Abstract
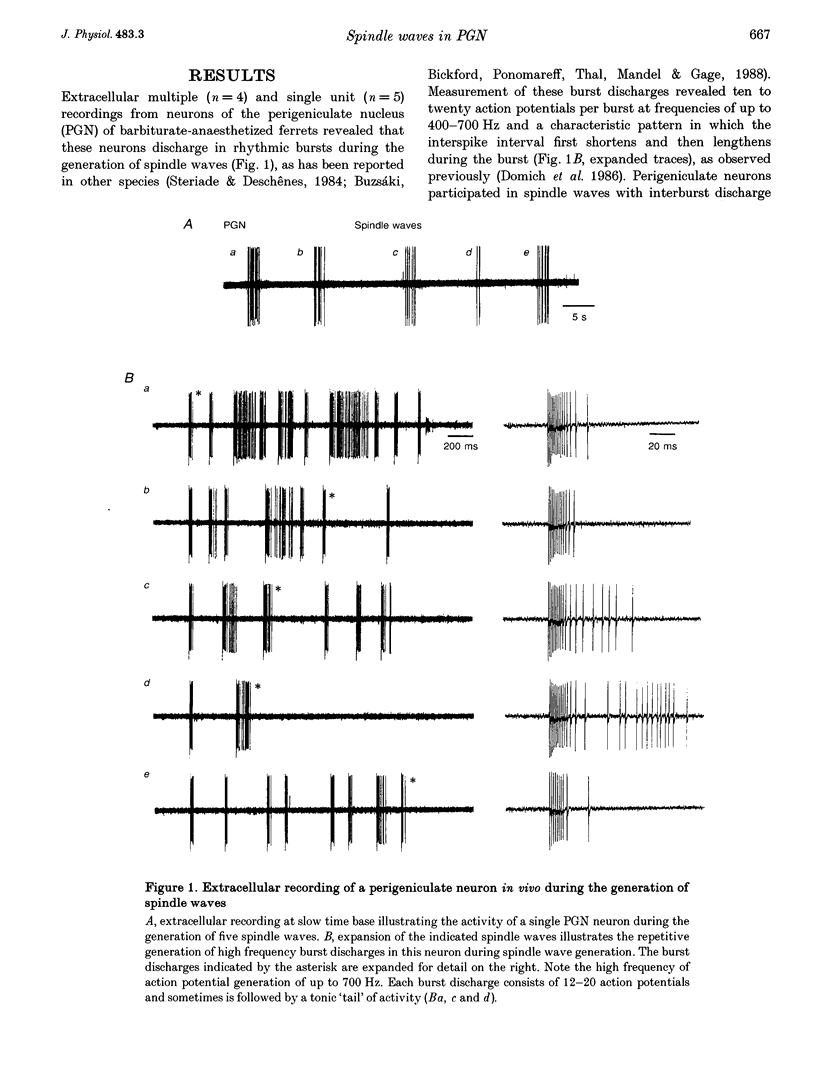
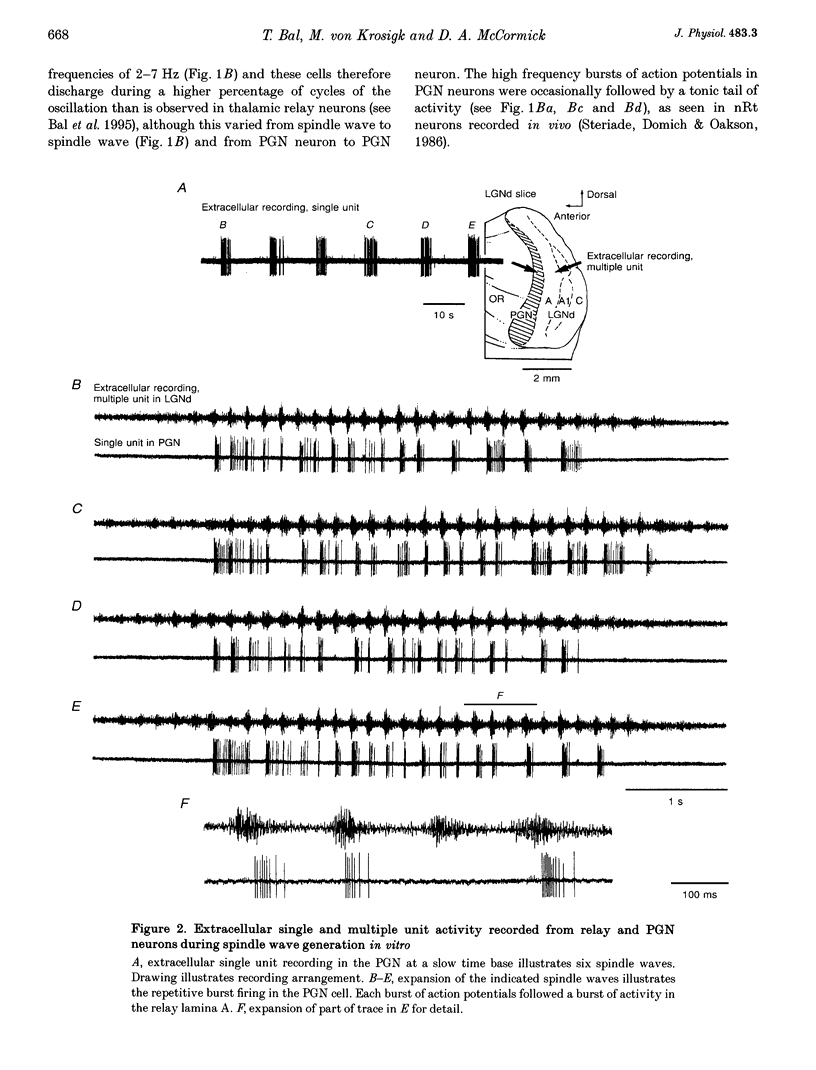
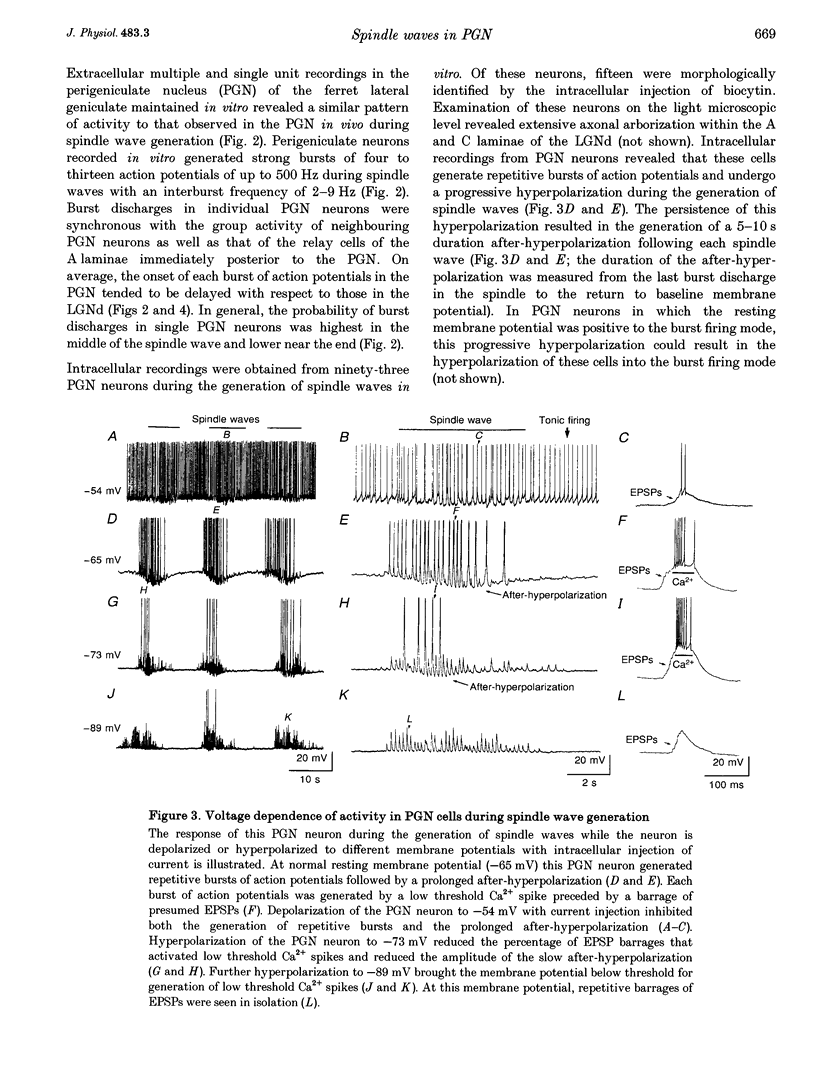
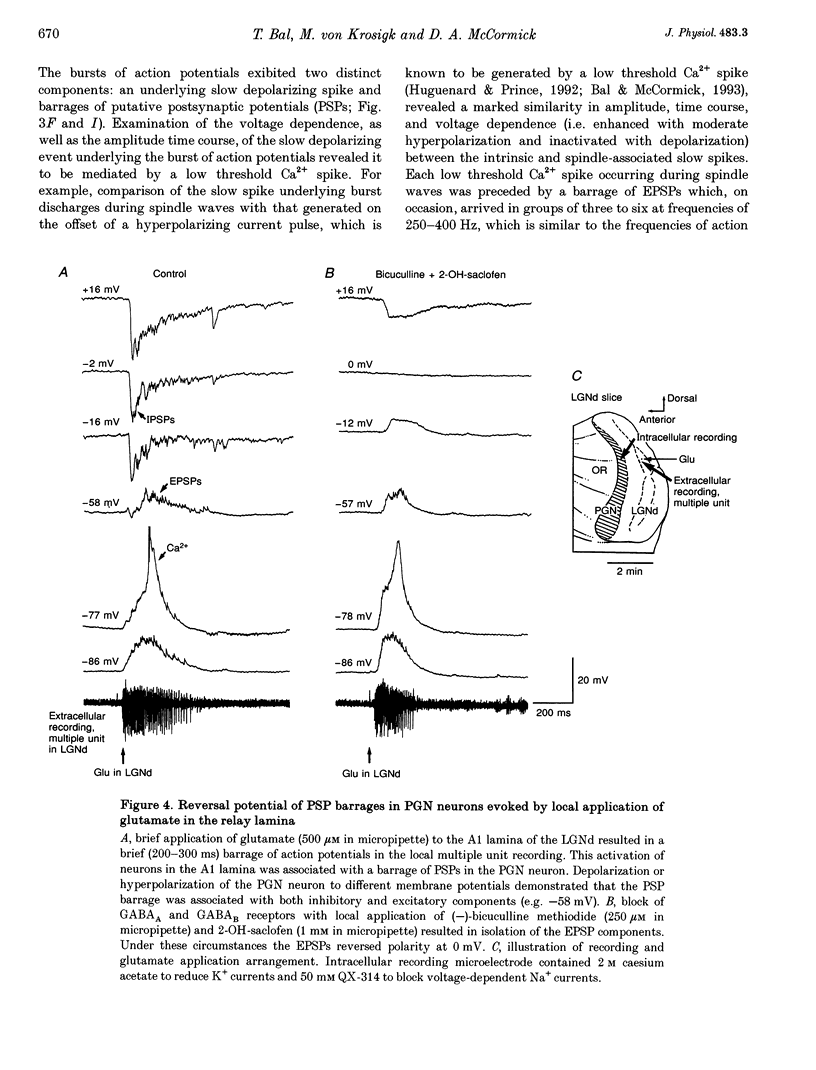
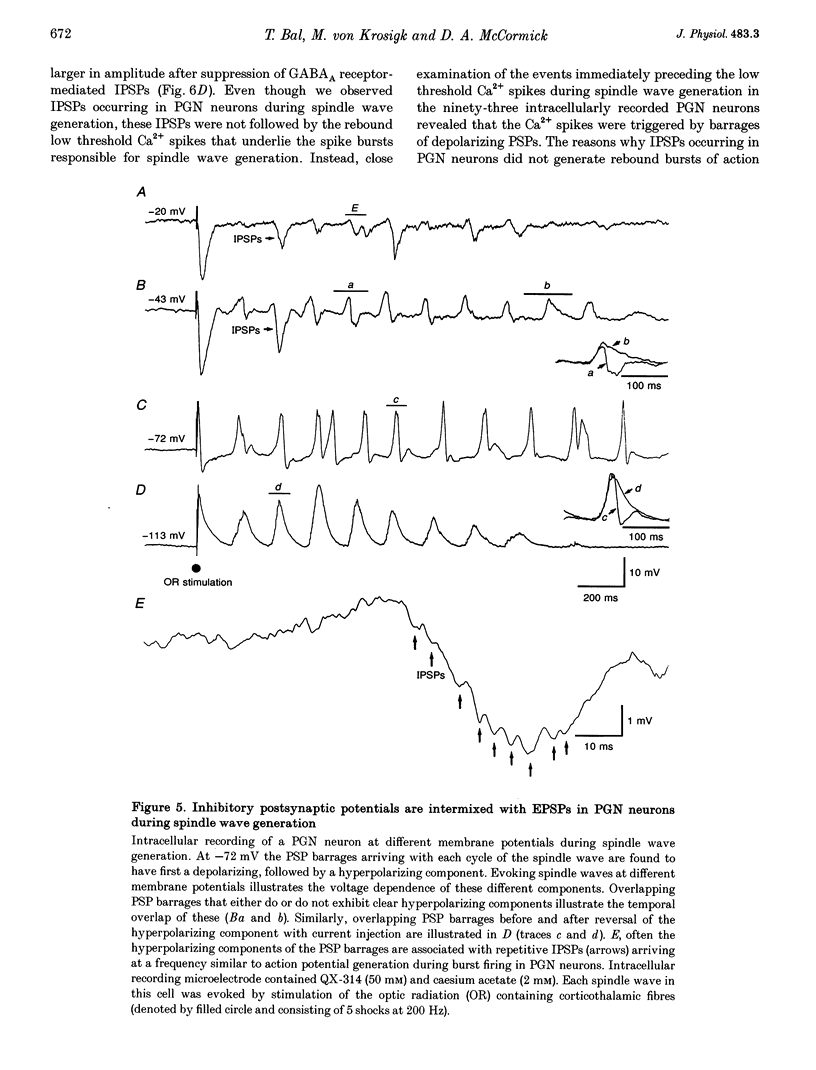
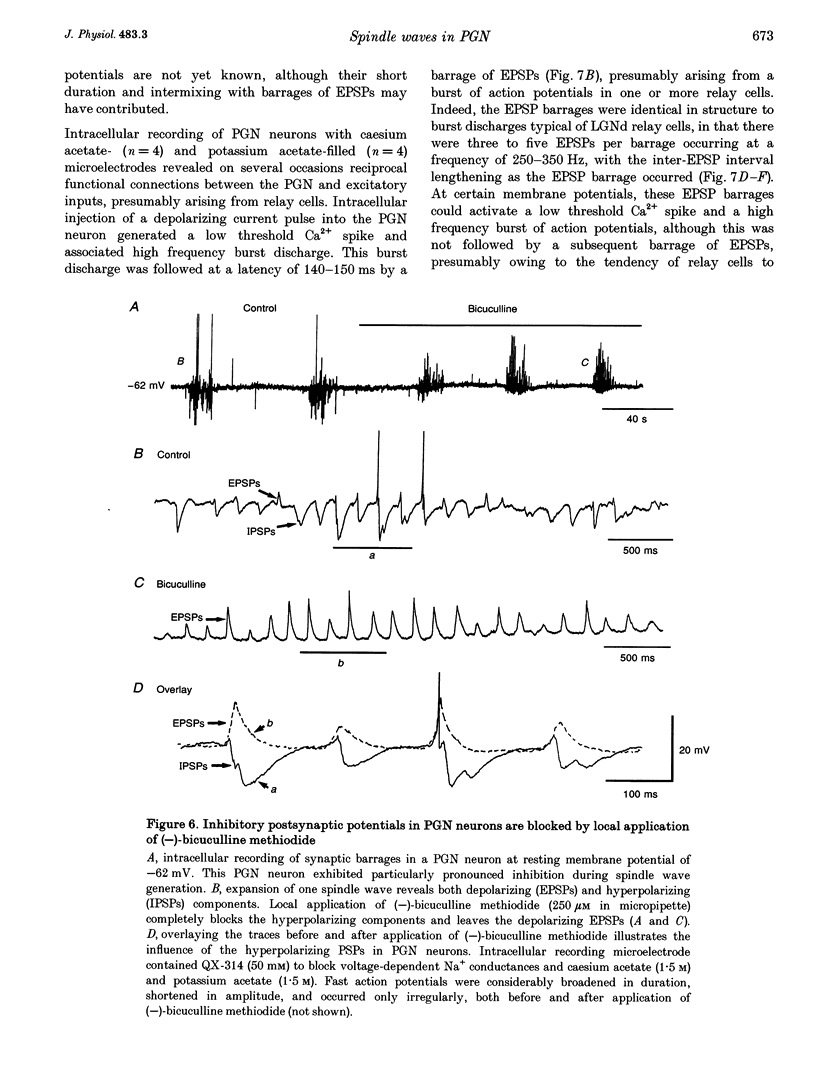
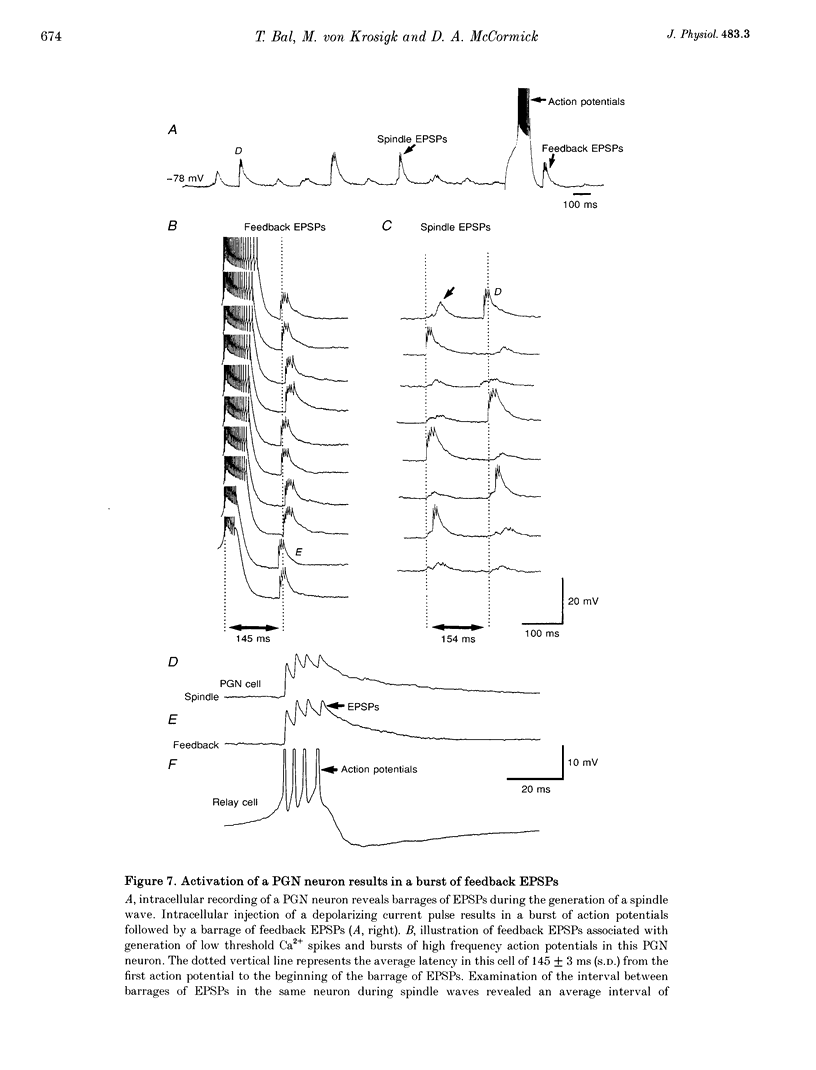
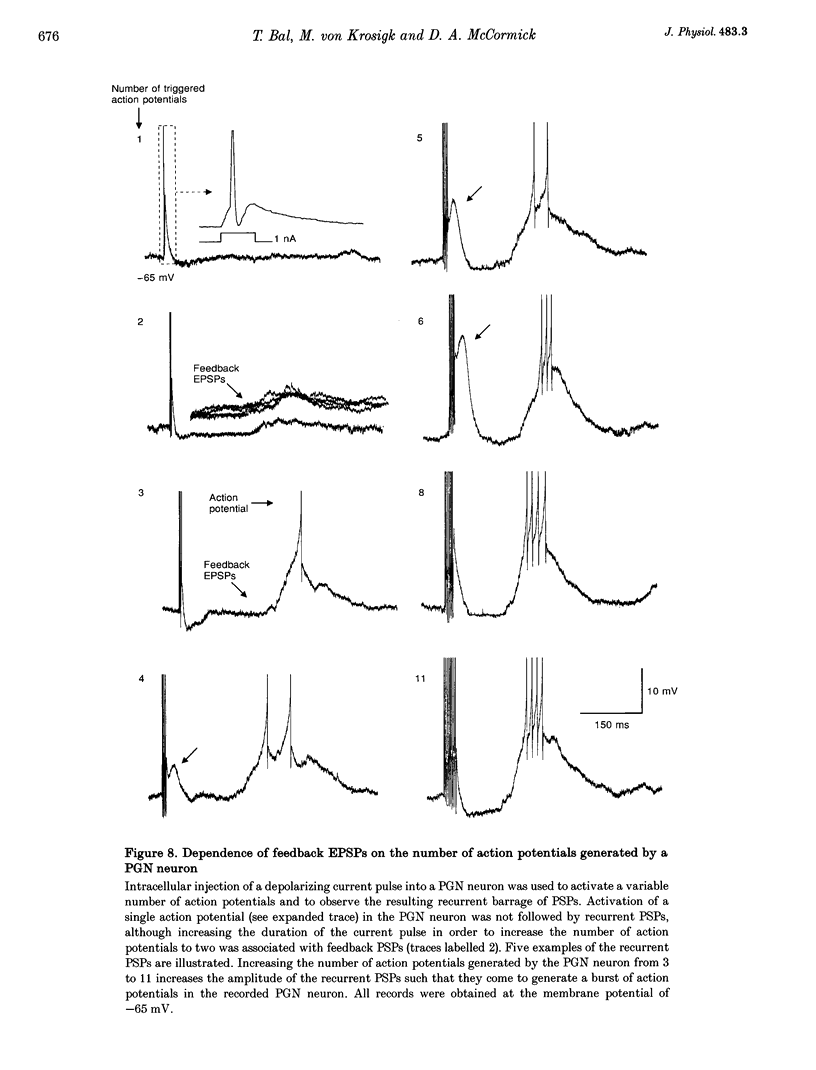
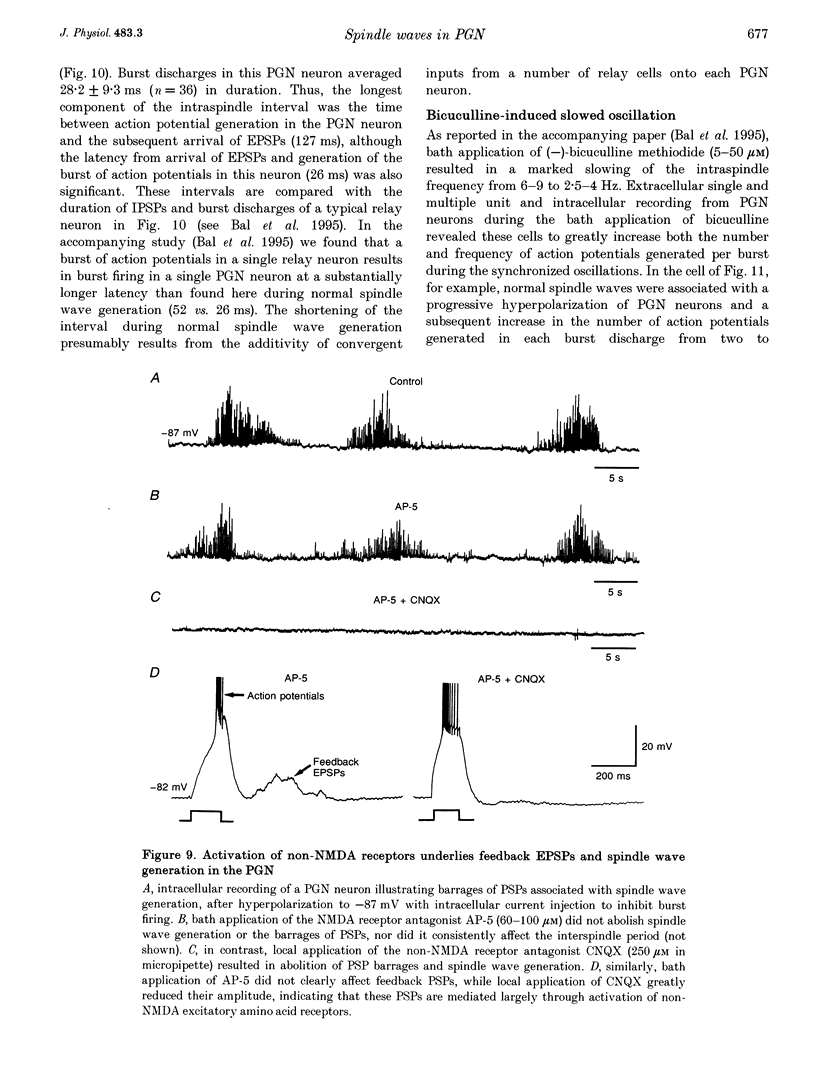
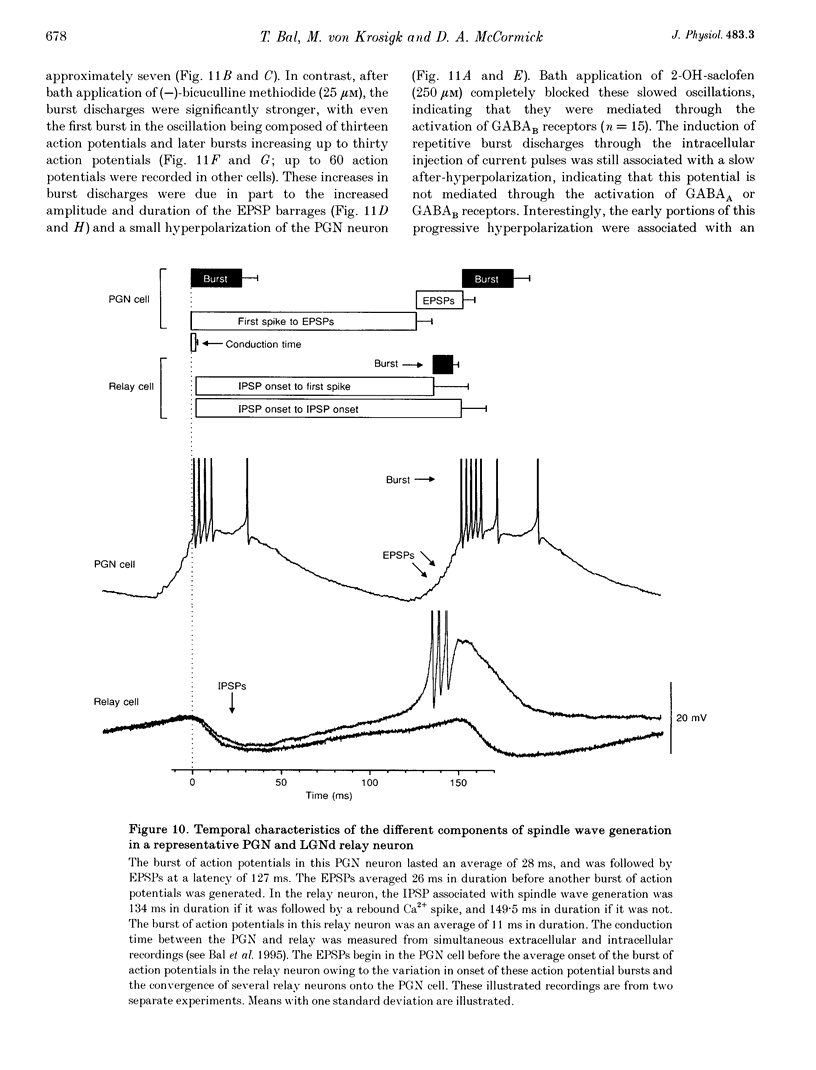
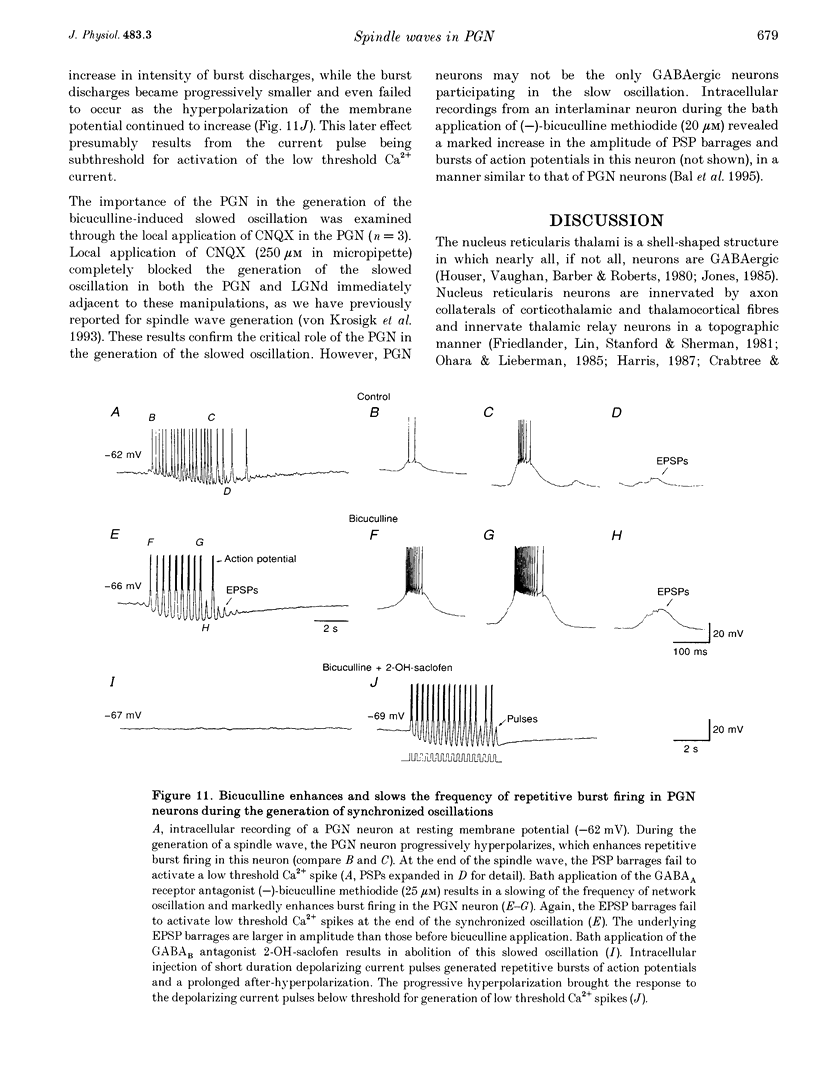
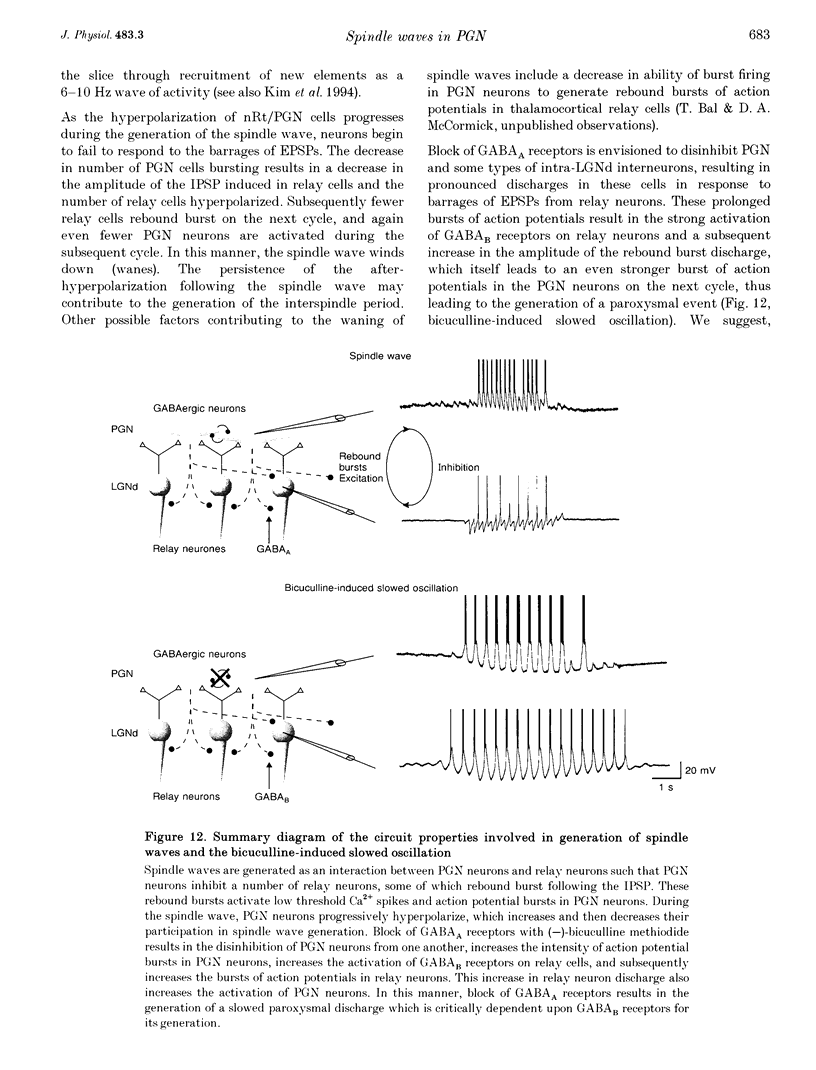
1. The cellular mechanisms by which neurons of the ferret perigeniculate nucleus (PGN) participate in the generation of spindle waves and slowed absence seizure-like oscillations were investigated with intracellular and extracellular recording techniques in geniculate slices maintained in vitro. 2. During spindle wave generation, PGN neurons generated repetitive (2-9 Hz) high frequency (up to 500 Hz) burst discharges mediated by the activation of a low threshold Ca2+ spike by the arrival of barrages of excitatory postsynaptic potentials (EPSPs). In most PGN cells at resting membrane potentials (-60 to -70 mV) spindle waves were associated with a progressive hyperpolarization that persisted as a prolonged after-hyperpolarization. 3. The EPSPs occurring in PGN cells were highly synchronized with burst firing in the neighbouring portion of the dorsal lateral geniculate nucleus (LGNd) and were intermixed with short duration inhibitory postsynaptic potentials (IPSPs). After block of GABAergic receptors, the EPSPs occurring during the generation of spindle waves reversed polarity at around 0 mV. In addition, these EPSPs were completely blocked with the bath application of the non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), as was spindle wave generation in both the PGN and LGNd. 4. Slowing the intraspindle frequency to 2-4 Hz with pharmacological block of GABAA receptors resulted in a marked increase in the intensity of burst firing by PGN cells such that the number of action potentials per burst increased from a maximum of thirteen to a maximum of sixty. Block of GABAA receptors also resulted in a marked increase in the amplitude and duration of the EPSP barrages arriving from the relay laminae during generation of the slowed oscillation. 5. These findings indicate that spindle waves are generated in the ferret LGNd in vitro through an interaction between the GABAergic neurons of the PGN and relay neurons, such that burst firing in relay neurons activates a barrage of EPSPs and a subsequent low threshold Ca2+ spike in PGN cells. This activation of PGN neurons inhibits a substantial number of relay cells, a few of which rebound burst after this IPSP, thus starting the cycle again. Block of GABAA receptors results in a marked enhancement of activity in PGN cells through increased excitation from relay cells and disinhibition from neighbouring PGN cells. This increased activity in PGN neurons results in a markedly enhanced activation of GABAB receptors in relay neurons and the subsequent generation of paroxysmal activity that is similar to that associated with absence seizures.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avanzini G., de Curtis M., Panzica F., Spreafico R. Intrinsic properties of nucleus reticularis thalami neurones of the rat studied in vitro. J Physiol. 1989 Sep;416:111–122. doi: 10.1113/jphysiol.1989.sp017752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M., Gloor P. Interaction of cortex and thalamus in spike and wave discharges of feline generalized penicillin epilepsy. Exp Neurol. 1982 Apr;76(1):196–217. doi: 10.1016/0014-4886(82)90112-1. [DOI] [PubMed] [Google Scholar]
- Avoli M., Kostopoulos G. Participation of corticothalamic cells in penicillin-induced generalized spike and wave discharges. Brain Res. 1982 Sep 9;247(1):159–163. doi: 10.1016/0006-8993(82)91042-3. [DOI] [PubMed] [Google Scholar]
- Bal T., McCormick D. A. Mechanisms of oscillatory activity in guinea-pig nucleus reticularis thalami in vitro: a mammalian pacemaker. J Physiol. 1993 Aug;468:669–691. doi: 10.1113/jphysiol.1993.sp019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T., von Krosigk M., McCormick D. A. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995 Mar 15;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Bickford R. G., Ponomareff G., Thal L. J., Mandel R., Gage F. H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988 Nov;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Smith A., Berger S., Fisher L. J., Gage F. H. Petit mal epilepsy and parkinsonian tremor: hypothesis of a common pacemaker. Neuroscience. 1990;36(1):1–14. doi: 10.1016/0306-4522(90)90345-5. [DOI] [PubMed] [Google Scholar]
- Contreras D., Curró Dossi R., Steriade M. Electrophysiological properties of cat reticular thalamic neurones in vivo. J Physiol. 1993 Oct;470:273–294. doi: 10.1113/jphysiol.1993.sp019858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree John W., Killackey Herbert P. The Topographic Organization and Axis of Projection within the Visual Sector of the Rabbit's Thalamic Reticular Nucleus. Eur J Neurosci. 1989 Jan;1(1):94–109. doi: 10.1111/j.1460-9568.1989.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Paradis M., Roy J. P., Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J Neurophysiol. 1984 Jun;51(6):1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- Destexhe A., McCormick D. A., Sejnowski T. J. A model for 8-10 Hz spindling in interconnected thalamic relay and reticularis neurons. Biophys J. 1993 Dec;65(6):2473–2477. doi: 10.1016/S0006-3495(93)81297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domich L., Oakson G., Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically projecting and reticularis neurones. J Physiol. 1986 Oct;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Gloor P., Quesney L. F., Zumstein H. Pathophysiology of generalized penicillin epilepsy in the cat: the role of cortical and subcortical structures. II. Topical application of penicillin to the cerebral cortex and to subcortical structures. Electroencephalogr Clin Neurophysiol. 1977 Jul;43(1):79–94. doi: 10.1016/0013-4694(77)90198-5. [DOI] [PubMed] [Google Scholar]
- Harris R. M. Axon collaterals in the thalamic reticular nucleus from thalamocortical neurons of the rat ventrobasal thalamus. J Comp Neurol. 1987 Apr 15;258(3):397–406. doi: 10.1002/cne.902580308. [DOI] [PubMed] [Google Scholar]
- Hosford D. A., Clark S., Cao Z., Wilson W. A., Jr, Lin F. H., Morrisett R. A., Huin A. The role of GABAB receptor activation in absence seizures of lethargic (lh/lh) mice. Science. 1992 Jul 17;257(5068):398–401. doi: 10.1126/science.1321503. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Vaughn J. E., Barber R. P., Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980 Nov 3;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Huguenard J. R., Prince D. A. A novel T-type current underlies prolonged Ca(2+)-dependent burst firing in GABAergic neurons of rat thalamic reticular nucleus. J Neurosci. 1992 Oct;12(10):3804–3817. doi: 10.1523/JNEUROSCI.12-10-03804.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol. 1984 Apr;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos G., Gloor P., Pellegrini A., Gotman J. A study of the transition from spindles to spike and wave discharge in feline generalized penicillin epilepsy: microphysiological features. Exp Neurol. 1981 Jul;73(1):55–77. doi: 10.1016/0014-4886(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Kostopoulos G., Gloor P., Pellegrini A., Siatitsas I. A study of the transition from spindles to spike and wave discharge in feline generalized penicillin epilepsy: EEG features. Exp Neurol. 1981 Jul;73(1):43–54. doi: 10.1016/0014-4886(81)90044-3. [DOI] [PubMed] [Google Scholar]
- Liu Z., Vergnes M., Depaulis A., Marescaux C. Involvement of intrathalamic GABAB neurotransmission in the control of absence seizures in the rat. Neuroscience. 1992;48(1):87–93. doi: 10.1016/0306-4522(92)90340-8. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Wang Z. Serotonin and noradrenaline excite GABAergic neurones of the guinea-pig and cat nucleus reticularis thalami. J Physiol. 1991 Oct;442:235–255. doi: 10.1113/jphysiol.1991.sp018791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C., Steriade M., Deschênes M. Absence of spindle oscillations in the cat anterior thalamic nuclei. Brain Res. 1985 May 13;334(1):169–171. doi: 10.1016/0006-8993(85)90581-5. [DOI] [PubMed] [Google Scholar]
- Ohara P. T., Lieberman A. R. The thalamic reticular nucleus of the adult rat: experimental anatomical studies. J Neurocytol. 1985 Jun;14(3):365–411. doi: 10.1007/BF01217752. [DOI] [PubMed] [Google Scholar]
- Roy J. P., Clercq M., Steriade M., Deschênes M. Electrophysiology of neurons of lateral thalamic nuclei in cat: mechanisms of long-lasting hyperpolarizations. J Neurophysiol. 1984 Jun;51(6):1220–1235. doi: 10.1152/jn.1984.51.6.1220. [DOI] [PubMed] [Google Scholar]
- Schwartz M. L., Meinecke D. L. Early expression of GABA-containing neurons in the prefrontal and visual cortices of rhesus monkeys. Cereb Cortex. 1992 Jan-Feb;2(1):16–37. doi: 10.1093/cercor/2.1.16. [DOI] [PubMed] [Google Scholar]
- Snead O. C., 3rd Evidence for GABAB-mediated mechanisms in experimental generalized absence seizures. Eur J Pharmacol. 1992 Mar 31;213(3):343–349. doi: 10.1016/0014-2999(92)90623-c. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Crunelli V. On the properties and origin of the GABAB inhibitory postsynaptic potential recorded in morphologically identified projection cells of the cat dorsal lateral geniculate nucleus. Neuroscience. 1989;33(1):23–33. doi: 10.1016/0306-4522(89)90307-2. [DOI] [PubMed] [Google Scholar]
- Spreafico R., Battaglia G., Frassoni C. The reticular thalamic nucleus (RTN) of the rat: cytoarchitectural, Golgi, immunocytochemical, and horseradish peroxidase study. J Comp Neurol. 1991 Feb 15;304(3):478–490. doi: 10.1002/cne.903040311. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschênes M., Domich L., Mulle C. Abolition of spindle oscillations in thalamic neurons disconnected from nucleus reticularis thalami. J Neurophysiol. 1985 Dec;54(6):1473–1497. doi: 10.1152/jn.1985.54.6.1473. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G., Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987 Jan;57(1):260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- Steriade M., Domich L., Oakson G. Reticularis thalami neurons revisited: activity changes during shifts in states of vigilance. J Neurosci. 1986 Jan;6(1):68–81. doi: 10.1523/JNEUROSCI.06-01-00068.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D. A., Sejnowski T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993 Oct 29;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Activity-dependent disinhibition. I. Repetitive stimulation reduces IPSP driving force and conductance in the hippocampus in vitro. J Neurophysiol. 1989 Mar;61(3):501–511. doi: 10.1152/jn.1989.61.3.501. [DOI] [PubMed] [Google Scholar]
- Uhlrich D. J., Cucchiaro J. B., Humphrey A. L., Sherman S. M. Morphology and axonal projection patterns of individual neurons in the cat perigeniculate nucleus. J Neurophysiol. 1991 Jun;65(6):1528–1541. doi: 10.1152/jn.1991.65.6.1528. [DOI] [PubMed] [Google Scholar]
- Yen C. T., Conley M., Hendry S. H., Jones E. G. The morphology of physiologically identified GABAergic neurons in the somatic sensory part of the thalamic reticular nucleus in the cat. J Neurosci. 1985 Aug;5(8):2254–2268. doi: 10.1523/JNEUROSCI.05-08-02254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M., Bal T., McCormick D. A. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993 Jul 16;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]



