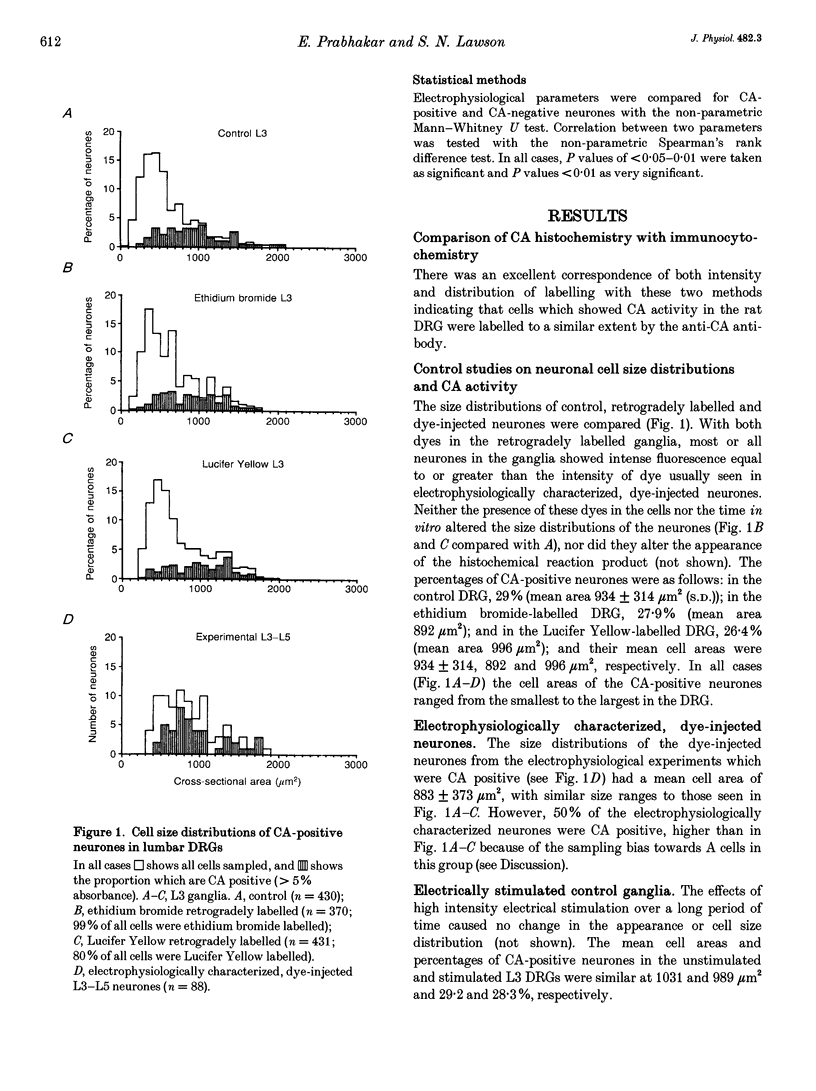
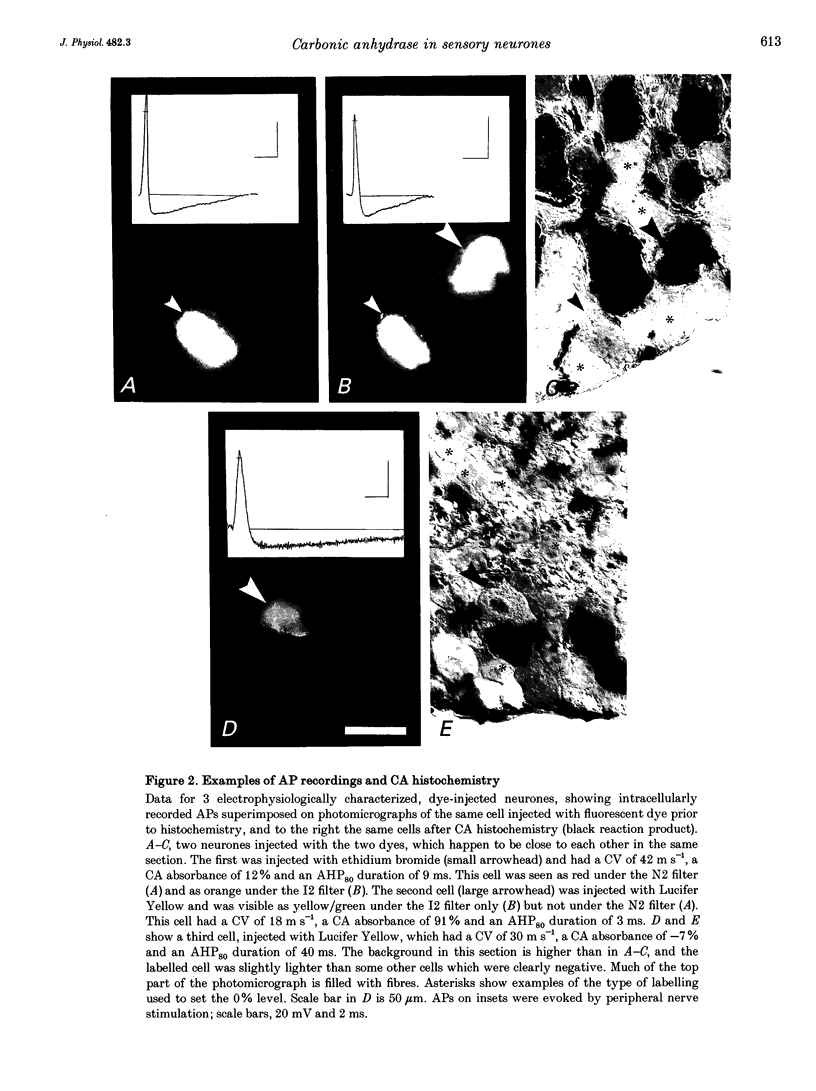
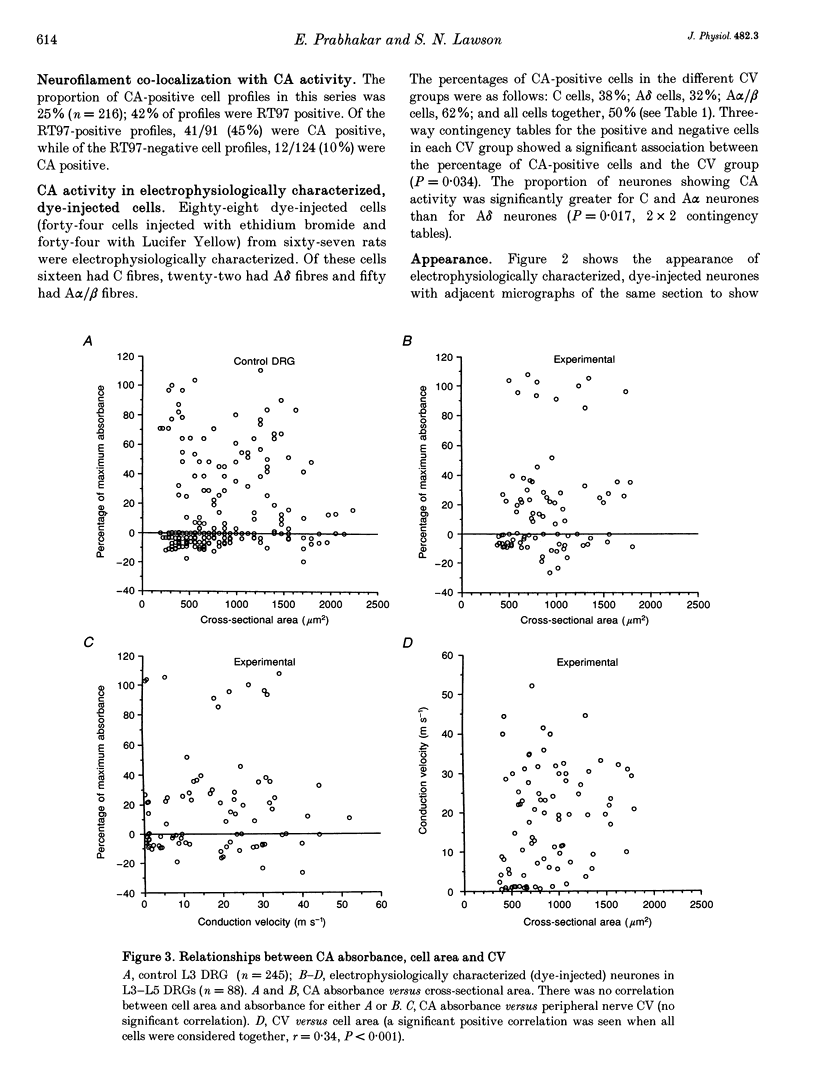
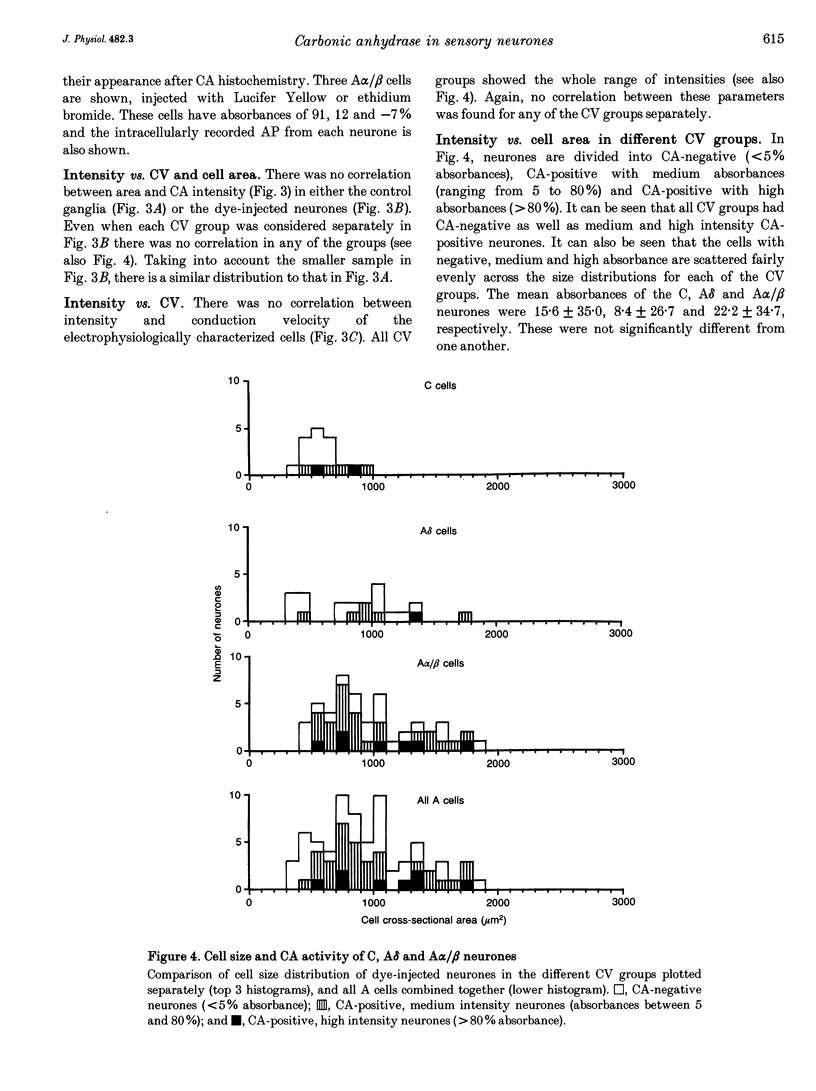
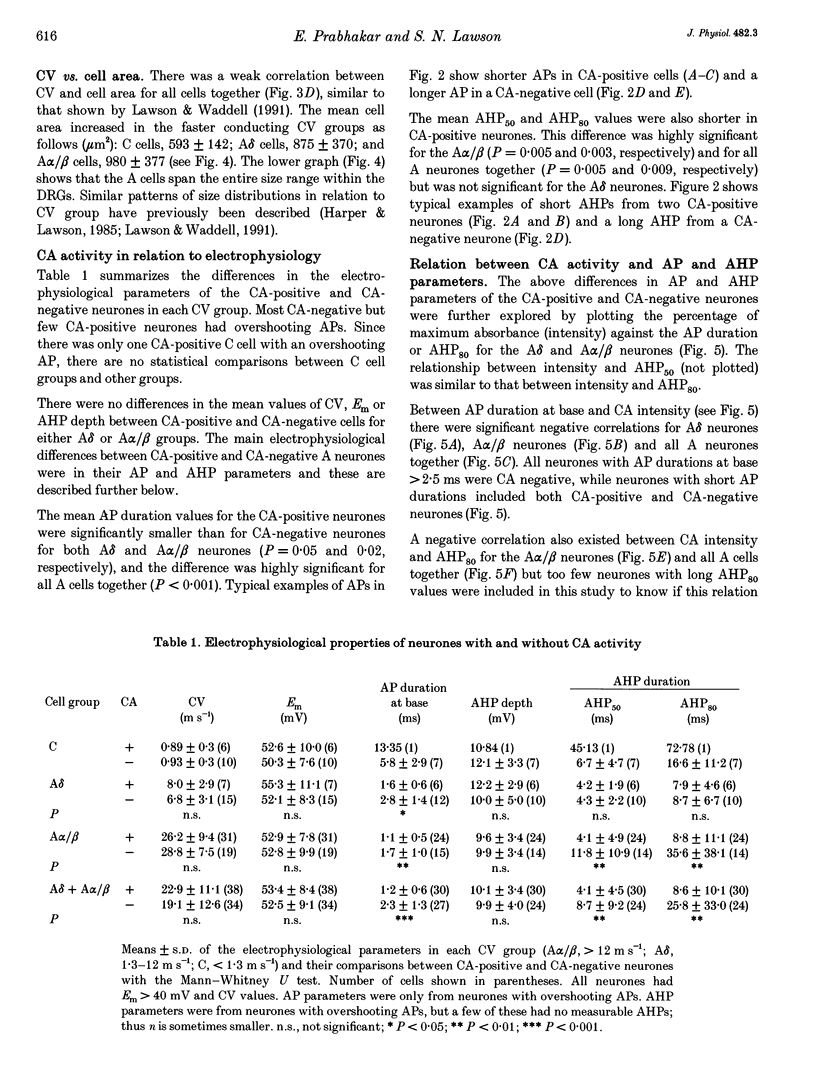
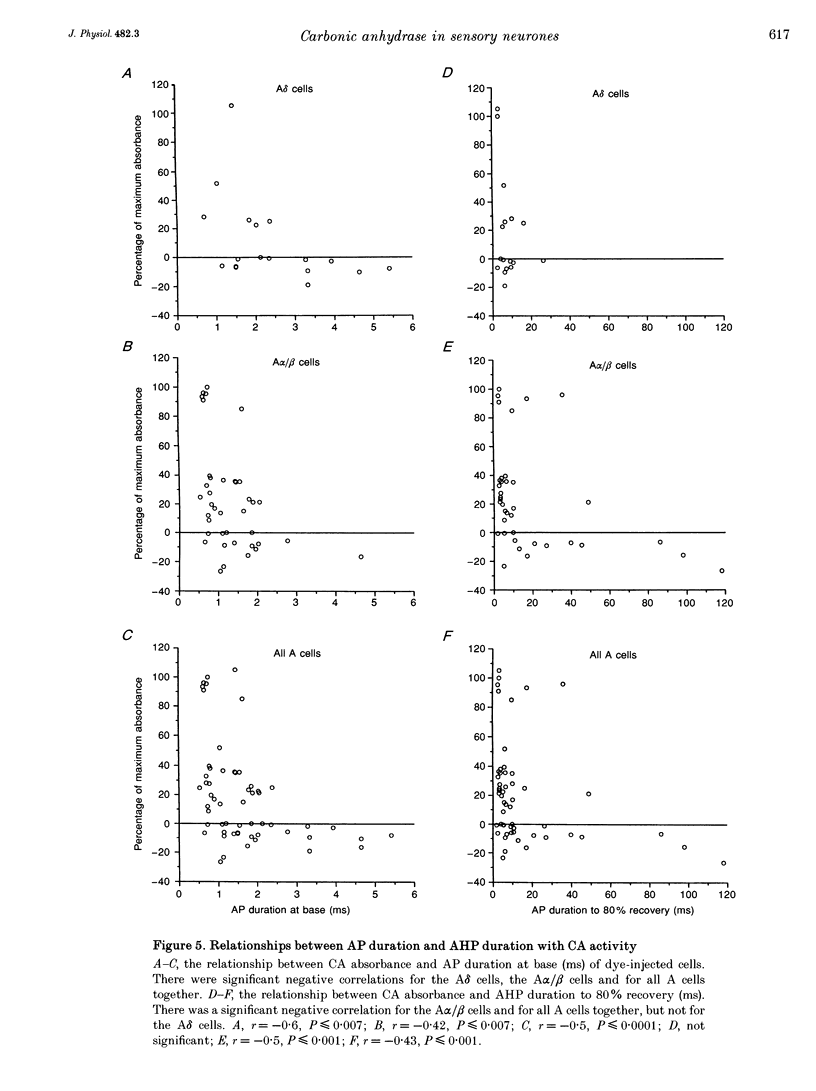
Abstract
1. Intracellular recordings of action potentials (APs) and after-hyperpolarizations (AHPs) were made from the L3, L4 and L5 dorsal root ganglia (DRGs) of 6- to 8-week-old anaesthetized female Wistar rats in vitro at 36.5 +/- 1 degree C. Neurones were classified by their conduction velocities (CVs) as A alpha/beta (> 12 m s-1), A delta (1.3-12 m s-1) or C fibre neurones (< 1.3 m s-1). 2. Following the recording, fluorescent dye was injected intracellularly. Sections of injected neurones were tested for carbonic anhydrase (CA) activity histochemically. Reaction product intensity and cell size were measured. Control experiments showed that intracellular dye, time in vitro, axotomy and electrical stimulation did not affect proportions of CA-positive neurones or their size distributions. 3. Approximately 28-30% of DRG neurones were CA positive. Their sizes were approximately normally distributed and covered the entire size range of DRG neurones with no correlation between size and CA intensity. A greater proportion of A alpha/beta cells (62%) than of A delta (32%) or C cells (38%) were CA positive, but CA intensity was not correlated with CV. 4. In A neurones mean AP duration was significantly shorter in CA-positive cells; for CA-positive and CA-negative cells, respectively, these values were 1.6 and 2.8 ms for A delta cells; 1.1 and 1.7 ms for A alpha/beta cells; and were 1.2 and 2.3 ms for all A cells. CA intensity was negatively correlated with AP duration at base in all these groups. 5. Again in A neurones, the mean AHP durations were significantly shorter in the CA-positive cells; the mean AHP durations to 80% recovery for positive and negative cells were 8.8 and 36 ms, respectively, for A alpha/beta cells and were 8.6 and 26 ms, respectively, for all A cells. CA intensity was negatively correlated with AHP duration in A alpha/beta cells and all A cells together. 6. A fibre cells with the longer AP and AHP durations were all CA negative, while cells with the shorter durations included both CA-positive and CA-negative cells. 7. CA-positive and CA-negative A fibre neurones therefore have different electrophysiological characteristics. It is suggested that CA-negative A fibre neurones may have slower somatic firing rates and different sensory functions from the CA-positive neurones.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin M. F., Hardin H. Interactions neurone-glie. Encephale. 1991 Sep-Oct;17(5):467–473. [PubMed] [Google Scholar]
- Carr P. A., Yamamoto T., Karmy G., Baimbridge K. G., Nagy J. I. Analysis of parvalbumin and calbindin D28k-immunoreactive neurons in dorsal root ganglia of rat in relation to their cytochrome oxidase and carbonic anhydrase content. Neuroscience. 1989;33(2):363–371. doi: 10.1016/0306-4522(89)90216-9. [DOI] [PubMed] [Google Scholar]
- Carr P. A., Yamamoto T., Staines W. A., Whittaker M. E., Nagy J. I. Quantitative histochemical analysis of cytochrome oxidase in rat dorsal root ganglia and its co-localization with carbonic anhydrase. Neuroscience. 1989;33(2):351–362. doi: 10.1016/0306-4522(89)90215-7. [DOI] [PubMed] [Google Scholar]
- Elder J. A., Lehninger A. L. Respiration-dependent transport of carbon dioxide into rat liver mitochondria. Biochemistry. 1973 Feb 27;12(5):976–982. doi: 10.1021/bi00729a029. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:431–446. doi: 10.1113/jphysiol.1985.sp015594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R. The ionic basis of action potentials in petrosal ganglion cells of the cat. J Physiol. 1983 Sep;342:591–602. doi: 10.1113/jphysiol.1983.sp014870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görke K., Pierau F. K. Spike potentials and membrane properties of dorsal root ganglion cells in pigeons. Pflugers Arch. 1980 Jul;386(1):21–28. doi: 10.1007/BF00584182. [DOI] [PubMed] [Google Scholar]
- Hansson H. P. Histochemical demonstration of carbonic anhydrase activity. Histochemie. 1967;11(2):112–128. doi: 10.1007/BF00571716. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Lawson S. N. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol. 1985 Feb;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazimierczak J., Sommer E. W., Philippe E., Droz B. Carbonic anhydrase activity in primary sensory neurons. I. Requirements for the cytochemical localization in the dorsal root ganglion of chicken and mouse by light and electron microscopy. Cell Tissue Res. 1986;245(3):487–495. doi: 10.1007/BF00218548. [DOI] [PubMed] [Google Scholar]
- Koerber H. R., Druzinsky R. E., Mendell L. M. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988 Nov;60(5):1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Veselovsky N. S., Fedulova S. A., Tsyndrenko A. Y. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons-III. Potassium currents. Neuroscience. 1981;6(12):2439–2444. doi: 10.1016/0306-4522(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Harper A. A., Harper E. I., Garson J. A., Anderton B. H. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984 Sep 10;228(2):263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Perry M. J., Prabhakar E., McCarthy P. W. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30(3-4):239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Lawson S. N., Waddell P. J. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol. 1991 Apr;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Marty A. The physiological role of calcium-dependent channels. Trends Neurosci. 1989 Nov;12(11):420–424. doi: 10.1016/0166-2236(89)90090-8. [DOI] [PubMed] [Google Scholar]
- McCarthy P. W., Lawson S. N. Differential intracellular labelling of identified neurones with two fluorescent dyes. Brain Res Bull. 1988 Feb;20(2):261–265. doi: 10.1016/0361-9230(88)90188-8. [DOI] [PubMed] [Google Scholar]
- Pacifico J. J., Scott S. A. The ontogeny of carbonic anhydrase in chick dorsal root ganglia and spinal cords. Brain Res Dev Brain Res. 1990 Dec 1;57(1):77–84. doi: 10.1016/0165-3806(90)90187-4. [DOI] [PubMed] [Google Scholar]
- Peyronnard J. M., Charron L. F., Messier J. P., Lavoie J. Differential effects of distal and proximal nerve lesions on carbonic anhydrase activity in rat primary sensory neurons, ventral and dorsal root axons. Exp Brain Res. 1988;70(3):550–560. doi: 10.1007/BF00247602. [DOI] [PubMed] [Google Scholar]
- Peyronnard J. M., Charron L., Lavoie J., Messier J. P., Dubreuil M. Carbonic anhydrase and horseradish peroxidase: double labelling of rat dorsal root ganglion neurons innervating motor and sensory peripheral nerves. Anat Embryol (Berl) 1988;177(4):353–359. doi: 10.1007/BF00315844. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y., Hanson M. Histochemical study of the distribution of carbonic anhydrase in the cat brain. Acta Physiol Scand. 1985 Aug;124(4):557–564. doi: 10.1111/j.1748-1716.1985.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Riley D. A., Ellis S., Bain J. L. Ultrastructural cytochemical localization of carbonic anhydrase activity in rat peripheral sensory and motor nerves, dorsal root ganglia and dorsal column nuclei. Neuroscience. 1984 Sep;13(1):189–206. doi: 10.1016/0306-4522(84)90269-0. [DOI] [PubMed] [Google Scholar]
- Ritter A. M., Mendell L. M. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992 Dec;68(6):2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Robertson B., Grant G. Immunocytochemical evidence for the localization of the GM1 ganglioside in carbonic anhydrase-containing and RT 97-immunoreactive rat primary sensory neurons. J Neurocytol. 1989 Feb;18(1):77–86. doi: 10.1007/BF01188426. [DOI] [PubMed] [Google Scholar]
- Sah P. Role of calcium influx and buffering in the kinetics of Ca(2+)-activated K+ current in rat vagal motoneurons. J Neurophysiol. 1992 Dec;68(6):2237–2247. doi: 10.1152/jn.1992.68.6.2237. [DOI] [PubMed] [Google Scholar]
- Sapirstein V. S., Strocchi P., Gilbert J. M. Properties and function of brain carbonic anhydrase. Ann N Y Acad Sci. 1984;429:481–493. doi: 10.1111/j.1749-6632.1984.tb12375.x. [DOI] [PubMed] [Google Scholar]
- Sommer E. W., Kazimierczak J., Droz B. Neuronal subpopulations in the dorsal root ganglion of the mouse as characterized by combination of ultrastructural and cytochemical features. Brain Res. 1985 Nov 4;346(2):310–326. doi: 10.1016/0006-8993(85)90865-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto T., Takemura M., Ichikawa H., Akai M. Carbonic anhydrase activity in the trigeminal primary afferent neuronal cell bodies with peripheral axons innervating the mandibular molar tooth pulps of the rat. Brain Res. 1989 Dec 29;505(2):354–357. doi: 10.1016/0006-8993(89)91468-6. [DOI] [PubMed] [Google Scholar]
- Szabolcs M. J., Kopp M., Schaden G. E. Carbonic anhydrase activity in the peripheral nervous system of rat: the enzyme as a marker for muscle afferents. Brain Res. 1989 Jul 17;492(1-2):129–138. doi: 10.1016/0006-8993(89)90895-0. [DOI] [PubMed] [Google Scholar]
- Tenser R. B. Sequential changes of sensory neuron (fluoride-resistant) acid phosphatase in dorsal root ganglion neurons following neurectomy and rhizotomy. Brain Res. 1985 Apr 22;332(2):386–389. doi: 10.1016/0006-8993(85)90610-9. [DOI] [PubMed] [Google Scholar]
- Tohyama K., Ide C. Carbonic anhydrase activity in axon terminals of sensory corpuscles. Arch Histol Jpn. 1987 Jul;50(3):325–333. doi: 10.1679/aohc.50.325. [DOI] [PubMed] [Google Scholar]
- Trachtenberg M. C., Sapirstein V. S. Carbonic anhydrase distributions in central and peripheral nervous system of the rat. Neurochem Res. 1980 May;5(5):573–581. doi: 10.1007/BF00964994. [DOI] [PubMed] [Google Scholar]
- Waddell P. J., Lawson S. N. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neuroscience. 1990;36(3):811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Wong V., Barrett C. P., Donati E. J., Eng L. F., Guth L. Carbonic anhydrase activity in first-order sensory neurons of the rat. J Histochem Cytochem. 1983 Feb;31(2):293–300. doi: 10.1177/31.2.6403607. [DOI] [PubMed] [Google Scholar]