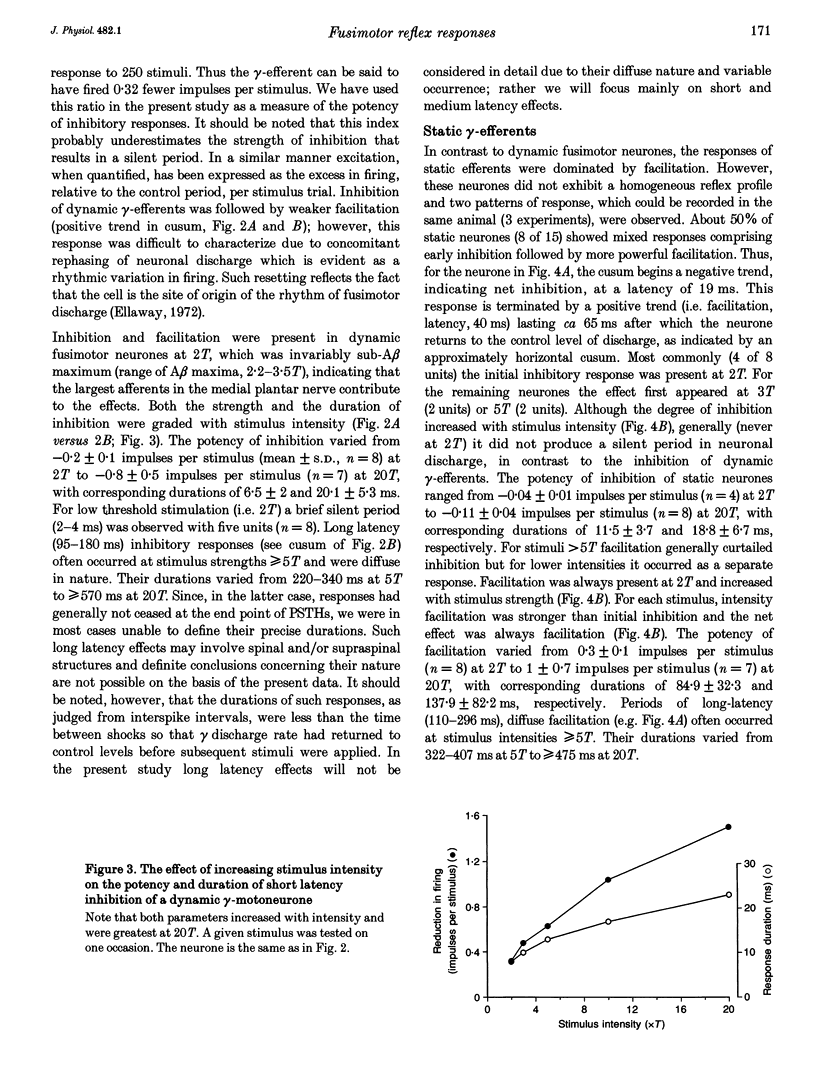
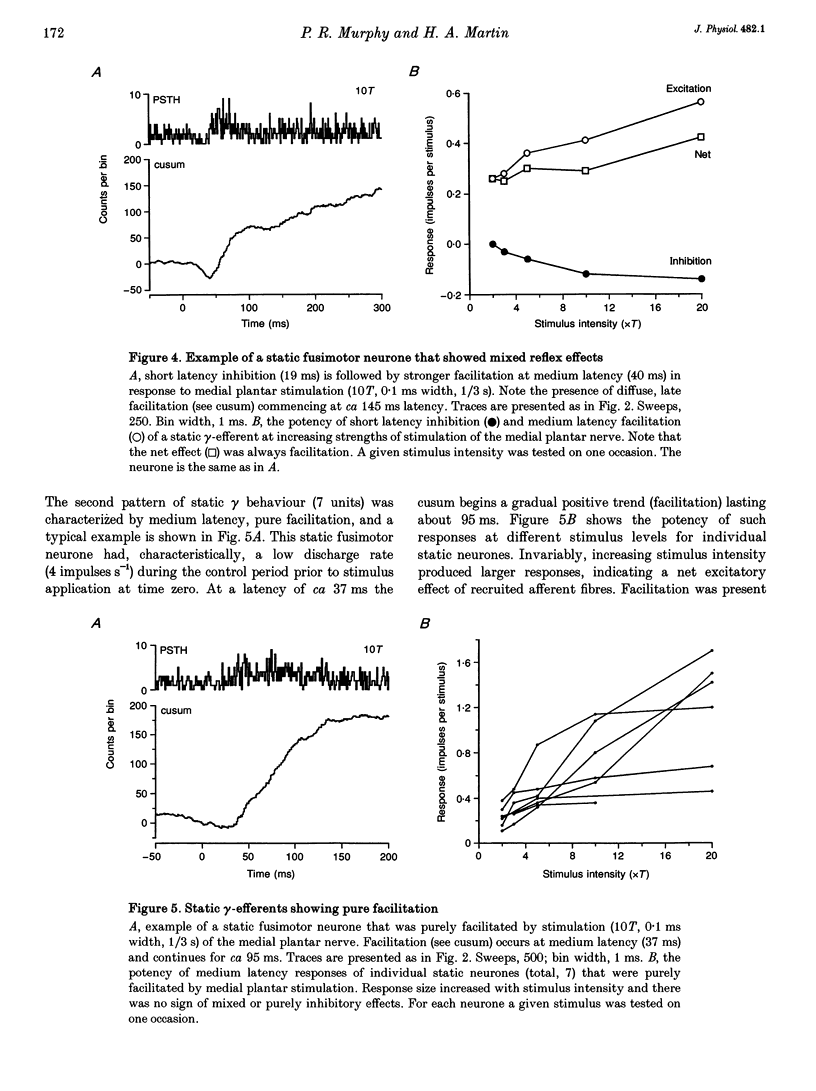
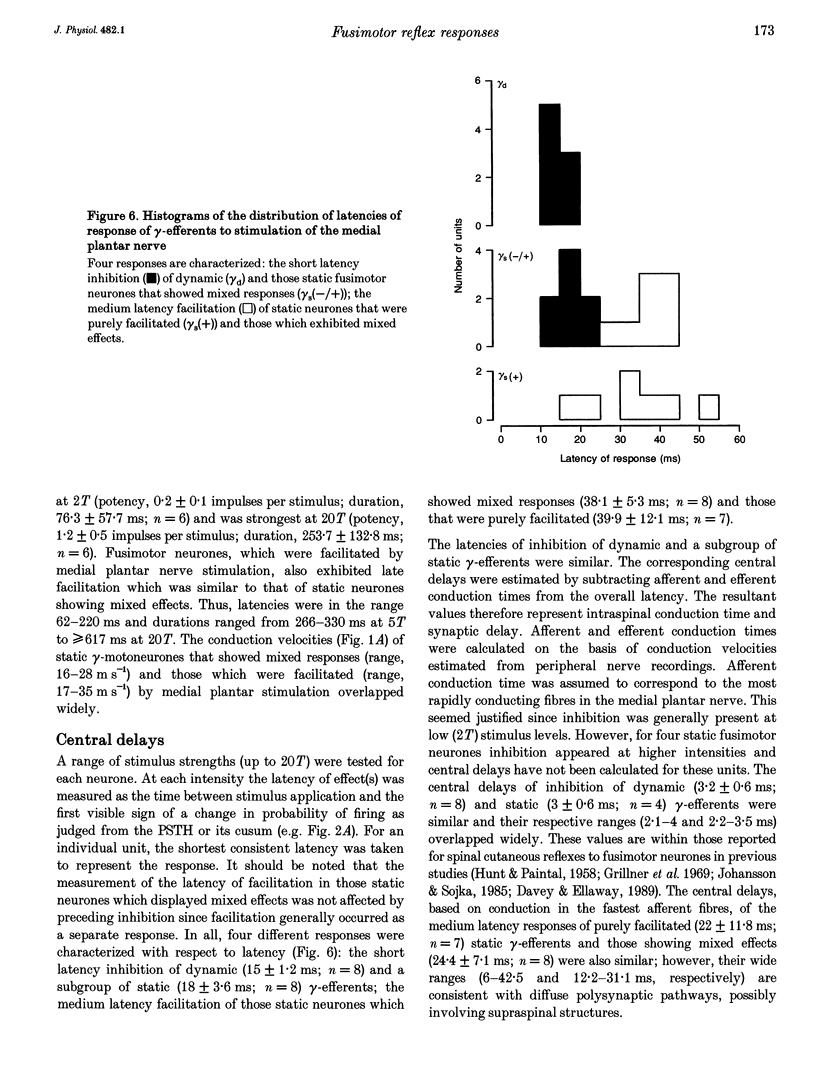
1. The effect of single shock electrical stimulation, up to 20 x threshold (T), of the medial plantar nerve on the discharges of single medial gastrocnemius static and dynamic gamma-efferents has been investigated in the decerebrate cat. 2. The neurones were classified as static (15) or dynamic (8) indirectly on the basis of their locomotor and/or resting discharge characteristics. 3. All gamma-efferents were affected by stimulation of the medial plantar nerve. Dynamic units showed net inhibition while facilitation dominated the responses of static neurones. 4. The responses of dynamic units consisted of powerful short latency (15 +/- 1.2 ms, mean +/- S.D.) spinal inhibition followed by weaker facilitation that was difficult to characterize due to concomitant rephasing of neuronal discharge. 5. Static neurones showed two patterns of response. Some units (7 of 15) were facilitated at medium latency (39.9 +/- 12.2 ms) while the remainder showed mixed effects in which short latency (18 +/- 3.6 ms) spinal inhibition was followed by stronger facilitation (latency, 38.1 +/- 5.3 ms). 6. Fusimotor facilitation and inhibition were generally present at 2T. The inhibition of dynamic and static gamma-efferents, and the facilitation of the latter type, increased with stimulus intensity. Thus low and high threshold afferents contributed to the effects without changing their qualitative nature. 7. We conclude that low threshold cutaneous mechanoreceptors in the plantar surface of the foot are capable of influencing the discharges of medial gastrocnemius static and dynamic gamma-efferents. Further, the cutaneous responses of fusimotor neurones appear to vary according to both the source of the afferent input and the type of unit involved. 8. The results are discussed in relation to the control and function of fusimotor neurones and the possible existence of subdivisions within the static system.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aniss A. M., Diener H. C., Hore J., Burke D., Gandevia S. C. Reflex activation of muscle spindles in human pretibial muscles during standing. J Neurophysiol. 1990 Aug;64(2):671–679. doi: 10.1152/jn.1990.64.2.671. [DOI] [PubMed] [Google Scholar]
- Banks R. W. The distribution of static gamma-axons in the tenuissimus muscle of the cat. J Physiol. 1991 Oct;442:489–512. doi: 10.1113/jphysiol.1991.sp018805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Reciprocal control of spontaneous activity and reflex effects in static and dynamic flexor gamma-motoneurones revealed by an injection of DOPA. Acta Physiol Scand. 1969 Sep-Oct;77(1):106–124. doi: 10.1111/j.1748-1716.1969.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Bessou P., Joffroy M., Pagès B. Efferents and afferents in an intact muscle nerve: background activity and effects of sural nerve stimulation in the cat. J Physiol. 1981 Nov;320:81–102. doi: 10.1113/jphysiol.1981.sp013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd I. A. Two types of static gamma-axon in cat muscle spindles. Q J Exp Physiol. 1986 Apr;71(2):307–327. doi: 10.1113/expphysiol.1986.sp002987. [DOI] [PubMed] [Google Scholar]
- Campbell G. D., Edwards F. R., Hirst G. D., O'Shea J. E. Effects of vagal stimulation and applied acetylcholine on pacemaker potentials in the guinea-pig heart. J Physiol. 1989 Aug;415:57–68. doi: 10.1113/jphysiol.1989.sp017711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catley D. M., Pascoe J. E. The reflex effects of sural nerve stimulation upon gastrocnemius fusimotor neurones of the rabbit [proceedings]. J Physiol. 1978 Mar;276:32P–32P. [PubMed] [Google Scholar]
- Davey N. J., Ellaway P. H. Facilitation of individual gamma-motoneurones by the discharge of single slowly adapting type 1 mechanoreceptors in cats. J Physiol. 1989 Apr;411:97–114. doi: 10.1113/jphysiol.1989.sp017563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., HAGBARTH K. E. Facilitation and inhibition of gamma efferents by stimulation of certain skin areas. J Neurophysiol. 1954 Jan;17(1):59–65. doi: 10.1152/jn.1954.17.1.59. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. The variability in discharge of fusimotor neurones in the decerebrate cat. Exp Brain Res. 1972;14(2):105–117. doi: 10.1007/BF00234794. [DOI] [PubMed] [Google Scholar]
- Emonet-Dénand F., Laporte Y., Matthews P. B., Petit J. On the subdivision of static and dynamic fusimotor actions on the primary ending of the cat muscle spindle. J Physiol. 1977 Jul;268(3):827–861. doi: 10.1113/jphysiol.1977.sp011884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden M. H., McWilliam P. N. The activity of intrafusal muscle fibres during cortical stimulation in the cat [proceedings]. J Physiol. 1977 Dec;273(2):28P–29P. [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffer J. A., Caputi A. A., Pose I. E., Griffiths R. I. Roles of muscle activity and load on the relationship between muscle spindle length and whole muscle length in the freely walking cat. Prog Brain Res. 1989;80:75–60. doi: 10.1016/s0079-6123(08)62201-3. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of cutaneous afferent fibres in the hind limb of the cat. J Physiol. 1985 Sep;366:343–363. doi: 10.1113/jphysiol.1985.sp015802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Schmidt R. F., Zimmermann M. Single unit responses and the total afferent outflow from the cat's foot pad upon mechanical stimulation. Exp Brain Res. 1968;6(2):100–115. doi: 10.1007/BF00239165. [DOI] [PubMed] [Google Scholar]
- Jänig W. The afferent innervation of the central pad of the cat's hind foot. Brain Res. 1971 May 7;28(2):203–216. doi: 10.1016/0006-8993(71)90655-x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Hoffer J. A., Marks W. B. Activity of spindle afferents from cat anterior thigh muscles. III. Effects of external stimuli. J Neurophysiol. 1985 Sep;54(3):578–591. doi: 10.1152/jn.1985.54.3.578. [DOI] [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Hammond G. R. Short latency cutaneous reflex responses of gamma-efferents in the decerebrate cat. Exp Brain Res. 1992;89(1):140–146. doi: 10.1007/BF00229011. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Hammond G. R. The role of cutaneous afferents in the control of gamma-motoneurones during locomotion in the decerebrate cat. J Physiol. 1991 Mar;434:529–547. doi: 10.1113/jphysiol.1991.sp018484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. R., Stein R. B., Taylor J. Phasic and tonic modulation of impulse rates in gamma-motoneurons during locomotion in premammillary cats. J Neurophysiol. 1984 Aug;52(2):228–243. doi: 10.1152/jn.1984.52.2.228. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J. F., Durbaba R., Taylor A., Fowle A. J. The use of sinusoidal and ramp stretch stimuli in characterizing fusimotor effects on cat muscle spindles. Exp Physiol. 1994 May;79(3):337–355. doi: 10.1113/expphysiol.1994.sp003769. [DOI] [PubMed] [Google Scholar]
- Taylor J., Stein R. B., Murphy P. R. Impulse rates and sensitivity to stretch of soleus muscle spindle afferent fibers during locomotion in premammillary cats. J Neurophysiol. 1985 Feb;53(2):341–360. doi: 10.1152/jn.1985.53.2.341. [DOI] [PubMed] [Google Scholar]
- VOORHOEVE P. E., van KANTEN R. Reflex behaviour of fusimotor neurones of the cat upon electrical stimulation of various afferent fibers. Acta Physiol Pharmacol Neerl. 1962;10:391–407. [PubMed] [Google Scholar]
- Wand P., Schwarz M. Two types of cat static fusimotor neurones under separate central control? Neurosci Lett. 1985 Jul 4;58(1):145–149. doi: 10.1016/0304-3940(85)90344-1. [DOI] [PubMed] [Google Scholar]


