Abstract
Background
Postoperative rehabilitation exercise is commonly prescribed after total hip arthroplasty (THA), but its efficacy compared to no or minimal rehabilitation exercise has been questioned. Preliminary efficacy would be indicated if a dose-response relationship exists between performed exercise dose and degree of postoperative recovery. The objective was to evaluate the preliminary efficacy of home-based rehabilitation using elastic band exercise on performance-based function after THA, based on the association between performed exercise dose and change in performance-based function (gait speed) from 3 (start of intervention) to 10 weeks (end of intervention) after surgery.
Methods
A prospective cohort study was conducted. Following primary THA, patients were prescribed home-based rehabilitation exercise using elastic bands. Performed exercise dose (repetitions/week) was objectively measured using attached sensor technology. Primary outcome was change in gait speed (40 m fast-paced walk test). Secondary outcomes included patient-reported hip disability. In the primary analysis, a linear regression model was used.
Results
Ninety-four patients (39 women) with a median age of 66.5 years performed a median of 339 exercise repetitions/week (1st-3rd quartile: 209–549). Across outcomes, participants significantly improved from 3 to 10-week follow-up. The association between performed exercise dose and change in mean gait speed was 0.01 m/s [95% CI: -0.01; 0.02] per 100 repetitions.
Conclusions
We found no indication of preliminary efficacy of home-based rehabilitation exercise using elastic bands, as no significant and clinically relevant associations between performed exercise dose and changes in outcomes were present. Trials comparing postoperative rehabilitation exercise with no exercise early after THA are warranted.
Trial registration
Pre-registered: ClinicalTrials.gov (Identifier: NCT03109821, 12/04/2017).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-024-08057-x.
Keywords: Hip arthroplasty, Exercise therapy, Rehabilitation, Compliance, Dose-response
Background
Total hip arthroplasty (THA) is commonly performed in patients with severe hip osteoarthritis (OA) to reduce pain and improve function [1], and projections show a significant increase in procedures [2, 3]. This challenges health care budgets [3] and calls for optimised clinical pathways. Functional performance and muscle strength are substantially reduced after THA [4, 5], and postoperative rehabilitation exercise has been recommended [6, 7]. However, an evidence-based rehabilitation exercise protocol has not been established [8], and the organization and content of postoperative rehabilitation varies greatly [9–12]. Using home-based rehabilitation exercise as usual clinical practice is in concordance with clinical guidelines [7, 13] and the findings from a recent systematic review with meta-analysis [14]. The systematic review reported, that out-patient rehabilitation exercise with close supervision (minimum two supervised sessions per week) is not superior to home-based rehabilitation exercise with no or very little supervision (a maximum of two supervised sessions after hospital discharge) for both patient-reported and performance-based function, pain and health-related quality of life [14].
Although postoperative rehabilitation exercise in some form is recommended (as opposed to no rehabilitation exercise), the evidence for its effectiveness is inconclusive. Some systematic reviews conclude, that rehabilitation exercise may be superior to no or very little rehabilitation exercise after THA, measured on gait speed and hip abduction muscle-strength [15, 16], as well as pain and self-reported function (Harris Hip Score) [16]. Opposed to that, a recent systematic review concluded, that rehabilitation exercise compared to usual care, or no or minimal intervention was not associated with improved patient-reported function or hip muscle strength [17]. A relevant question, which was not addressed in these systematic reviews, is how much exercise patients performed and if the exercise dose is related to the postoperative outcome. Evidence regarding this is sparse, and previous studies have reported conflicting results [18, 19].
The presence of a dose–response gradient is recognized as a criterion for believing in a causal effect [20]. Therefore, preliminary efficacy would be indicated if a dose-response relationship exists between the amount of performed exercise and degree of postoperative recovery. Recovery will primarily be evaluated based on function and pain, since these outcomes reflect the main reasons for performing THA surgery [1] as well as for prescribing exercise therapy [21]. To investigate a dose-response relationship between post-operative home-based rehabilitation exercise and recovery after THA, objective measures of exercise compliance are needed [22]. We have previously validated an in-built sensor attached to an elastic exercise band to monitor compliance to home-based exercise in healthy subjects [23–25], and started using it for intervention research in clinical populations [26–29]. By using this sensor technology, it is possible to objectively quantify performed exercise dose, which improves the validity in studies assessing dose-response relationships and evaluating preliminary efficacy of interventions.
Objectives
The primary objective was to evaluate the preliminary efficacy of home-based rehabilitation using elastic band exercise on performance-based function after THA, based on the relationship between the performed exercise dose and the change in performance-based function (gait speed measured by 40-m fast-paced walk test) from 3 (start of intervention) to 10 weeks (end of intervention) after surgery [30]. The secondary objective was to investigate if a dose-response relationship exists between the performed exercise dose and changes in: hip-related disability, lower-extremity functional performance, and hip muscle strength [30].
Methods
Study design and ethics
A pragmatic, single-center, prospective cohort study – “The Pragmatic Home-Based Exercise after Total Hip Arthroplasty – Silkeborg study (PHETHAS-1)” – was conducted. We included patients who were prescribed home-based rehabilitation exercise after THA (usual care) and used sensor technology to objectively measure performed exercise dose (exposure) during a 7-week intervention period from 3 weeks (start of home-based strengthening exercise, baseline) to 10 weeks (follow-up) after THA. Performance-based and patient-reported outcomes were measured at baseline and follow-up.
This is the primary study report for PHETHAS-1, which adheres to the STROBE (Strengthening the reporting of observational studies in epidemiology) statement [31, 32]. It uses the checklist for cohort studies [32, 33] as well as applicable items from the CONSORT (Consolidated Standards of Reporting Trials) statement [34, 35] and the REPORT trial guide [36]. Written informed consent was obtained from all participants. The study was reported to The Central Denmark Region Committee on Health Research Ethics and was reviewed as non-notifiable (Inquiry 270/2017). The study was approved by the Danish Data Protection Agency (ref. no: 1-16-02-589-15) and preregistered with ClinicalTrials.gov (Identifier: NCT03109821 12/04/2017) [37]. The full study protocol was published open access, 14 Oct 2019 [30].
Setting
The present study was conducted from 21 April 2017 to 8 January 2020 at a public Danish hospital (Elective Surgery Centre, Silkeborg Regional Hospital). At the hospital, THA is performed using a posterolateral surgical approach and the following clinical rehabilitation practice is used; during admission, patients are instructed to perform unloaded exercises (not part of the intervention studied) at home until their scheduled follow-up visit at the hospital three weeks after surgery. Here, they are instructed in a home-based rehabilitation exercise program including strengthening exercises, that should be performed at home without further supervision. Referral to supervised outpatient rehabilitation is initiated in approximately 30% of the patients, based on individual needs. There are no clear-cut criteria for referral to the supervised pathway, but the patient’s preference, rehabilitation goal, functional ability in daily activities, reduced cognitive function and comorbidities are factors influencing the decision.
Intervention
The intervention was the home-based rehabilitation exercise program used in clinical practice at the Elective Surgery Centre, thus, a pragmatic approach was used. All patients received identical instruction in the exercise program, which was initiated immediately after the outcome assessment at baseline (3 weeks after surgery). The physiotherapists who did the exercise instruction all had at least 6 months of experience working with THA. In a previously published protocol paper [30], we outlined the intervention in great detail using the exercise-specific Consensus on Exercise Reporting Template (CERT) [38] as well as the Template for Intervention Description and Replication (TIDieR) [39] – both supplemented with the full set of strength training descriptors as suggested by Toigo and Boutellier [40] (replicated in Appendix A, Table A2). We refer to the published protocol for details [30], but a summarised description is presented below.
Strengthening exercises included in the program were: standing hip abduction, flexion and extension with elastic band resistance and sit-to-stands. The elastic band exercises were performed in a standing position with the elastic band placed as a loop around the ankles, and the participants were instructed to perform the exercises with both the operated and non-operated leg. In each repetition, the prescribed time-under-tension was 5 seconds (2s concentric, 1s isometric and 2 s excentric). The prescribed training dosage was two sets (one set in week 1) with repetitions to contraction failure in each set and a relative load of 10 to 20 repetition maximum (RM), which should be performed every second day (3–4 times a week). Participants were instructed to change the elastic band color and obtain a higher load, if they were able to perform more than 20 repetitions in two of three elastic band exercises. Supplemental exercises were daily stretching of hip flexor muscles (stretch 2 × 30 seconds or lying 5–10 min in prone position) and balance exercise (one-legged stance – gradually progressing to 1 min). From week two, the prescribed dosage of elastic band exercises was a mean of 630 repetitions per week (range 420 to 840 repetitions per week), but based on previous research [41] and a pilot study conducted prior to this trial (unpublished), a larger variation in actually performed number of repetitions was expected.
The participants were also advised to gradually increase their physical activity level after surgery to comply with the Danish Health and Medicines Authority’s recommendations on physical activity. Furthermore, participants were given instructions on how to handle pain during exercises (reduction of load) [42] and recreational activities. The pain management guide is available online as extended data for the published protocol [30, 42].
Participants
The inclusion criteria were: age above 18 years, scheduled for primary THA due to OA and ability to understand written and spoken Danish. The exclusion criterion was: referral to supervised rehabilitation in the municipality (instead of the usual care, home-based rehabilitation exercise used in the present study).
A limited number of sensors used to measure exercise dose and physical activity made restricted inclusion necessary and only 2–3 participants could be recruited per week. To mitigate the risk of selection bias, participants were consecutively sampled from random pre-specified assessment programs in the outpatient department. Patients were allocated at random to these assessment programs by a secretary without any influence from personnel involved in the study.
Data collection
Demographics and supplementary descriptive participant variables were collected at baseline (3 weeks after surgery) by the physiotherapist conducting the outcome assessments. Variables collected were: Age, gender, height, weight, ASA (The American Society of Anesthesiologists physical status classification system) classification, prosthesis type, prior total joint replacement and length of hospital stay.
Exposure
Performed exercise dose was quantified as the total physiological exercise stimulus (number of repetitions per week) recorded by a sensor (www.Bandcizer.io) attached to the elastic exercise band [23, 24]. The sensor automatically switches on, records, and stores exercise data when the elastic exercise band that it is attached to is used. Previously, it has been found valid in measuring date, time of day, number of repetitions, single repetition time-under-tension (TUT), and total TUT during home-based strength training exercises for the lower extremity [24]. Performed exercise dose was also quantified as the number of days per week with strengthening exercises being performed, both based on sensor data and patient-reported data from exercise diaries (see description in Appendix A, Table A3, details on ‘mean change in pain after each exercise session’).
To minimize sensor-induced influence on exercise compliance and to reduce expectation bias, the participants were informed that the sensor was used to measure how they exercised. They were not told that the focus was on how much exercise was performed nor were they told what the study hypothesis was.
Outcomes
Performance-based outcome assessments were conducted at baseline and follow-up (10 weeks after surgery) by three physiotherapists who had been thoroughly trained in performing the assessments and who were blinded to exercise compliance data. Patient-reported outcome measures were collected pre-surgery, at baseline and at follow-up (see participant timeline in Appendix A, Table A1, replicated from the published protocol [30]).
The primary outcome was the change in gait speed from 3 to 10 weeks after surgery measured by the 40-m fast-paced walk test [43, 44]. This test was chosen, since it measures performance-based function and is recommended by Osteoarthritis Research Society International (OARSI) as part of the core set of tests to assess physical function in people with hip or knee OA [43, 44]. Furthermore, patients undergoing THA surgery have reported walking ability to be the most important function to improve [45].
Secondary outcomes were absolute gait speed at 10 week, change in patient-reported function, pain, symptoms and hip-related quality of life measured by Hip disability and Osteoarthritis Outcome Score (HOOS) [46], change in maximal isometric hip flexion and hip abduction strength and change in performance-based lower extremity function measured by the 30-s chair stand test [43, 44]. A detailed description of all study outcomes and measurement tools with supplementary details were presented in the published protocol [30] and are also available in Appendix A, Table A3.
Data collection was continued for participants who stopped exercising but was discontinued if participants explicitly withdrew from the study or if major events or diseases prevented the outcome assessments.
Data management
Demograhics, supplementary descriptive variables and outcome measurements were entered in EpiData 3.1. Anonymous coding with ID numbers and range checks for data values were used to minimize typing errors. Instead of double entering data as planned, 20% of the participants’s data were validated by two research assistants. Very few and minor errors were found, and further validation was not considered relevant.
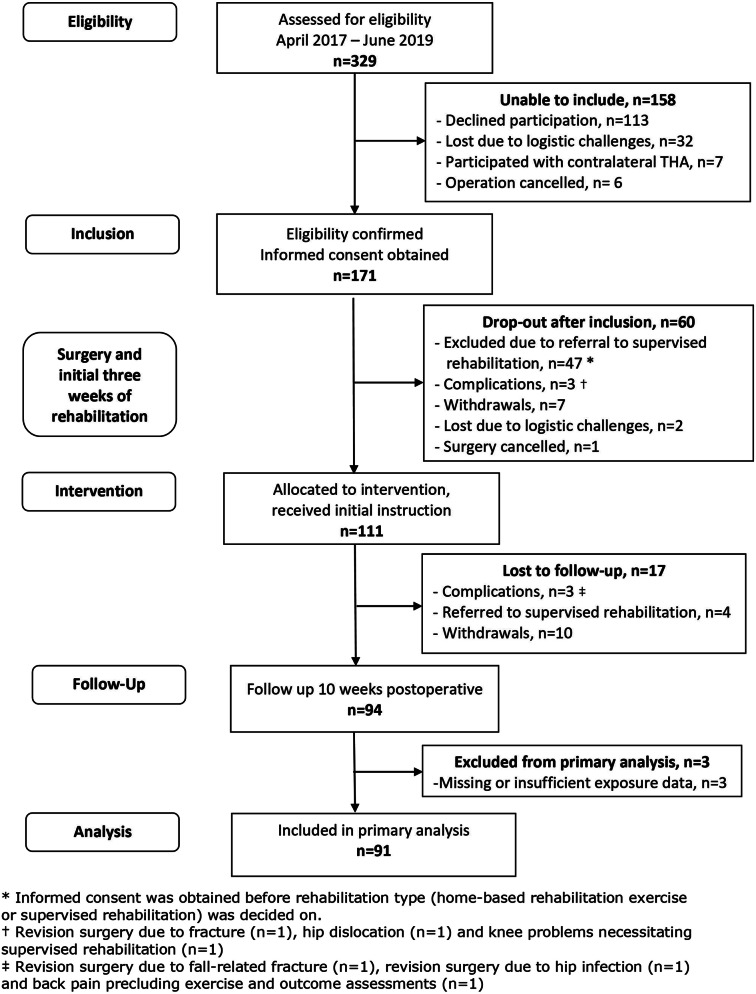
Raw Bandcizer data was uploaded to a secure online database. Here, data were accessed, and graphical illustrations of exercise sessions and repetitions were visually inspected. Hereafter performed exercise dose was determined. Due to invalid time-under-tension (TUT) data, and in accordance with the pre-defined contingency plan [30], the exposure variable was changed from TUT to number of repetitions. Reasons are described in detail in the section ‘Deviations from the trial registration and protocol’. The quantification of exercise dose was still challenged, as substantial differences between automatically software-generated and manually counted number of repetitions were found. To ensure data validity, we therefore manually counted every single repetition for all exercise sets. Also, in case heterogeneity of illustrated repetitions made counting challenging an interpretation level was assigned. Details on this process are available in Appendix A. In one case, data quality was too poor to calculate or count repetitions, and in further two cases, due to sensor failure, no data were obtained. Hence, the latter three cases were not included in the primary analysis (Fig. 1).
Fig. 1.
Participant flow
Sample size
The sample size calculation was outlined in the published protocol, and the procedure is elaborated below. It was based on a linear regression model with exercise dose as a continuous independent parameter and gait speed as the dependent parameter. A minimal clinically important difference (MCID) in the slope for change in gait speed as a function of exercise dose was needed. This MCID slope was based on a previous MCID value for gait speed of 0.2 m/s [47] and the difference between highest and lowest exercise dose, which was estimated to 4 hours (difference in total TUT during the intervention period), based on results from a pilot study conducted prior to this trial (unpublished). Using these values, a MCID slope of 0.05 m/s per hour of exercise dose (0.2 m/s/4 hours) was used. Together with a standard deviation (SD) for exercise dose of 1.06, a SD for gait speed of 0.16, a power of 90% and a level of significance of 5%, a sample size of 88 participants was required. SDs for exercise dose and gait speed were obtained from the previously mentioned pilot study. The sample size calculation was done using the Stata command: sampsi_reg [48].
Statistical methods
A full statistical analysis plan was published as part of the published protocol and formed the basis for the analyses [30]. All deviations – with reasons – are provided below. The main analyses are summarized below, while description of exploratory analyses and handling of quantitative continuous and categorical data (e.g. grouping and transformation) are available in Appendix A.
Descriptive statistics
Descriptive statistics were performed for demographics, supplementary descriptive variables, pre-surgery HOOS, pre-surgery self-efficacy, exposure, all outcomes (at 3-week, at 10 week and change values) and other pre-specified variables. Categorical variables were presented as frequencies with percentages and continuous variables as means with standard deviation (SD) or medians with 1st and 3rd quartile, depending on data distribution being parametric or not.
Primary analysis
The analysis of a dose-response relationship between performed exercise dose and change in gait speed from 3 to 10 weeks after surgery was investigated according to the analysis plan described in the published protocol [30]. Based on scatterplots the starting model was a linear regression model with a fixed increase in outcome. R-squared value was low, thus more complex regression models were tested, but without resulting in a model fitting data better. Correlation between change in gait speed and gait speed at baseline was evaluated by scatterplot, and no regression to the mean was indicated. Furthermore, inclusion of the predefined possible confounding variables (self-efficacy at baseline, physical activity during intervention (mean upright time/day and mean number of steps/day), and gait speed at baseline) were evaluated in the model by comparing the dose-response estimates with and without the confounding variables. The normality assumption of the model was evaluated by a quantile-quantile plot and histogram. Estimate of change in gait speed is presented as mean with 95% confidence intervals (CI).
Sensitivity analyses of the primary analysis outlined above were performed to test robustness of the estimate. First, outliers in change in gait speed were excluded before estimating the relationship between performed exercise dose and change in gait speed. Secondly, participants, where a high level of interpretation had been used in the count of repetitions, were excluded from the analysis. Thirdly, participants not having used the sensor technology every time or most of the time during exercising were excluded from the analysis. Finally, mean number of exercise days per week (both based on sensor data and data from the exercise diary) was used as performed exercise dose variable in the analysis.
Secondary analyses
Models similar to the ones used in the primary analysis were used to analyze the dose-response relationship between performed exercise dose and change in patient-reported function measured by the subscale Activity of Daily Living in HOOS (HOOS-adl). The scatterplot of change in HOOS-adl score against performed number of repetitions showed a widespread distribution of data, but linear association was considered the best model for testing association. The scatterplot of change in HOOS-adl against HOOS-adl baseline score indicated regression to the mean, hence, the baseline score was included in the regression model.
Linear regression models were also used to analyze association between gait speed at 10 weeks and performed exercise dose, self-efficacy at baseline, physical activity (mean upright time/day and mean number of steps/day) and gait speed at baseline.
Change in gait speed, HOOS subscales, 30-s chair stand test and maximal isometric hip muscle strength were estimated within each quartile of performed exercise, presented as mean with CI and graphically as boxplots.
Handling of missing data
As recommended in guidelines, <50% missing items in each subscale of HOOS was accepted [46] and ≤3 missing items in the General Self-efficacy scale was accepted [49]. For the physical activity data, ≥4 days of data collection with the ActivPAL movement sensor was considered sufficient to calculate mean upright time/day and steps/day [50]. In some cases, participants had to stop the performance-based outcome assessments tests due to pain. In these situations, data from the best performance were used no matter if the pre-defined number of test repetitions was met.
In general, we did not use imputation procedures on exposure, but in one case an exception was made. In this case, failure of the BandCizer occurred, leaving the particpant without a sensor for a week before being provided with a new sensor. The participant had perfectly congruence between objectively measured exercise days and self-reported exercise days in the diary, hence, last-observation-carried forward and next-observation carried backwards were imputed to the missing exercise days. As no confounding variables were included in the primary analysis model, no data imputation of possible confounders was performed. Participants lost to follow-up were excluded from the analyses.
Deviations from the trial registration and published protocol
All predefined analyses have been performed. The exploratory analyses were not pre-defined and registered with ClinicalTrials.gov. However, they were defined in the protocol paper [30], which was published before the end of recruitment and before running any data analyses. Changes to outcomes between registration and protocol publication are reported in the protocol paper [30], hence, only deviations from the published protocol are described in detail below.
First, exposure is presented as number of repetitions per week instead of TUT/week. This change was made according to our pre-defined contingency plan for outcomes [30] and based on the following thorough data assessment. The visual inspection showed a great deal of heterogeneity both within and between exercise sessions and individuals. In general, repetitions seemed to be of shorter duration than recommended and performed with relatively small range of motion. Based on this, we decided to test a sample of exercise sessions, to investigate whether the software’s automatically generated number of repetitions and TUT could be validated against manually calculated TUT and visually-counted number of repetitions. Based on this test, we realized, that TUT was too imprecise to be considered a valid measure in this study.
Secondly, due to data distribution and data quality, we performed sensitivity analyses for the primary analysis. The sensitivity analyses are described in detail in the section ‘Statistical methods, primary analysis’.
Thirdly, adverse events were grouped as serious adverse events (SAE) and non-serious adverse events. This was decided to provide the reader with the most transparent and clinically relevant overview of data, since several different non-serious events were registered in the category “other”. Classification of SAE was done according to definitions by the U.S Food & Drug Administration [51], International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) [52] and Ioannidis et al. [53].
Finally, presentation of summary statistics on body mass index (BMI), pre-surgery HOOS scores, physical activity level and patient-perceived result of surgery were added. Also, supplementary description on pain and exercise data were provided to allow the reader a more detailed insight in data management and exercise compliance.
Results
Participants and exposure
Informed consent was obtained from 171 patients, of which 60 were excluded before baseline assessment at intervention start (3 week postoperative). The main reason for exclusion was referral to supervised rehabilitation (n=47). A total of 94 participants completed the study (see Fig. 1). Demographics and participant characteristics are presented in Table 1 and Appendix B, Table B1.
Table 1.
Summary statistics on demographic variables and supplementary descriptive variables*
| Demographic variables | Age (years) | |
| median (1st; 3rd quartile) | 66.5 (62; 72) | |
| Gender | ||
| number (percentage) | ||
| - Male | 55 (59) | |
| - Female | 39 (41) | |
| Height (m), n=93 | ||
| mean (SD) | 1.75 (0.09) | |
| Weight (kg), n=93 | ||
| median (1st; 3rd quartile) | 81 (72; 95) | |
| BMI (kg/m2), n=93 | ||
| median (1st; 3rd quartile) | 26.6 (24.3; 29.4) | |
| ASA classification, n=92 | ||
| number (percentage) | ||
| - ASA 1 | 26 (28) | |
| - ASA 2 | 54 (59) | |
| - ASA 3 | 12 (13) | |
| Supplementary descriptive variables | Length of hospital stay (days), n=93 | |
| number (percentage) | ||
| −0† | 23 (25) | |
| −1 | 65 (70) | |
| −2 | 5 (5) | |
| Self-efficacy (mean per answered question) | ||
| median (1st; 3rd quartile) | ||
| - Pre-surgery, n=89 | 3.3 (2.8; 3.7) | |
| - Baseline (3 weeks), n=88 | 3.5 (3; 3.8) | |
| HOOS – pre-surgery | ||
| mean (SD) | ||
| - ADL | 49.3 (15.5) | |
| - Pain | 46.5 (14.5) | |
| - Symptoms | 41.3 (16.0) | |
| - QOL | 29.7 (13.2) | |
| Physical activity level, n=81 | ||
| mean (SD) | ||
| - Upright time per day (hours) | 5.5 (1.5) | |
| - Steps per day (numbers) | 6619 (2700) |
* N=94 unless otherwise stated; † Discharge on the day of surgery
Abbreviations: SD: Standard deviation; BMI: Body mass index; ASA: The American Society of Anesthesiologists physical status classification system; HOOS: Hip disability and Osteoarthritis Outcome Score; ADL: Activities of daily living; QOL: Quality of life
The participants performed a median of 2.7 exercise sessions (1st and 3rd quartile: (2.0; 3.2)) and a median of 339 repetitions per week (1st and 3rd quartile: (209; 549)). Hence, compared to the prescribed exercise dose more than 50% of the participants performed less than the lower limit of recommended number of repetitions per week. Further details on exercise dose are available in Appendix B.
Primary analysis
Ninety-one participants were included in the analysis. Gait speed improved from a median of 1.45 m/s (1st and 3rd quartile: (0.21; 1.69)) at baseline to 1.74 (1st and 3rd quartile: (1.50; 2.04)) at follow-up, median change: 0.31 (1st and 3rd quartile: (0.21; 0.42), p<0.001). Crude analysis showed a non-significant increase in mean change of gait speed on 0.01 m/s [CI: -0.01; 0.02, p=0.22] per 100 extra repetitions performed per week. Inclusion of the pre-defined possible confounders changed the estimate to values between 0.005 m/s and 0.012 m/s per 100 extra repetitions performed per week. These changes did not change interpretation of the estimate, hence, none of the confounders were included in the analysis model.
Sensitivity analyses for the primary analysis
Omitting the six outliers in speed change changed the estimate to 0.005 m/s ([CI: -0.005; 0.014], p=0.34) per 100 extra performed repetitions per week, while excluding the three participants where a high degree of interpretation for the estimation of exercise dose was used, changed the estimate to 0.01 ([CI: -0.004; 0.023], p=0.16). Thus, sensitivity analyses changed the estimates, but not to a degree that led to a different interpretation of the results. When using self-reported exercise dose (number of exercise days per week registered in diaries) as exposure, the analysis showed a non-significant mean change in gait speed of 0.03 m/s [CI: -0.01; 0.07], p=0.20) per extra exercise day per week.
Summary statistics and secondary analyses
Summary statistics for HOOS, 30-s chair stand, and isometric hip muscle strength are presented in Table 2.
Table 2.
Summary statistics for HOOS, 30-s chair stand and isometric hip muscle strength
| Outcomes | Baseline | Follow-up | Change |
|---|---|---|---|
| HOOS | |||
| median (1st; 3rd quartile) | |||
| - ADL | 75 (63; 85)* | 91 (85; 95)* | 13 (6; 21)† |
| - Symptoms | 70 (60; 80)* | 85 (75; 90) | 10 (5; 20)* |
| - Pain | 75 (65; 88)* | 93 (85; 98) | 12 (3; 23)* |
| - QOL | 56 (44; 63)* | 75 (63; 94)* | 19 (6.5; 31)† |
| 30-s chair stand test | |||
| median (1st;3rd quartile) | 12 (10; 15) | 16 (13; 20) | 4 (2; 7) |
| Isometric hip muscle strength (Nm/kg) | |||
| mean (95% CI) | |||
| - Abduction | 0.76 | 1.02 | 0.25 |
| (0.71; 0.82)† | (0.96; 1.08)† | (0.21; 0.29)‡ | |
| - Flexion | 0.88 | 1.1 | 0.22 |
| (0.82; 0.94)* | (1.03; 1.16)† | (0.18; 0.26)† |
The table presents values at baseline and follow-up as well as change (baseline to follow-up, 3–10 weeks after surgery) in Hip disability and Osteoarthritis Outcome Score (HOOS), 30-s chair stand and isometric hip muscle strength. N=94 unless otherwise stated. * n=93; † n=92; ‡ n=91
A non-significant increase in the HOOS-adl score change of 0.58 ([CI: -0.11;1.26], p=0.10) per 100 extra repetitions performed per week was found. Adjusting for possible confounders resulted in estimates between 0.29 and 0.64 per 100 extra repetitions performed per week.
Excluding the most extreme outlier increased change in HOOS-adl to a statistically significant association of 0.75 ([CI: 0.14; 1.36], p=0.02) per 100 extra repetitions performed per week. When excluding all three outliers, the estimated association was 0.67 ([CI: 0.12;1.21], p=0.02).
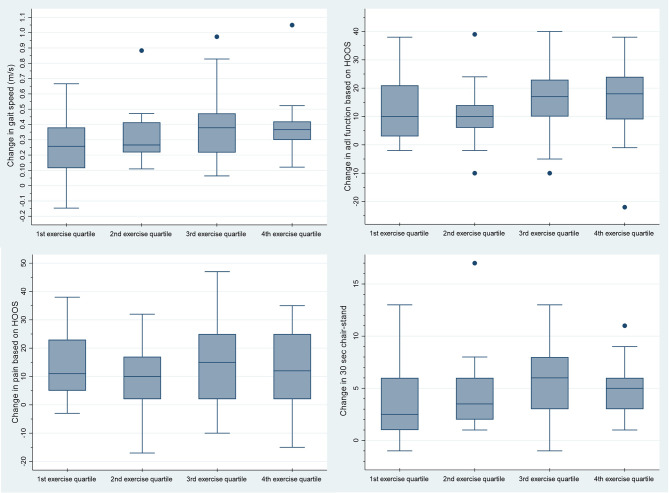
Change in gait speed, HOOS subscales, 30s chair-stand and maximal isometric hip muscle strength (flexion and abduction) distributed on quartiles of performed exercise dose are presented graphically in Fig. 2 and Appendix C, Figures C1-C4. For all outcomes, the exact estimates of change distributed on quartile of performed exercise dose are available in Appendix C, Table C1.
Fig. 2.
Change in four outcomes distributed on quartile groups of exercise dosages. The figure shows box plots of the median change (baseline to follow-up, 3–10 weeks after surgery) in four outcomes distributed on quartile groups of exercise dosages (number of performed repetitions per week). The four outcomes are: change in gait speed (m/s) measured by 40 m fast-paced walk test, change in function measured by Hip disability and Osteoarthritis Outcome Score (HOOS) (subscale adl), change in pain measured by HOOS and change in lower-extremity function measured by 30s-chair-stand. The dots represent outlying results
In the multiple regression analysis of associations between gait speed at 10 weeks follow up and self-efficacy at baseline, 24-hour physical activity, performed exercise dose and gait speed at baseline, small but statistically significant associations were found for the variables: mean upright time/day, mean number of steps/day and gait speed at baseline. Specific results are presented in Appendix C, Table C2.
Pain and adverse events
Based on each participant’s mean change in pain per exercise session (pain after exercise minus before exercise), the population median change in pain was an increase of 1.5 mm on the visual analogue scale (VAS) (1st and 3rd quartile: (0.2; 3.7)). During intervention period, a total of 57 pain flares (pain change≥20 mm VAS) in 21 participants occurred following an exercise session. Further details on exercise-related pain are available in Appendix B, Table B5. Among the 94 participants completing the study, two were readmitted due to bleeding or wound seepage (to avoid risk of indirect identification, no further details are reported). In further five participants wound seepage and/or wound infection occurred. Summary statistics on serious and non-serious adverse events are described in detail in Appendix B, Table B6.
Motivation for and evaluation of exercise, and patient-perceived result of surgery
At baseline, all but one participant were either very motivated or to some degree motivated to perform home-based rehabilitation exercise and 99% of all participants were very or almost certain, that they would comply to the prescribed exercise program. Further summary statistics on items regarding motivation for home-based rehabilitation exercise measured at baseline are available in Appendix B, Table B3.
At follow-up, when evaluating the prescribed exercises, 94% of the participants were satisfied or very satisfied, and 76% reported that because of participating in the study, they had exercised more than they would have done otherwise. Further summary statistics on items evaluating prescribed exercise are available in Appendix B, Table B4.
The patient-perceived result of surgery at the 10week follow up was rated excellent by 76% of the participants, while 4% reported a fair or poor result. 88% of the participants perceived their hip problem as much better than before surgery, while one participant reported the hip problem to be a little worse. Further details on patient-perceived results are available in Appendix B, Table B4 and Figure B1. The remaining exploratory analyses outlined in Appendix A are presented in Appendix C (Tables C3-7 and Figures C5-7).
Discussion
Main findings
The participants showed both clinically and statistically significant improvements in maximum gait speed from baseline to follow-up, but no significant linear dose-response association was found between change in gait speed and performed number of repetitions per week. Clinically, the estimated increase in mean change of gait speed on 0.01 m/s [CI: -0.01; 0.02] corresponds to a needed increase of 2000 repetitions per week (1000 reps/week if using the upper limit of the CI) to achieve a minimal clinically important difference of 0.2 m/s [47]. Hence, the observed associations were not statistically significant, nor were they clinically relevant. Also, no significant linear association was found between the change in HOOS-adl score and the number of performed repetitions per week. However, a sensitivity analysis on HOOS-adl indicates, that a significant but not clinically meaningful association might be present (0.75 [CI=0.14; 1.36] per 100 extra repetitions per week). We found no indications of a dose-response relationship when evaluating changes in gait speed, HOOS subscales, 30s chair-stand and isometric maximal hip muscle strength across quartiles of performed exercise. Based on these key findings, preliminary efficacy of home-based rehabilitation exercise was not indicated. No confirmatory conclusions on exercise efficacy can be drawn due to the cohort design without a non-exercise comparator.
Comparison with previous findings
Two other studies have evaluated associations between exercise dose and clinical improvements in THA populations. Zech et al. [19] reported that clinical improvements were not associated with the intensity and duration of postoperative exercise therapy in the early phase after THA [19], which is in concordance with our findings. Jan et al. found contrasting results in their randomized trial comparing participants performing a home exercise program to a control group receiving no exercise [18]. Participants in the intervention group who exercised more than 50% of the days in the intervention period, achieved greater improvements in muscle strength, gait speed and function, compared to the control group as well as the participants in the intervention group who exercised less than 50% of the days in the intervention period [18]. The results indicate a dose-response relationship, but the study was conducted more than 1.5 years after surgery [18] where spontaneous recovery after surgery likely had no confounding effect. This late timing is in contrast with our focus on early rehabilitation which reflects current clinical practice.
Explanation of results
The reduced compliance to the prescribed exercises did not seem to affect the overall recovery when compared to reference values [54–56] and to outcomes in previous Danish studies reported at similar time-points [41, 57]. We have previously shown that patients with THA perceive exercises as a means to achieve their goals, and that they modify the exercise recommendations according to their needs and individual goals [58]. This could be part of the reason why the performed exercise-dose varied substantially among participants. More than 50% of the participants did not perform the number of prescribed repetitions per week, and still more than 75% of the population had an increase in gait speed above the reported clinically meaningful improvement of 0.2 m/s [47]. Additionally, at 10 weeks, the patients achieved a median maximum gait speed of 1.74 m/s, which is comparable to reference values previously reported in US populations [54, 55]. 91% of the population reported an excellent or very good patient-perceived result of surgery, 88% rated their hip problem to be much better, and the 10-week scores on HOOS subscales varied between 75 and 93 (100 being the best possible). For the HOOS subscales adl, pain and symptoms the achieved results were at the same level as the Danish reference population [56].
This may indicate that a dose-response relationship for rehabilitation exercise and post-operative recovery in the early phase after THA does not exist. We speculate that spontaneous recovery determines the recovery trajectory for the most part.
The literature does not provide clear answers as to whether a dose-response relationship between exercise and postoperative outcome should be expected in the THA population. A meta-analysis by Borde et al. investigated resistance training in populations of healthy old adults [59] and reported a dose-response relationship between both TUT (per repetition) and training intensity (load) and the effect size for muscle strength [59]. In addition, a meta-analysis by Ralston et al. reported an association between weekly set volume and strength gain [60]. Based on these studies in healthy subjects [59, 60], a dose-response relationship could have been expected, although exercise responses may differ between healthy adults and adults with severe osteoarthritis recovering from surgery. The American College of Sports Medicine does state that individuals respond differently to resistance training based on training status, past experience and joint health [61] and that a variety of exercise intensities may be effective in the elderly population especially when they start exercising [61]. Much like the effect size of spontaneous recovery may blur or exclude an exercise dose-response relationship after THA, the effect size of starting resistance exercise (going from nothing to something) may also blur or exclude an exercise dose-response relationship in previously untrained adults.
Strengths, limitations and generalizability
A main strength of our study is the use of objectively measured, performed exercise dose. Even though, data quality forced us to use the performed number of repetitions instead of TUT, we still consider the use of performed number of repetitions based on sensor technology to be much more valid than patient-reported data, which can be challenged by non-timely reporting [62] and inaccuracy [63, 64]. We are aware, that it could be a limitation, that some interpretation was used in the counting of repetitions, and that 15% of the participants did not use the sensor technology every time or most of the time during exercising. However, interpretation was only used in a minority of cases, and the sensitivity analyses on both issues did not change the interpretation of the primary result. Hence, we do not consider these issues concerning.
A limitation of the study is that about one third of the patients assessed for eligibility declined to participate, inducing a potential risk of selection bias. Possibly, resourceful patients with generally good health are more likely to accept study participation than patients with less resources. Furthermore, 30% of the participants were excluded due to referral to supervised rehabilitation in the municipality. As described in the introduction, there is no clear-cut criteria for this referral, but the excluded participants may be less resourceful, than the group of participants receiving usual care (home-based rehabilitation exercise). Thus, the study results may not be generalized to the less resourceful group of patients receiving a THA.
Conclusions
We found no indication of preliminary efficacy of home-based rehabilitation exercise using elastic bands, as no significant and clinically relevant associations between performed exercise dose and changes in the primary outcome gait speed or HOOS-adl were present. Although no exercise-dose outcome-response relationships were observed, the whole cohort of participants improved significantly from baseline to follow-up across outcomes. Confirmatory efficacy of home-based rehabilitation exercise after THA needs to be assessed in a future randomized controlled trial using a non-exercise comparator.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Appendix A - Supplements to the methods section.
Supplementary Material 2: Appendix B - Supplementary descriptive results.
Supplementary Material 3: Appendix C - Supplementary secondary and exploratory results.
Acknowledgements
The authors would like to thank physiotherapist, Rikke Dahl Nyholm and research assistant Trine Astrup Bech Vestergaard for their invaluable dedication, expertise and help throughout the research process. We would also like to thank the staff and chairs at The Elective Surgery Centre, Silkeborg Regional Hospital for supporting the project, with a special thanks to the involved research assistants, secretaries, nurses and physiotherapists, who’s input and engagement made this study possible. In addition, we would like to extend our sincere gratitude to all the patients who generously participated and gave their time and effort during this study. Lastly, we would like to thank the funding parties, for providing the necessary financial support.
Abbreviations
- ADL
Activities of daily living
- ASA
The American Society of Anesthesiologists physical status classification system
- BMI
Body mass index
- CERT
Specific Consensus on Exercise Reporting Template
- CI
95% confidence intervals
- CONSORT
Consolidated Standards of Reporting Trials
- HOOS
Hip disability and Osteoarthritis Outcome Score
- HOOS-adl
The Subscale Activity of Daily Living in The Hip disability and Osteoarthritis Outcome Score
- ICH
International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use
- MCID
Minimal clinically important difference
- OA
Osteoarthritis
- PHETHAS-1
The Pragmatic Home-Based Exercise after Total Hip Arthroplasty – Silkeborg study
- QOL
Quality of life
- SAE
Serious adverse events
- SD
Standard deviation
- STROBE
Strengthening the reporting of observational studies in epidemiology
- THA
Total hip arthroplasty
- TIDieR
Template for Intervention Description and Replication
- TUT
Time-under-tension
- VAS
Visual analogue scale
Author contributions
Conception of the study: LRM, MSR, KT and TB; Design and methodology: All authors; Project administration: MNM and LRM; Data acquisition: MNM; Analysis and interpretation of the data: All authors; Statistical expertise: TK; Drafting of the article: MNM; Critical revision of the article for important intellectual content: All authors. All authors read and approved the final manuscript. Guarantors: Mrs. Madsen (merete.madsen@clin.au.dk) and Prof. Bandholm (thomas.quaade.bandholm@regionh.dk) takes responsibility for the integrity of the work as a whole.
Funding
The PHETHAS-1 study has received grants from: Regional Hospital Central Jutland Research Foundation, The Danish Rheumatism Association, The Association of Danish Physiotherapists, The Aase and Ejnar Danielsen Foundation and The family Kjærsgaard foundation. Furthermore, the PHETHAS-1 study has become part of a PhD project, which has received funding from: Regional Hospital Central Jutland Research Foundation, The Danish Rheumatism Association, The Association of Danish Physiotherapists, The Faculty of Health, Aarhus University, The Department of Clinical Medicine, Aarhus University and The Frimodt-Heineke Foundation. The funding sources had no part in the design, conduction or reporting of the trial, thus there is no conflict of interests.
Data availability
The dataset generated and analysed to provide Figure 1, Table 1 and Appendix B, Table B1 (demographics and participant characteristics) cannot be fully anonymized. Hence, public deposition of these data points is not possible due to Denmarks national legislation (Data Protection Act § 10 and Data Disclosure Proclamation Act) which outline that we can only transfer pseudonymized data to the Journal after the Data Protection Authorities approval (Data Protection Act § 10, section 3, nr. 3.). Reviewers and others may obtain access to the data (used in the primary and secondary analyses) by reasonable request to Mrs. Madsen (merete.madsen@clin.au.dk), on condition that the Danish Data Protection Agency has approved of the data transfer from the Central Jutland Region to the Journal. If others are to gain access to the data, the Journal shall ensure that there is an adequate legal basis to share the Central Jutland Regions data and ensure that the data is only being processed for scientific research purposes.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all participants. The study was reported to The Central Denmark Region Committee on Health Research Ethics and was reviewed as non-notifiable (Inquiry 270/2017). The study was approved by the Danish Data Protection Agency (ref. no: 1-16-02-589-15). The Danish Data Protection Agency is a separate institution, which only supervises compliance with the rules on protection of personal data English(http://www.datatilsynet.dk).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gossec L, Paternotte S, Maillefert JF, Combescure C, Conaghan PG, Davis AM, et al. The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: an international cross-sectional study of 1909 patients. Report of the OARSI-OMERACT Task Force on total joint replacement. Osteoarthr Cartil. 2011;19(2):147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am. 2018;100(17):1455–60. [DOI] [PubMed] [Google Scholar]
- 3.Pabinger C, Lothaller H, Portner N, Geissler A. Projections of hip arthroplasty in OECD countries up to 2050. Hip Int. 2018;28(5):498–506. [DOI] [PubMed] [Google Scholar]
- 4.Holm B, Thorborg K, Husted H, Kehlet H, Bandholm T. Surgery-induced changes and early recovery of hip-muscle strength, leg-press power, and functional performance after fast-track total hip arthroplasty: a prospective cohort study. PLoS ONE. 2013;8(4):e62109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd DL, Dennis DA, Thomas AC, Wolfe P, Dayton MR, Stevens-Lapsley JE. Muscle strength and functional recovery during the first year after THA. Clin Orthop Relat Res. 2014;472(2):654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westby MD, Brittain A, Backman CL. Expert consensus on best practices for post-acute rehabilitation after total hip and knee arthroplasty: a Canada and United States Delphi study. Arthritis Care Res (Hoboken). 2014;66(3):411–23. [DOI] [PubMed] [Google Scholar]
- 7.NICE. Joint replacement (primary): hip, knee and shoulder. [London]: National Institute for Health and Care Excellence; 2020 [updated 4 June 2020. https://www.nice.org.uk/guidance/ng157/resources/joint-replacement-primary-hip-knee-and-shoulder-pdf-66141845322181 [PubMed]
- 8.Di Monaco M, Castiglioni C. Which type of exercise therapy is effective after hip arthroplasty? A systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 2013;49(6):893–907. quiz 21-3. [PubMed] [Google Scholar]
- 9.Smith TO, Dainty JR, Clark EM, Whitehouse MR, Price AJ, MacGregor AJ. Demographic and geographical variability in physiotherapy provision following hip and knee replacement. An analysis from the National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Physiotherapy. 2020;106:1–11. [DOI] [PubMed] [Google Scholar]
- 10.Jones CA, Martin RS, Westby MD, Beaupre LA. Total joint arthroplasty: practice variation of physiotherapy across the continuum of care in Alberta. BMC Health Serv Res. 2016;16(1):627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eulenburg C, Rahlf AL, Kutasow A, Zech A. Agreements and disagreements in exercise therapy prescriptions after hip replacement among rehabilitation professionals: a multicenter survey. BMC Musculoskelet Disord. 2015;16:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feilberg S. Udviklingen i antallet af genoptræningsplaner: benchmark af genoptræningsplaner på nationalt, regionalt og kommunalt niveau fra 2007–2014: KORA Danmarks Evalueringsinstitut; 2016. http://www.kora.dk/media/4936315/10024_udviklingen-i-antallet-af-genoptraeningsplaner.pdf
- 13.Sundhedsstyrelsen. National Klinisk retningslinje for hofteartrose: ikke-kirurgisk behandling og genoptræning efter total hoftealloplastik. 2 ed. Sundhedsstyrelsen [Danish Health Authority]; 2021.
- 14.Hansen S, Aaboe J, Mechlenburg I, Overgaard S, Mikkelsen LR. Effects of supervised exercise compared to non-supervised exercise early after total hip replacement on patient-reported function, pain, health-related quality of life and performance-based function - a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2019;33(1):13–23. [DOI] [PubMed] [Google Scholar]
- 15.Coulter CL, Scarvell JM, Neeman TM, Smith PN. Physiotherapist-directed rehabilitation exercises in the outpatient or home setting improve strength, gait speed and cadence after elective total hip replacement: a systematic review. J Physiotherapy. 2013;59(4):219–26. [DOI] [PubMed] [Google Scholar]
- 16.Wu JQ, Mao LB, Wu J. Efficacy of exercise for improving functional outcomes for patients undergoing total hip arthroplasty: a meta-analysis. Medicine. 2019;98(10):e14591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saueressig T, Owen PJ, Zebisch J, Herbst M, Belavy DL. Evaluation of Exercise interventions and outcomes after hip arthroplasty: a systematic review and Meta-analysis. JAMA Netw Open. 2021;4(2):e210254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jan MH, Hung JY, Lin JC, Wang SF, Liu TK, Tang PF. Effects of a home program on strength, walking speed, and function after total hip replacement. Arch Phys Med Rehabil. 2004;85(12):1943–51. [DOI] [PubMed] [Google Scholar]
- 19.Zech A, Hendrich S, Pfeifer K. Association between Exercise Therapy dose and functional improvements in the early postoperative phase after hip and knee arthroplasty: an observational study. PM R: J Injury Function Rehabilitation. 2015;7(10):1064–72. [DOI] [PubMed] [Google Scholar]
- 20.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58(5):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MeSH term. Exercise Therapy: The National Center for Biotechnology Information; [ https://www.ncbi.nlm.nih.gov/mesh/?term=exercise+therapy
- 22.Bollen JC, Dean SG, Siegert RJ, Howe TE, Goodwin VA. A systematic review of measures of self-reported adherence to unsupervised home-based rehabilitation exercise programmes, and their psychometric properties. BMJ open. 2014;4(6):e005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathleff MS, Bandholm T, Ahrendt P, Olesen JL, Thorborg K. Novel stretch-sensor technology allows quantification of adherence and quality of home-exercises: a validation study. Br J Sports Med. 2014;48(8):724–8. [DOI] [PubMed] [Google Scholar]
- 24.Rathleff MS, Thorborg K, Rode LA, McGirr KA, Sorensen AS, Bogild A, et al. Adherence to commonly prescribed, home-based strength training exercises for the lower extremity can be objectively monitored using the bandcizer. J Strength Conditioning Res. 2015;29(3):627–36. [DOI] [PubMed] [Google Scholar]
- 25.Skovdal Rathleff M, Thorborg K, Bandholm T. Concentric and eccentric Time-Under-Tension during strengthening exercises: Validity and Reliability of Stretch-Sensor recordings from an Elastic Exercise-Band. PLoS ONE. 2013;8(6):e68172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clausen MB, Bandholm T, Rathleff MS, Christensen KB, Zebis MK, Graven-Nielsen T et al. The Strengthening Exercises in Shoulder Impingement trial (The SExSI-trial) investigating the effectiveness of a simple add-on shoulder strengthening exercise programme in patients with long-lasting subacromial impingement syndrome: Study protocol for a pragmatic, assessor blinded, parallel-group, randomised, controlled trial. Trials. 2018;19(1):154-018-2509-7. [DOI] [PMC free article] [PubMed]
- 27.Husted RS, Troelsen A, Thorborg K, Rathleff MS, Husted H, Bandholm T. Efficacy of pre-operative quadriceps strength training on knee-extensor strength before and shortly following total knee arthroplasty: protocol for a randomized, dose-response trial (the QUADX-1 trial). Trials. 2018;19(1):47–017. [DOI] [PMC free article] [PubMed]
- 28.Rathleff MS, Bandholm T, McGirr KA, Harring SI, Sorensen AS, Thorborg K. New exercise-integrated technology can monitor the dosage and quality of exercise performed against an elastic resistance band by adolescents with patellofemoral pain: an observational study. J Physiotherapy. 2016;62(3):159–63. [DOI] [PubMed] [Google Scholar]
- 29.Riel H, Matthews M, Vicenzino B, Bandholm T, Thorborg K, Rathleff MS. Feedback leads to Better Exercise Quality in adolescents with Patellofemoral Pain. Med Sci Sports Exerc. 2018;50(1):28–35. [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen LR, Madsen MN, Rathleff MS, Thorborg K, Rossen CB, Kallemose T, et al. Pragmatic home-based Exercise after total hip arthroplasty - silkeborg: protocol for a prospective cohort study (PHETHAS-1). F1000Res. 2019;8:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of Observational studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4(10):e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9. [DOI] [PubMed] [Google Scholar]
- 33.STROBE. STROBE Checklists [ https://www.strobe-statement.org/checklists/
- 34.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 35.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bandholm T, Thorborg K, Ardern CL, Christensen R, Henriksen M. Writing up your clinical trial report for a scientific journal: the REPORT trial guide for effective and transparent research reporting without spin. Br J Sports Med. 2022. [DOI] [PMC free article] [PubMed]
- 37.ClinicalTrials.gov. Home-based Rehabilitation Following a Total Hip Replacement ClinicalTrials.gov. [cited 2023 June 20]. https://clinicaltrials.gov/show/NCT03109821
- 38.Slade SC, Dionne CE, Underwood M, Buchbinder R. Consensus on Exercise Reporting Template (CERT): explanation and Elaboration Statement. Br J Sports Med. 2016;50(23):1428–37. [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (Clinical Res ed). 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
- 40.Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97(6):643–63. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen LR, Mechlenburg I, Soballe K, Jorgensen LB, Mikkelsen S, Bandholm T, et al. Effect of early supervised progressive resistance training compared to unsupervised home-based exercise after fast-track total hip replacement applied to patients with preoperative functional limitations. A single-blinded randomised controlled trial. Osteoarthr Cartil. 2014;22(12):2051–8. [DOI] [PubMed] [Google Scholar]
- 42.Mikkelsen LR. PHETHAS-1 protocol. figshare. 2019. 10.6084/m9.figshare.8256014.v1
- 43.Dobson F, Bennell KL, Hinman RS, Abbott JH, Roos EM. Recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. 2013. [DOI] [PubMed]
- 44.Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthr Cartil. 2013;21(8):1042–52. [DOI] [PubMed] [Google Scholar]
- 45.Heiberg KE, Ekeland A, Mengshoel AM. Functional improvements desired by patients before and in the first year after total hip arthroplasty. BMC musculoskeletal disorders. 2013;14:243-2474-14-243. [DOI] [PMC free article] [PubMed]
- 46.Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4(10). [DOI] [PMC free article] [PubMed]
- 47.Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41(5):319–27. [DOI] [PubMed] [Google Scholar]
- 48.Mander A, Sampsi_reg. [ http://fmwww.bc.edu/RePEc/bocode/s/sampsi_reg.html
- 49.Schwarzer R. Everything you wanted to know about the General Self-Efficacy Scale but were afraid to ask 2014 [ http://userpage.fu-berlin.de/~health/faq_gse.pdf
- 50.Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nystrom C, Mora-Gonzalez J, Lof M, et al. Accelerometer Data Collection and Processing Criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med (Auckland NZ). 2017;47(9):1821–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.FDA. What is a Serious Adverse Event? U.S Food & Drug Administration; 2016 [ https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event
- 52.ICH. Clinical safety data management: Definitions and standards for expedited reporting E2A. ICH Harmonised tripartite guideline: International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use 1994 [Step 4 version dated 27 October 1994:[ https://database.ich.org/sites/default/files/E2A_Guideline.pdf
- 53.Ioannidis JP, Evans SJ, Gøtzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–8. [DOI] [PubMed] [Google Scholar]
- 54.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997;26(1):15–9. [DOI] [PubMed] [Google Scholar]
- 55.Bohannon RW, Wang YC. Four-meter gait speed: normative values and reliability determined for adults participating in the NIH Toolbox Study. Arch Phys Med Rehabil. 2019;100(3):509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Larsen P, Rathleff MS, Roos EM, Elsoe R. National population-based reference data for the hip disability and osteoarthritis outcome score (HOOS). Arch Orthop Trauma Surg. 2023;143(11):6865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mark-Christensen T, Kehlet H. Assessment of functional recovery after total hip and knee arthroplasty: an observational study of 95 patients. Musculoskelet Care. 2019;17(4):300–12. [DOI] [PubMed] [Google Scholar]
- 58.Poulsen AG, Gravesen JD, Madsen MN, Mikkelsen LR, Bandholm T, Rossen CB. Patient perspectives on home-based rehabilitation exercise and general physical activity after total hip arthroplasty: a qualitative study (PHETHAS-2). F1000Res. 2021;10:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borde R, Hortobágyi T, Granacher U. Dose-response relationships of Resistance Training in Healthy Old adults: a systematic review and Meta-analysis. Sports Med. 2015;45(12):1693–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ralston GW, Kilgore L, Wyatt FB, Baker JS. The Effect of Weekly Set volume on Strength Gain: a Meta-analysis. Sports Med. 2017;47(12):2585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.ACSM. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. [DOI] [PubMed] [Google Scholar]
- 62.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24(2):182–99. [DOI] [PubMed] [Google Scholar]
- 63.Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bassett SF. The assessment of patient adherence to physiotherapy rehabilitation. Phys Ther. 2003;31(6).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Appendix A - Supplements to the methods section.
Supplementary Material 2: Appendix B - Supplementary descriptive results.
Supplementary Material 3: Appendix C - Supplementary secondary and exploratory results.
Data Availability Statement
The dataset generated and analysed to provide Figure 1, Table 1 and Appendix B, Table B1 (demographics and participant characteristics) cannot be fully anonymized. Hence, public deposition of these data points is not possible due to Denmarks national legislation (Data Protection Act § 10 and Data Disclosure Proclamation Act) which outline that we can only transfer pseudonymized data to the Journal after the Data Protection Authorities approval (Data Protection Act § 10, section 3, nr. 3.). Reviewers and others may obtain access to the data (used in the primary and secondary analyses) by reasonable request to Mrs. Madsen (merete.madsen@clin.au.dk), on condition that the Danish Data Protection Agency has approved of the data transfer from the Central Jutland Region to the Journal. If others are to gain access to the data, the Journal shall ensure that there is an adequate legal basis to share the Central Jutland Regions data and ensure that the data is only being processed for scientific research purposes.