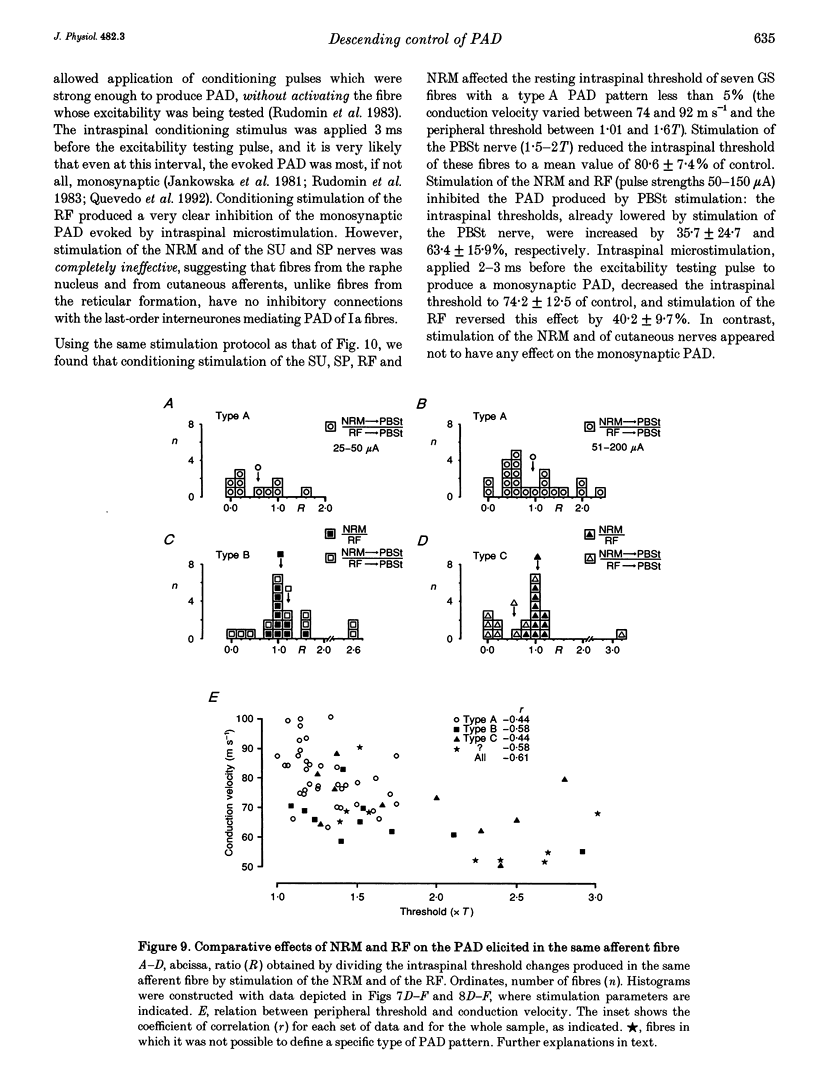
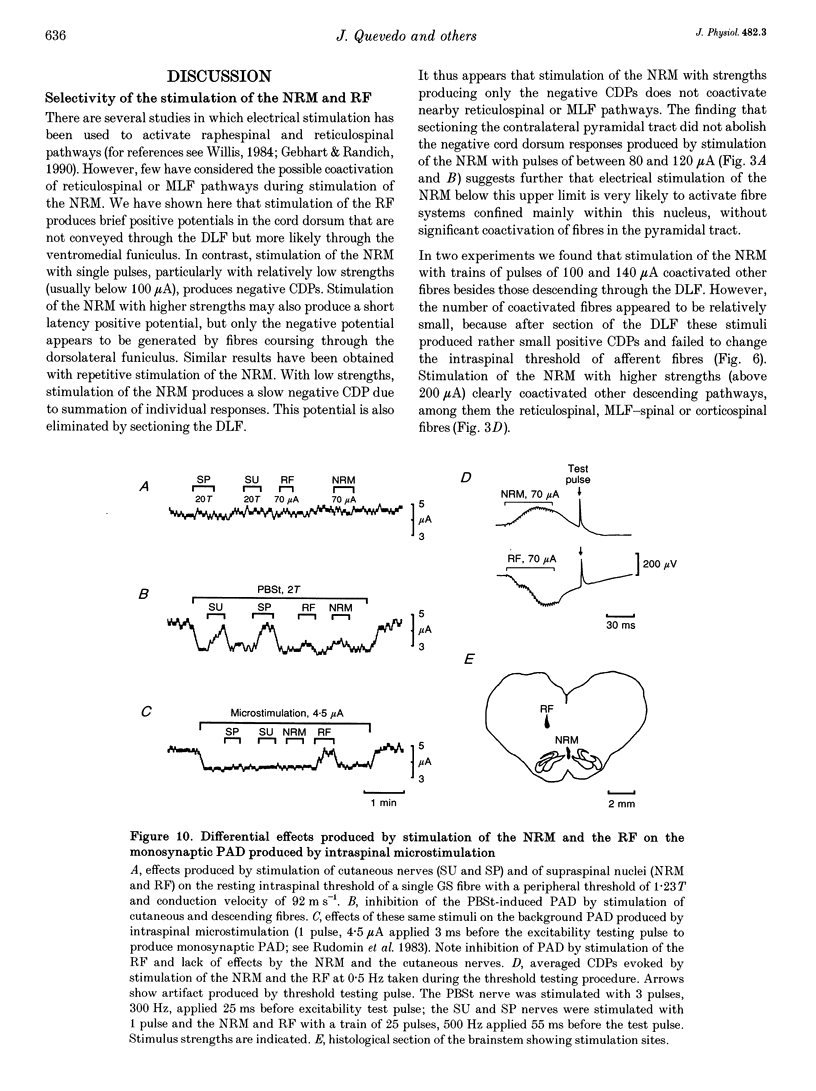
Abstract
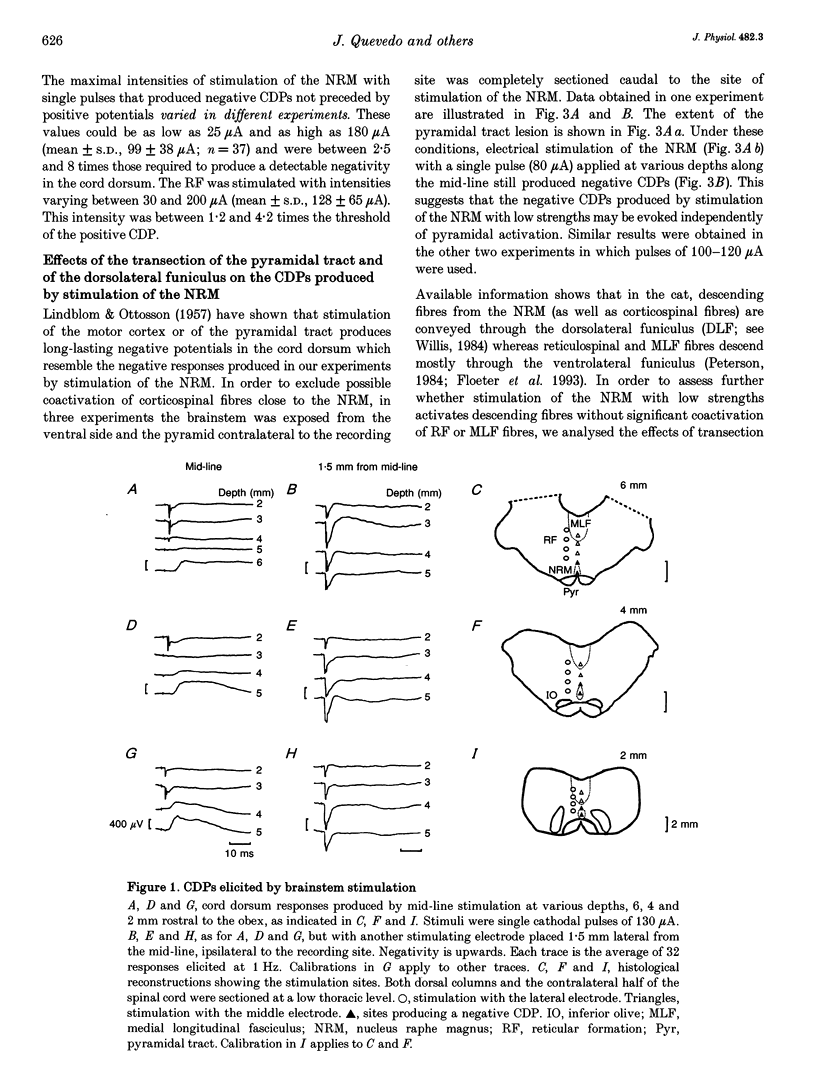
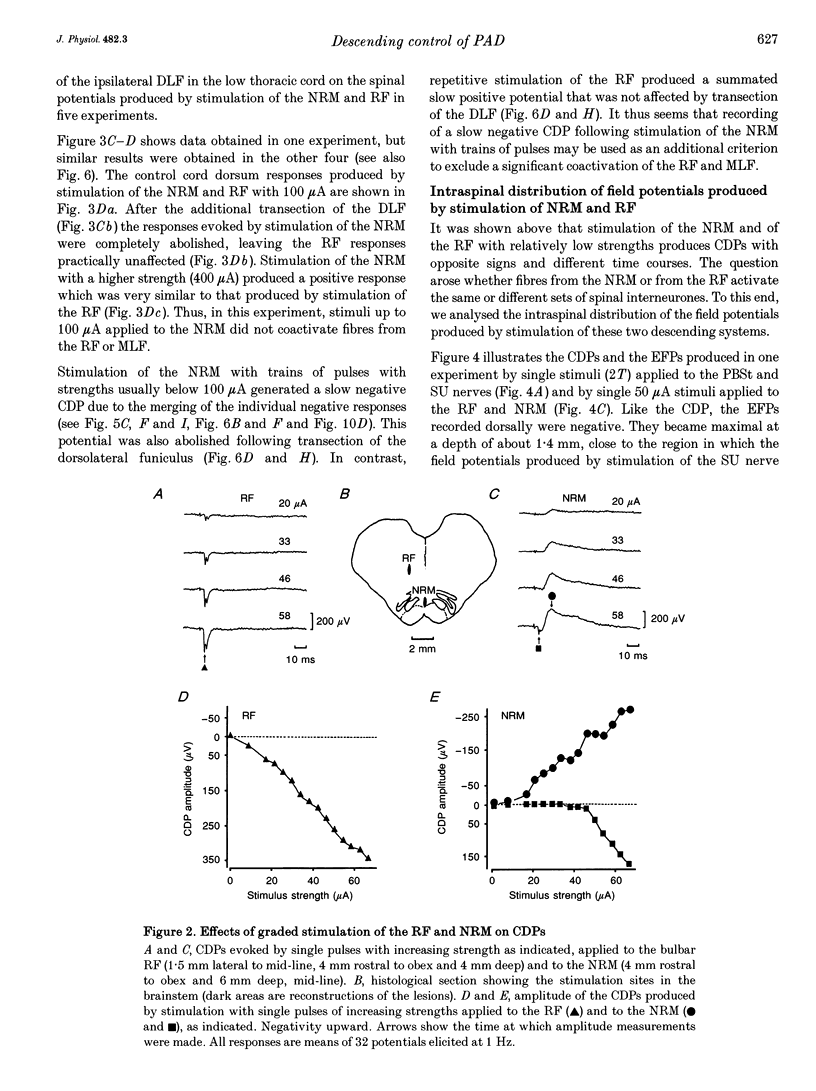
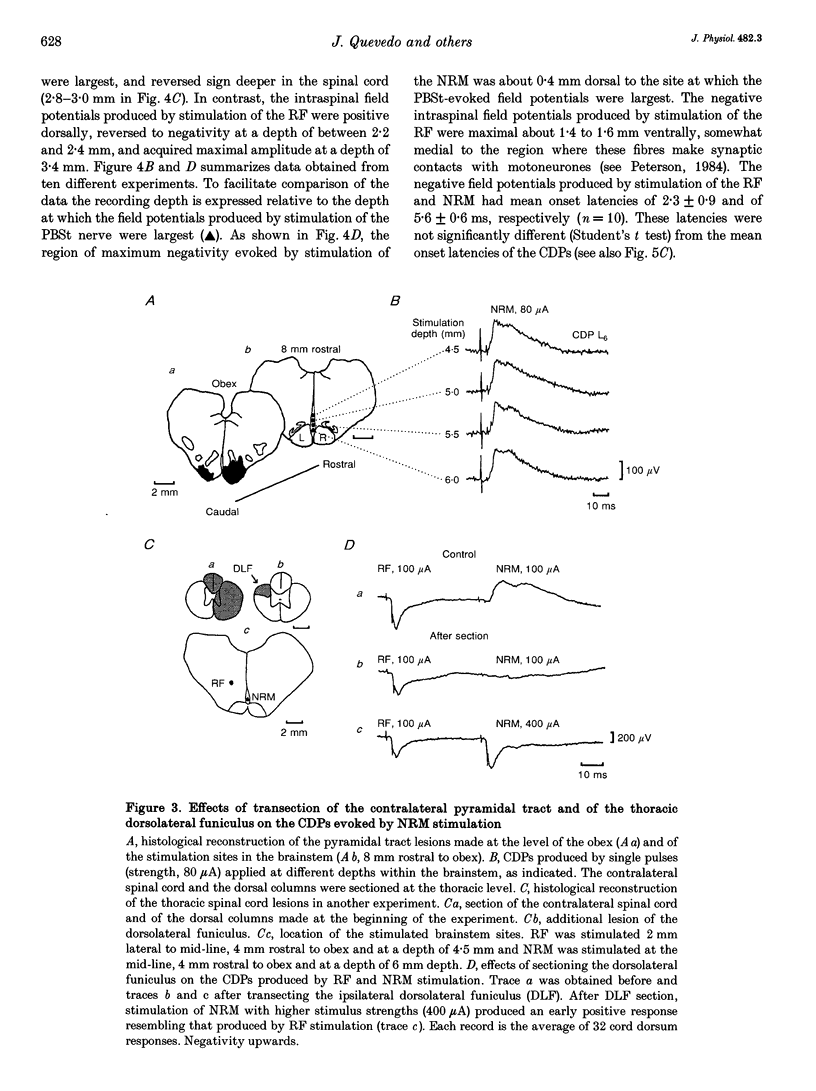
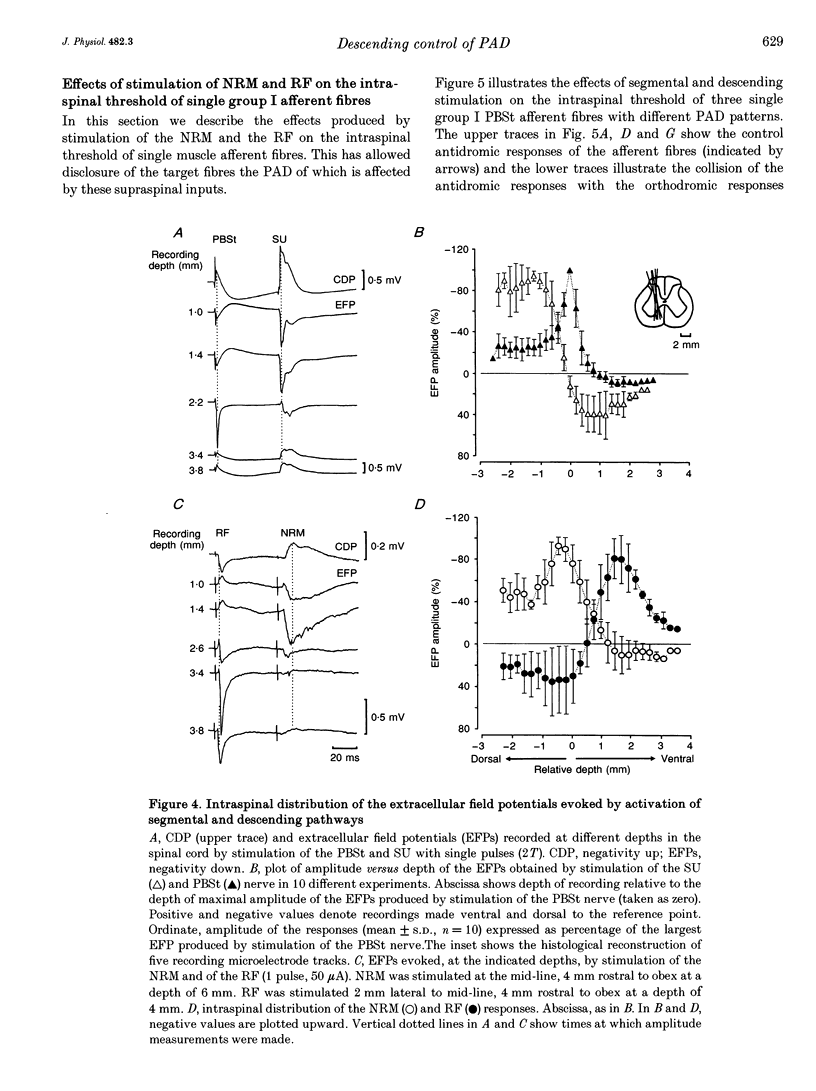
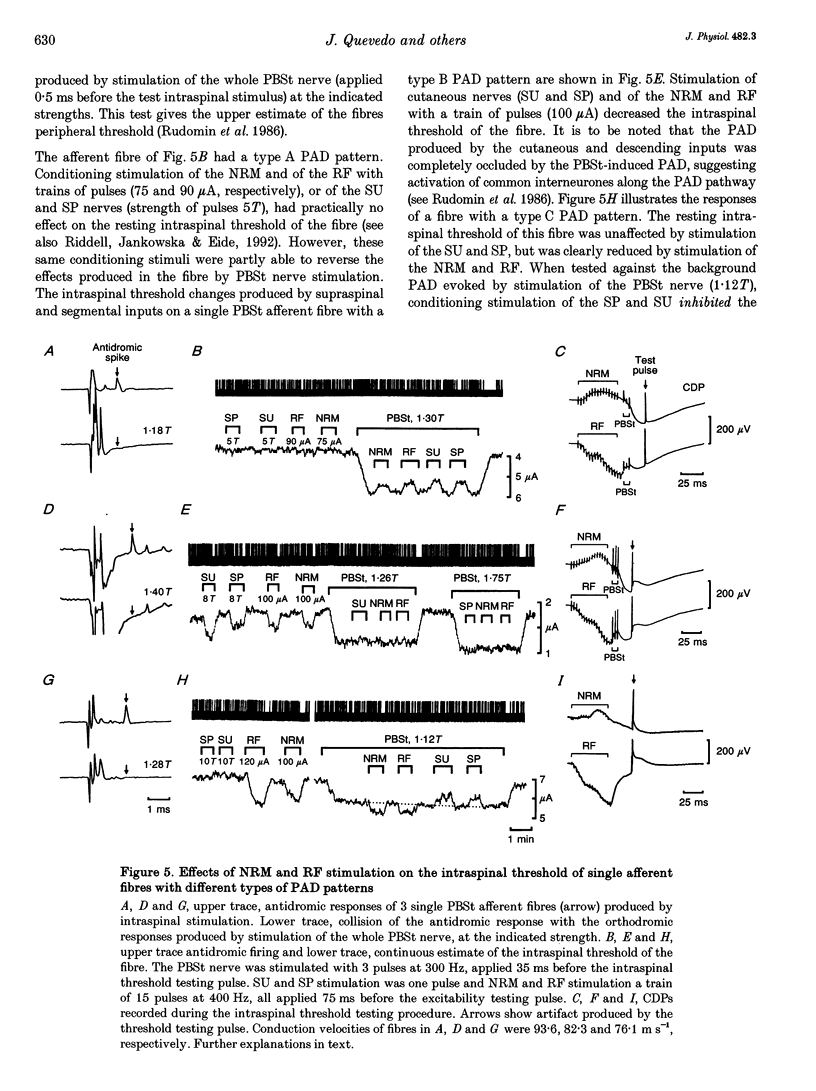
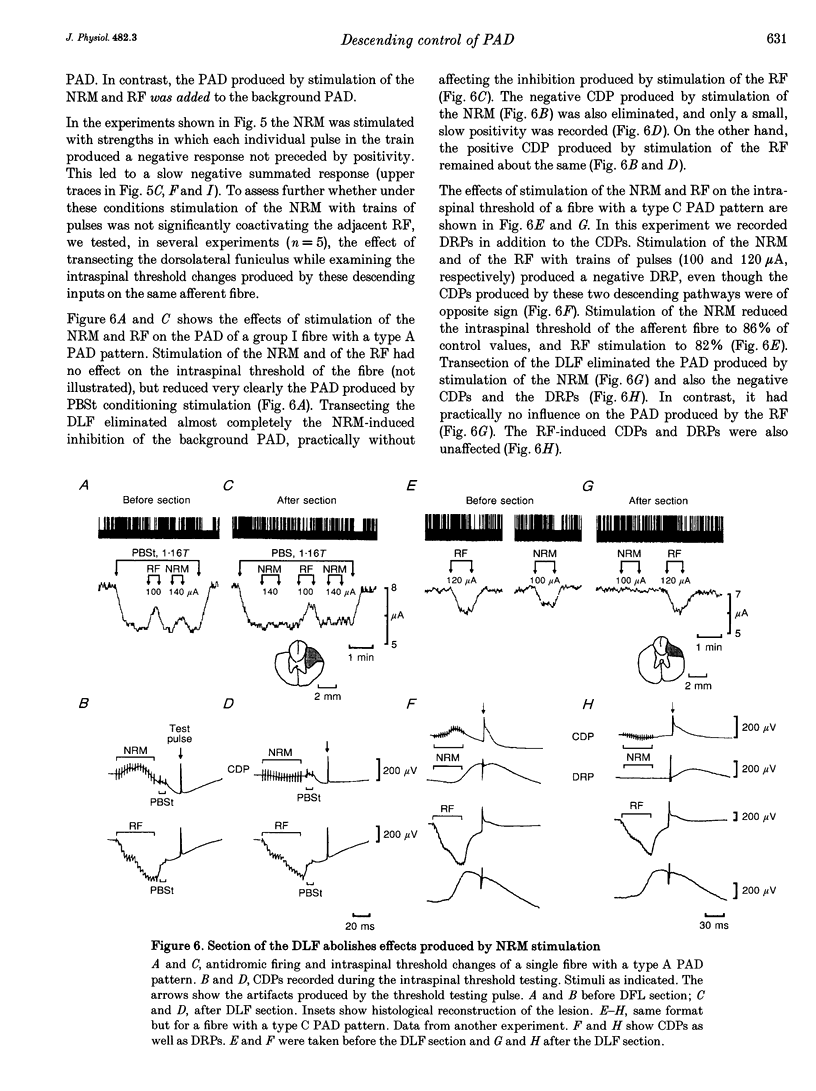
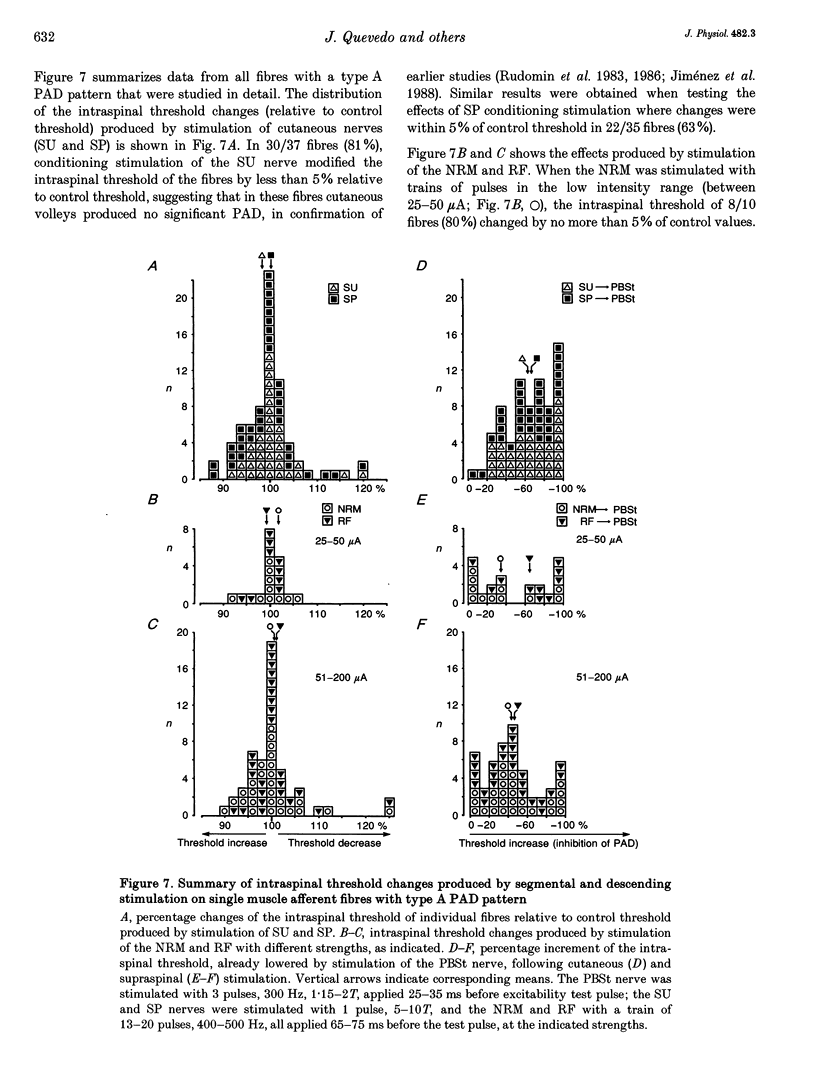
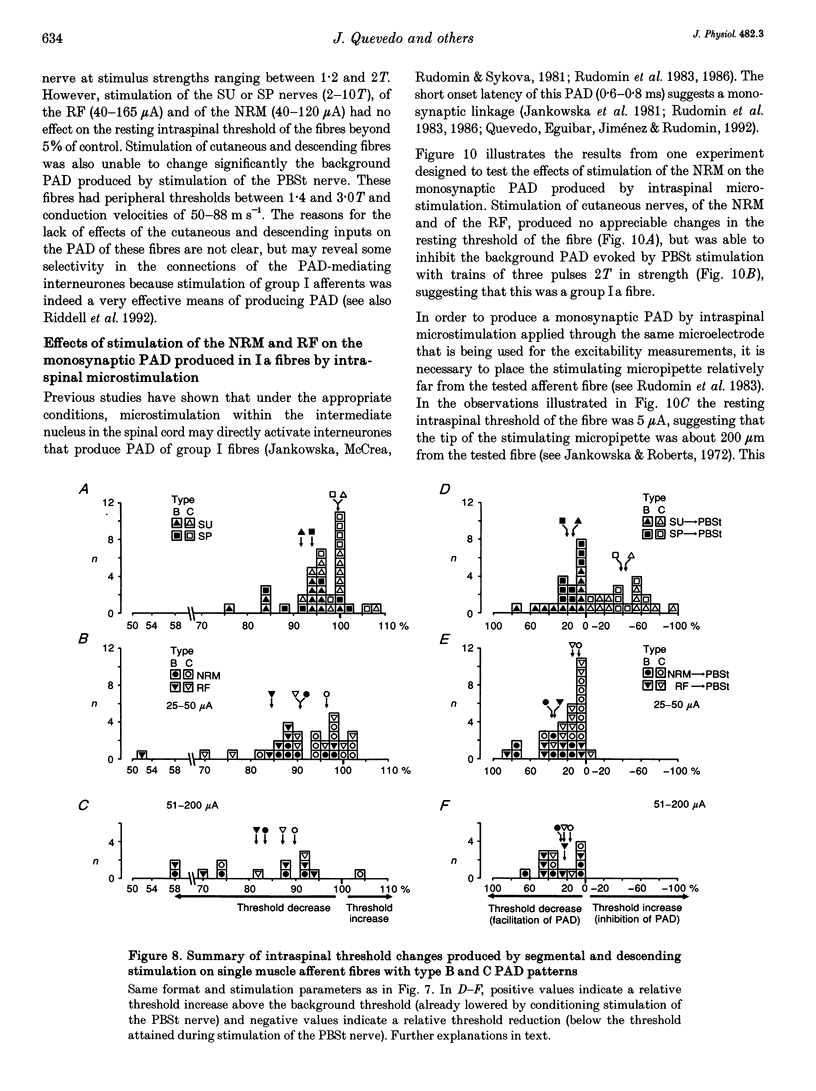
1. In the anaesthetized cat, electrical stimulation of the bulbar reticular formation produced a short latency (2.1 +/- 0.3 ms) positive potential in the cord dorsum. In contrast, stimulation of the nucleus raphe magnus with strengths below 50 microA evoked a slow negative potential with a mean latency of 5.5 +/- 0.6 ms that persisted after sectioning the contralateral pyramid and was abolished by sectioning the ipsilateral dorsolateral funiculus. 2. The field potentials evoked by stimulation of the bulbar reticular formation and of the nucleus raphe magnus had a different intraspinal distribution, suggesting activation of different sets of segmental interneurones. 3. Stimulation of these two supraspinal nuclei produced primary afferent depolarization (PAD) in single Ib fibres and inhibited the PAD elicited by group I volleys in single Ia fibres. The inhibition of the PAD of Ia fibres produced by reticulospinal and raphespinal inputs appears to be exerted on different interneurones along the PAD pathway. 4. It is concluded that, although reticulospinal and raphespinal pathways have similar inhibitory effects on PAD of Ia fibres, and similar excitatory effects on the PAD of Ib fibres, their actions are conveyed by partly independent pathways. This would allow their separate involvement in the control of posture and movement.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basbaum A. I., Clanton C. H., Fields H. L. Three bulbospinal pathways from the rostral medulla of the cat: an autoradiographic study of pain modulating systems. J Comp Neurol. 1978 Mar 15;178(2):209–224. doi: 10.1002/cne.901780203. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W., Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Res. 1978 Aug 4;151(2):307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Bras H., Jankowska E., Noga B., Skoog B. Comparison of Effects of Various Types of NA and 5-HT Agonists on Transmission from Group II Muscle Afferents in the Cat. Eur J Neurosci. 1990;2(12):1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Curtis D. R. A method for continuously monitoring the electrical threshold of single intraspinal nerve fibers. Electroencephalogr Clin Neurophysiol. 1979 Oct;47(4):503–506. doi: 10.1016/0013-4694(79)90167-6. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Leah J. D., Peet M. J. Effects of noradrenaline and 5-hydroxytryptamine on spinal Ia afferent terminations. Brain Res. 1983 Jan 10;258(2):328–332. doi: 10.1016/0006-8993(83)91160-5. [DOI] [PubMed] [Google Scholar]
- Eccles J. C., Magni F., Willis W. D. Depolarization of central terminals of Group I afferent fibres from muscle. J Physiol. 1962 Jan;160(1):62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floeter M. K., Sholomenko G. N., Gossard J. P., Burke R. E. Disynaptic excitation from the medial longitudinal fasciculus to lumbosacral motoneurons: modulation by repetitive activation, descending pathways, and locomotion. Exp Brain Res. 1993;92(3):407–419. doi: 10.1007/BF00229029. [DOI] [PubMed] [Google Scholar]
- Fung S. J., Barnes C. D. Raphé-produced excitation of spinal cord motoneurons in the cat. Neurosci Lett. 1989 Aug 28;103(2):185–190. doi: 10.1016/0304-3940(89)90573-9. [DOI] [PubMed] [Google Scholar]
- Hammond D. L., Yaksh T. L. Antagonism of stimulation-produced antinociception by intrathecal administration of methysergide or phentolamine. Brain Res. 1984 Apr 30;298(2):329–337. doi: 10.1016/0006-8993(84)91432-x. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Lund S., Lundberg A., Pompeiano O. Inhibitory effects evoked through ventral reticulospinal pathways. Arch Ital Biol. 1968 May;106(2):124–140. [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Rudomín P., Sykova E. Observations on neuronal pathways subserving primary afferent depolarization. J Neurophysiol. 1981 Sep;46(3):506–516. doi: 10.1152/jn.1981.46.3.506. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M. Mechanisms involved in the depolarization of cutaneous afferents produced by segmental and descending inputs in the cat spinal cord. Exp Brain Res. 1987;69(1):195–207. doi: 10.1007/BF00247042. [DOI] [PubMed] [Google Scholar]
- Jiménez I., Rudomin P., Solodkin M. PAD patterns of physiologically identified afferent fibres from the medial gastrocnemius muscle. Exp Brain Res. 1988;71(3):643–657. doi: 10.1007/BF00248758. [DOI] [PubMed] [Google Scholar]
- LINDBLOM U. F., OTTOSSON J. O. Influence of pyramidal stimulation upon the relay of coarse cutaneous afferents in the dorsal horn. Acta Physiol Scand. 1957 Mar 7;38(3-4):309–318. doi: 10.1111/j.1748-1716.1957.tb01394.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A. SUPRASPINAL CONTROL OF TRANSMISSION IN REFLEX PATHS TO MOTONEURONES AND PRIMARY AFFERENTS. Prog Brain Res. 1964;12:197–221. doi: 10.1016/s0079-6123(08)60624-x. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Vyklický L. Inhibition of transmission to primary afferents by electrical stimulation of the brain stem. Arch Ital Biol. 1966 Mar;104(1):86–97. [PubMed] [Google Scholar]
- Madrid J., Alvarado J., Dutton H., Rudomín P. A method for the dynamic continuous estimation of excitability changes of single fiber terminals in the central nervous system. Neurosci Lett. 1979 Mar;11(3):253–258. doi: 10.1016/0304-3940(79)90003-x. [DOI] [PubMed] [Google Scholar]
- Martin R. F., Haber L. H., Willis W. D. Primary afferent depolarization of identified cutaneous fibers following stimulation in medial brain stem. J Neurophysiol. 1979 May;42(3):779–790. doi: 10.1152/jn.1979.42.3.779. [DOI] [PubMed] [Google Scholar]
- McCreery D. B., Bloedel J. R. Reduction of the response of cat spinothalamic neurons to graded mechanical stimuli by electrical stimulation of the lower brain stem. Brain Res. 1975 Oct 24;97(1):151–156. doi: 10.1016/0006-8993(75)90923-3. [DOI] [PubMed] [Google Scholar]
- Mokha S. S., McMillan J. A., Iggo A. Descending control of spinal nociceptive transmission. Actions produced on spinal multireceptive neurones from the nuclei locus coeruleus (LC) and raphe magnus (NRM). Exp Brain Res. 1985;58(2):213–226. doi: 10.1007/BF00235305. [DOI] [PubMed] [Google Scholar]
- Mokha S. S., McMillan J. A., Iggo A. Pathways mediating descending control of spinal nociceptive transmission from the nuclei locus coeruleus (LC) and raphe magnus (NRM) in the cat. Exp Brain Res. 1986;61(3):597–606. doi: 10.1007/BF00237586. [DOI] [PubMed] [Google Scholar]
- Nicholas A. P., Hancock M. B. Evidence for projections from the rostral medullary raphe onto medullary catecholamine neurons in the rat. Neurosci Lett. 1990 Jan 1;108(1-2):22–28. doi: 10.1016/0304-3940(90)90700-j. [DOI] [PubMed] [Google Scholar]
- Noga B. R., Bras H., Jankowska E. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kölliker-Fuse and raphe nuclei in the cat. Exp Brain Res. 1992;88(3):502–516. doi: 10.1007/BF00228180. [DOI] [PubMed] [Google Scholar]
- Proudfit H. K., Anderson E. G. New long latency bulbospinal evoked potentials blocked by serotonin antagonists. Brain Res. 1974 Jan 18;65(3):542–546. doi: 10.1016/0006-8993(74)90246-7. [DOI] [PubMed] [Google Scholar]
- Quevedo J., Eguibar J. R., Jiménez I., Rudomin P. Differential action of (-)-baclofen on the primary afferent depolarization produced by segmental and descending inputs. Exp Brain Res. 1992;91(1):29–45. doi: 10.1007/BF00230011. [DOI] [PubMed] [Google Scholar]
- Riddell J. S., Jankowska E., Eide E. Depolarization of group II muscle afferents by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol. 1993 Feb;461:723–741. doi: 10.1113/jphysiol.1993.sp019538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P. Presynaptic inhibition of muscle spindle and tendon organ afferents in the mammalian spinal cord. Trends Neurosci. 1990 Dec;13(12):499–505. doi: 10.1016/0166-2236(90)90084-n. [DOI] [PubMed] [Google Scholar]
- Rudomin P., Solodkin M., Jiménez I. PAD and PAH response patterns of group Ia- and Ib-fibers to cutaneous and descending inputs in the cat spinal cord. J Neurophysiol. 1986 Oct;56(4):987–1006. doi: 10.1152/jn.1986.56.4.987. [DOI] [PubMed] [Google Scholar]
- Rudomín P., Jiménez I., Solodkin M., Dueñas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol. 1983 Oct;50(4):743–769. doi: 10.1152/jn.1983.50.4.743. [DOI] [PubMed] [Google Scholar]
- Takakusaki K., Ohta Y., Mori S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp Brain Res. 1989;74(1):11–23. doi: 10.1007/BF00248276. [DOI] [PubMed] [Google Scholar]
- Wessendorf M. W., Proudfit H. K., Anderson E. G. The identification of serotonergic neurons in the nucleus raphe magnus by conduction velocity. Brain Res. 1981 Jun 9;214(1):168–173. doi: 10.1016/0006-8993(81)90449-2. [DOI] [PubMed] [Google Scholar]
- Yates B. J., Goto T., Bolton P. S. Responses of neurons in the caudal medullary raphe nuclei of the cat to stimulation of the vestibular nerve. Exp Brain Res. 1992;89(2):323–332. doi: 10.1007/BF00228248. [DOI] [PubMed] [Google Scholar]



