Abstract
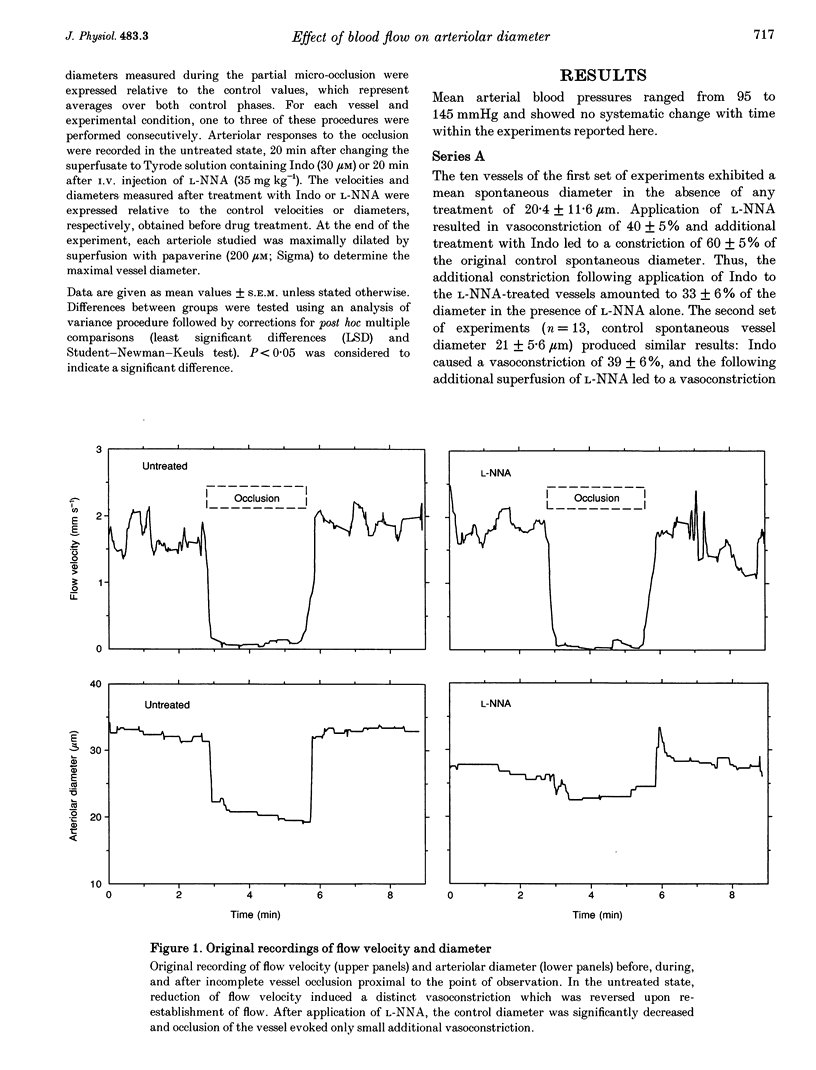
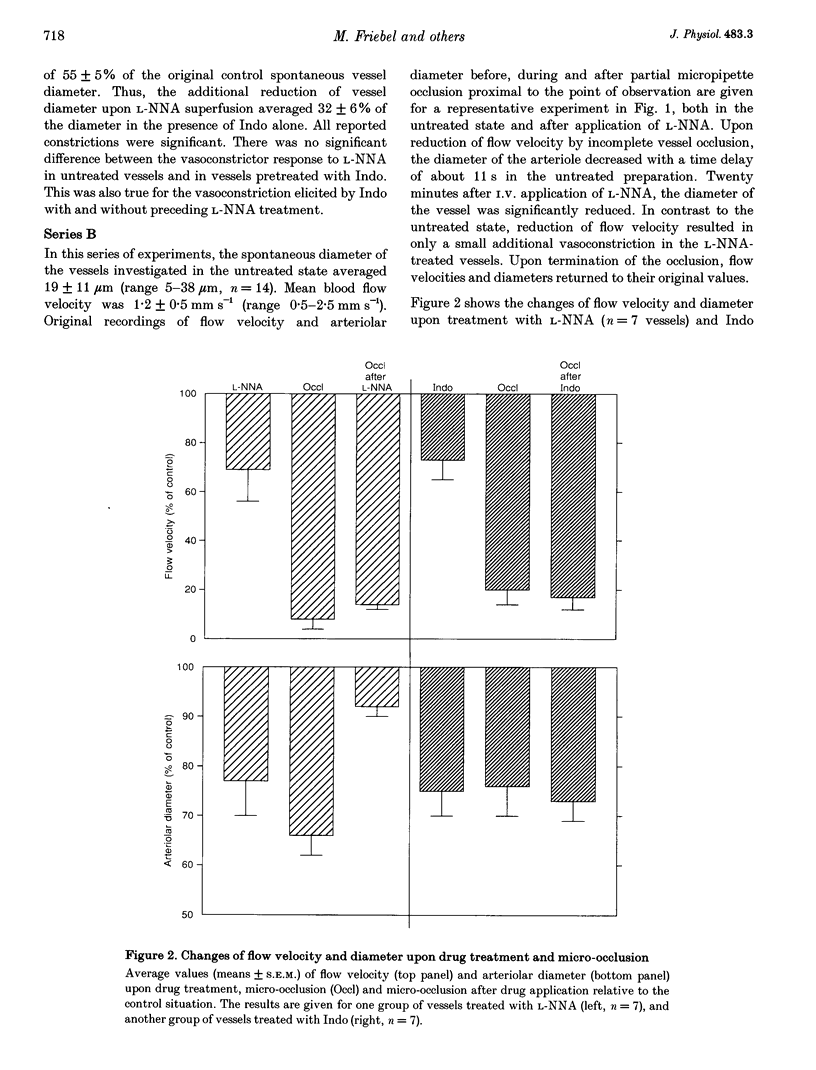
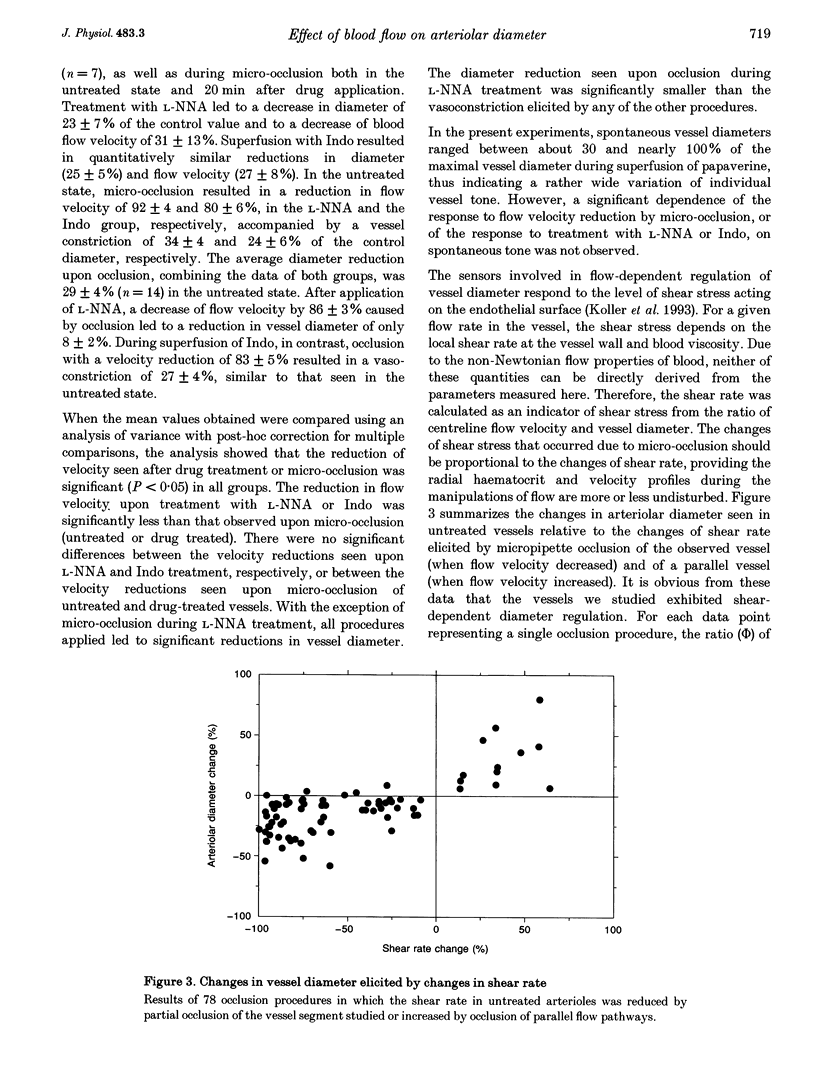
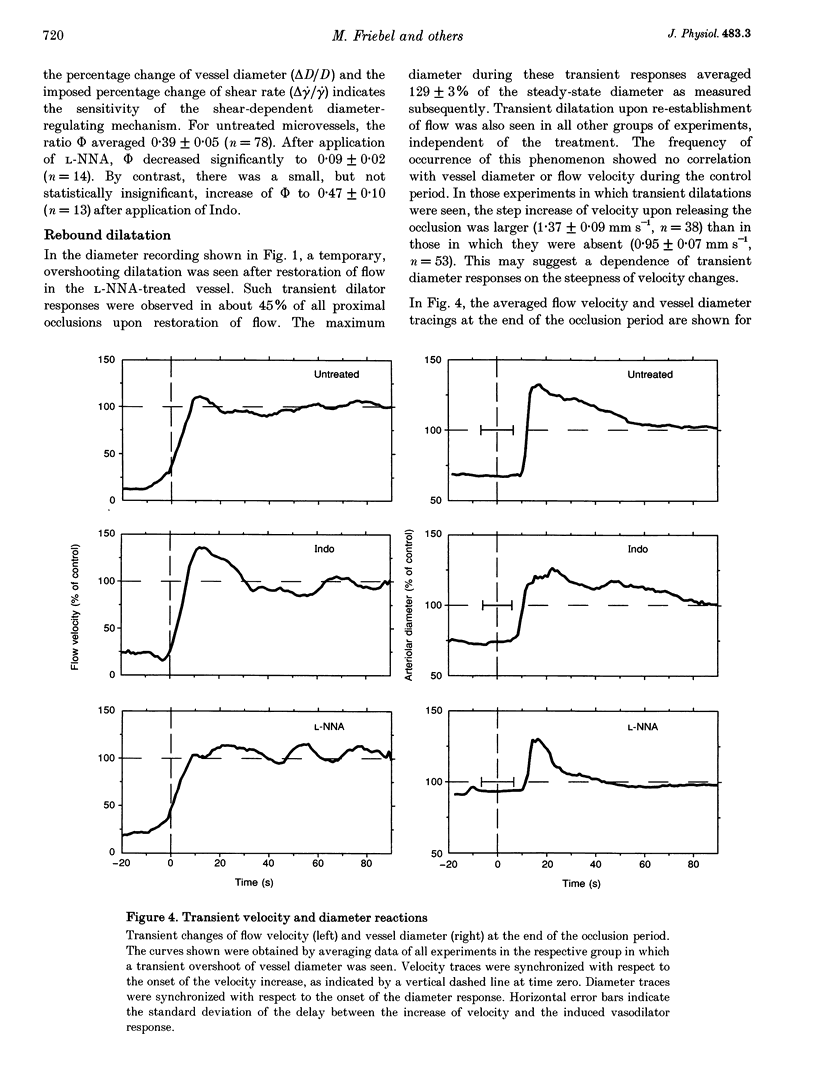
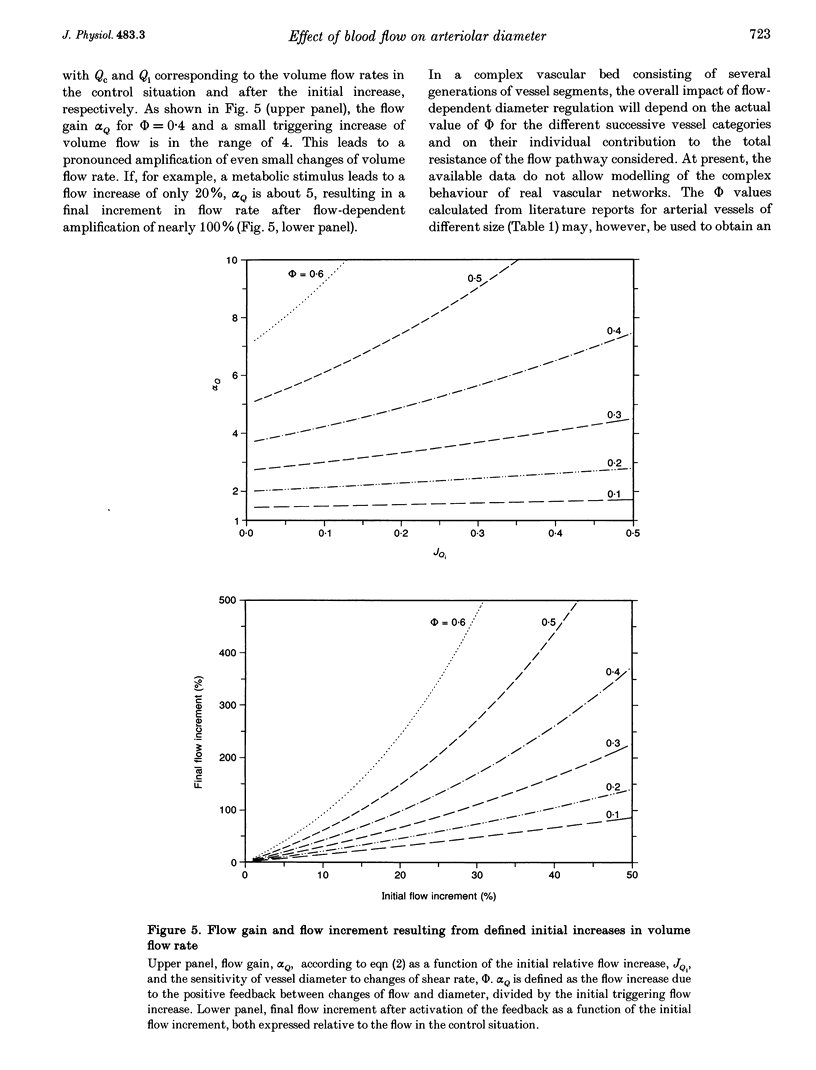
1. Arteriolar diameter in the resting rat spinotrapezius muscle was studied by intravital video microscopy before and after blockade of the L-arginine-EDRF (NG-nitro-L-arginine, L-NNA) or the cyclo-oxygenase-prostacyclin (indomethacin) pathway. Blockade of either pathway leads to a decrease of arteriolar diameter of 25-40%, while the combined blockade of both results in vasoconstriction of 50-60%. 2. Alteration of blood flow velocity elicited by partial micropipette occlusion induces corresponding changes of vessel diameter. The flow-dependent diameter response is reduced by about 80% by L-NNA. By contrast, blockade of prostanoid production shows no significant influence on vessel response to blood flow alteration in the range tested. 3. Transient overshooting vasodilatation is seen for about 1 min following the sudden restoration of flow velocity subsequent to occlusion. In contrast to the initial phase of this response, the late phase is blocked by L-NNA. 4. The findings suggest that basal release of endothelium-derived relaxing factor (EDRF) and prostanoids leads to additive and independent dilator effects, and that flow-dependent diameter changes are primarily mediated by EDRF. 5. If present data are compared with literature reports, it appears that arterial flow sensitivity is most pronounced in the smallest vessels. In such vessels, flow-dependent dilatation will amplify even small changes of volume flow by more than four times.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan J. A., Joyce E. H., Wellman G. C. Flow-dependent dilation in a resistance artery still occurs after endothelium removal. Circ Res. 1988 Nov;63(5):980–985. doi: 10.1161/01.res.63.5.980. [DOI] [PubMed] [Google Scholar]
- Faraci F. M. Role of endothelium-derived relaxing factor in cerebral circulation: large arteries vs. microcirculation. Am J Physiol. 1991 Oct;261(4 Pt 2):H1038–H1042. doi: 10.1152/ajpheart.1991.261.4.H1038. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogerwerf N., van der Linden P. J., Westerhof N., Sipkema P. A new mounting technique for perfusion of isolated small arteries: the effects of flow and oxygen on diameter. Microvasc Res. 1992 Jul;44(1):49–60. doi: 10.1016/0026-2862(92)90101-t. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Harbison R. G., Wood K. S., Kadowitz P. J. Activation of purified soluble guanylate cyclase by endothelium-derived relaxing factor from intrapulmonary artery and vein: stimulation by acetylcholine, bradykinin and arachidonic acid. J Pharmacol Exp Ther. 1986 Jun;237(3):893–900. [PubMed] [Google Scholar]
- Inoue T., Tomoike H., Hisano K., Nakamura M. Endothelium determines flow-dependent dilation of the epicardial coronary artery in dogs. J Am Coll Cardiol. 1988 Jan;11(1):187–191. doi: 10.1016/0735-1097(88)90188-x. [DOI] [PubMed] [Google Scholar]
- Kaley G., Koller A., Rodenburg J. M., Messina E. J., Wolin M. S. Regulation of arteriolar tone and responses via L-arginine pathway in skeletal muscle. Am J Physiol. 1992 Apr;262(4 Pt 2):H987–H992. doi: 10.1152/ajpheart.1992.262.4.H987. [DOI] [PubMed] [Google Scholar]
- Klotz K. F., Pries A. R., Jepsen H., Gossrau R., Gaehtgens P. A new approach to intravital videomicroscopy of rat spinotrapezius muscle. Int J Microcirc Clin Exp. 1991 Aug;10(3):205–218. [PubMed] [Google Scholar]
- Koller A., Kaley G. Endothelial regulation of wall shear stress and blood flow in skeletal muscle microcirculation. Am J Physiol. 1991 Mar;260(3 Pt 2):H862–H868. doi: 10.1152/ajpheart.1991.260.3.H862. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol. 1990 Mar;258(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1990.258.3.H916. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990 Aug;67(2):529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- Koller A., Sun D., Kaley G. Role of shear stress and endothelial prostaglandins in flow- and viscosity-induced dilation of arterioles in vitro. Circ Res. 1993 Jun;72(6):1276–1284. doi: 10.1161/01.res.72.6.1276. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Kuo L., Chilian W. M., Davis M. J. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991 Dec;261(6 Pt 2):H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- Lie M., Sejersted O. M., Kiil F. Local regulation of vascular cross section during changes in femoral arterial blood flow in dogs. Circ Res. 1970 Nov;27(5):727–737. doi: 10.1161/01.res.27.5.727. [DOI] [PubMed] [Google Scholar]
- Lindén A., Eriksson M., Hansen S., Uvnäs-Moberg K. Suckling-induced release of cholecystokinin into plasma in the lactating rat: effects of abdominal vagotomy and lesions of central pathways concerned with milk ejection. J Endocrinol. 1990 Nov;127(2):257–263. doi: 10.1677/joe.0.1270257. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- OLSON R. M. IN VIVO BLOOD VISCOSITY AND HINDRANCE. Am J Physiol. 1964 May;206:955–961. doi: 10.1152/ajplegacy.1964.206.5.955. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pohl U., Herlan K., Huang A., Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol. 1991 Dec;261(6 Pt 2):H2016–H2023. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- Pohl U., Holtz J., Busse R., Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986 Jan;8(1):37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Pries A. R., Gaehtgens P. Digital video image-shearing device for continuous microvessel diameter measurement. Microvasc Res. 1987 Sep;34(2):260–267. doi: 10.1016/0026-2862(87)90060-4. [DOI] [PubMed] [Google Scholar]
- Shrier I., Magder S. Response of arterial resistance and critical pressure to changes in perfusion pressure in canine hindlimb. Am J Physiol. 1993 Dec;265(6 Pt 2):H1939–H1945. doi: 10.1152/ajpheart.1993.265.6.H1939. [DOI] [PubMed] [Google Scholar]
- Smiesko V., Khayutin V. M., Kozík J., Rogoza A. N. Flow-induced dilation of the dog gracilis muscle artery. Physiol Bohemoslov. 1987;36(4):289–300. [PubMed] [Google Scholar]
- Smiesko V., Lang D. J., Johnson P. C. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. Am J Physiol. 1989 Dec;257(6 Pt 2):H1958–H1965. doi: 10.1152/ajpheart.1989.257.6.H1958. [DOI] [PubMed] [Google Scholar]


