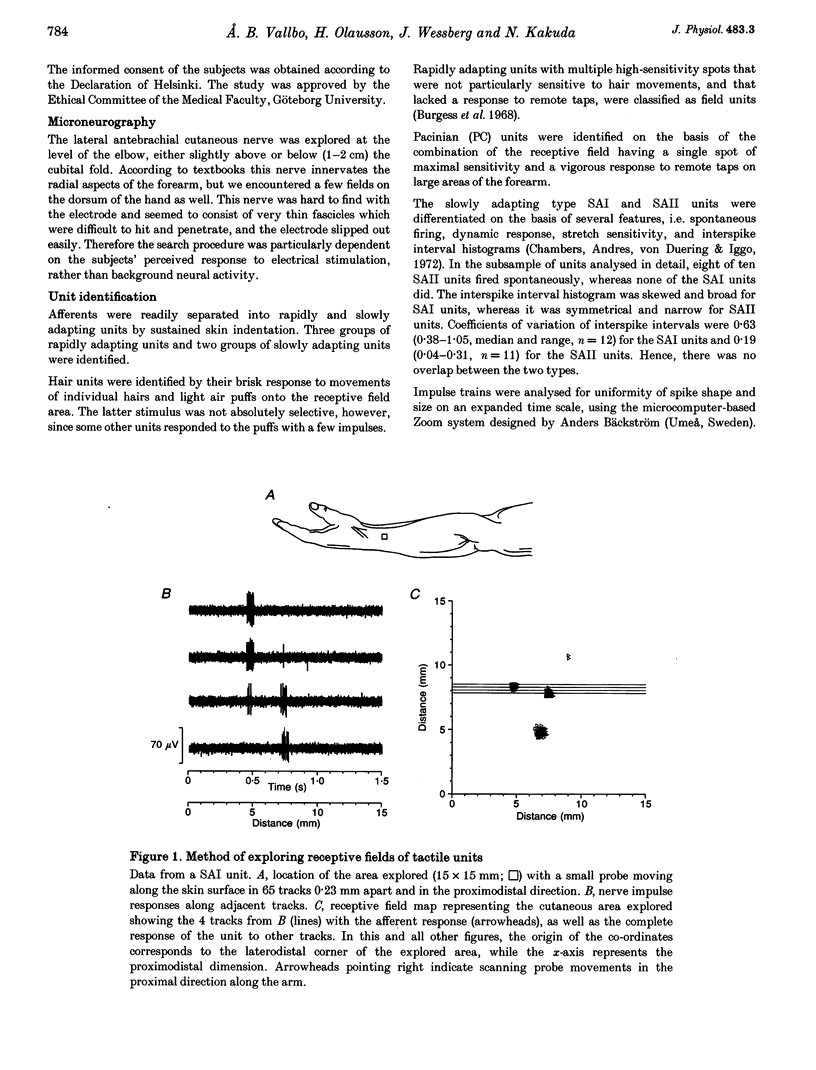
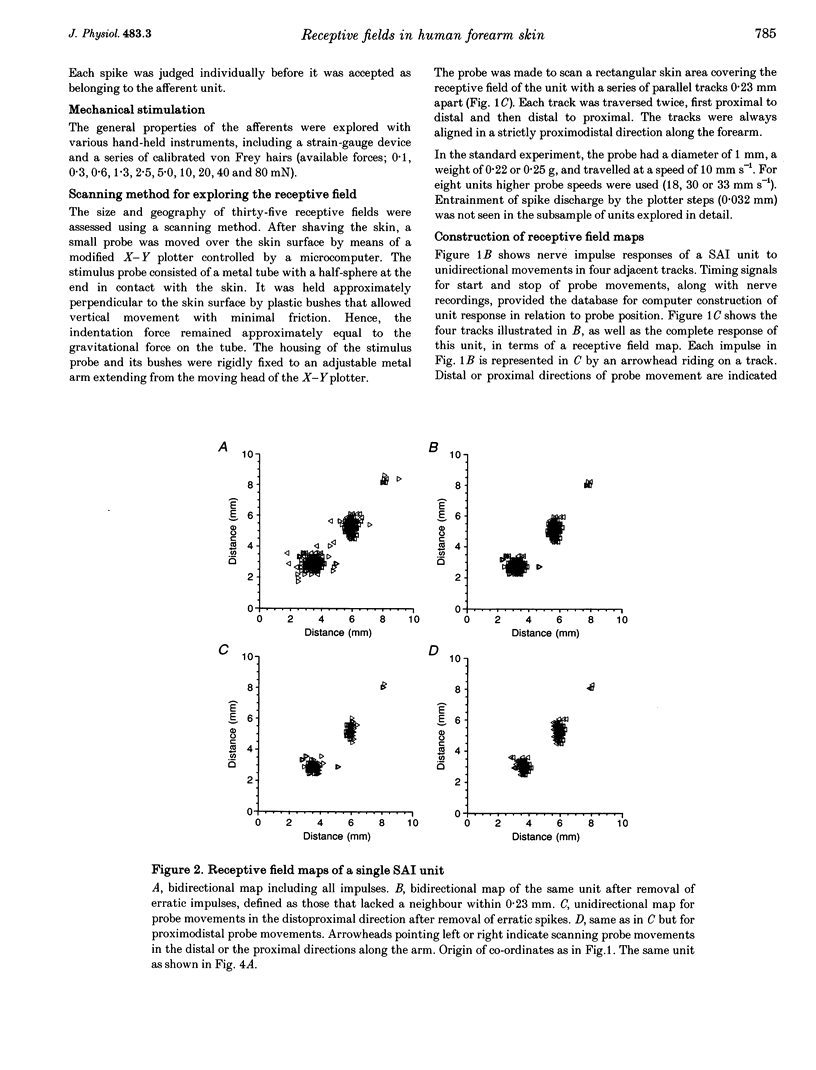
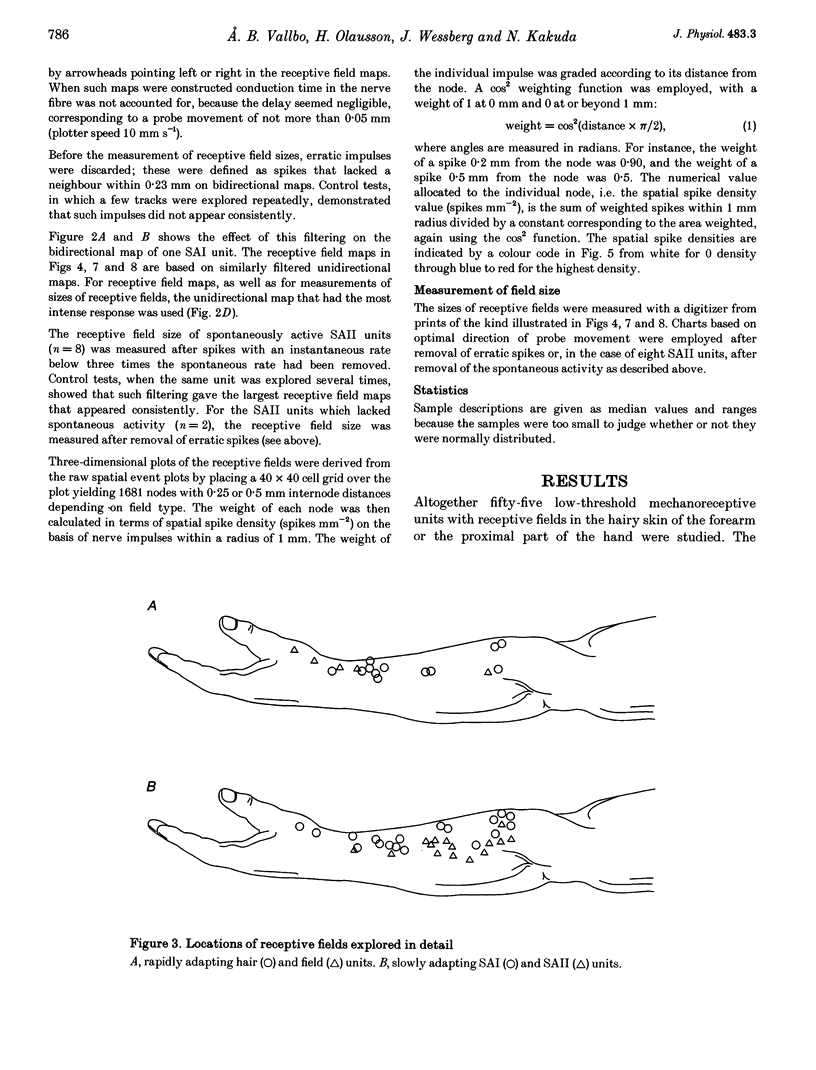
Abstract
1. Impulses in single nerve fibres from the lateral antebrachial cutaneous nerve were recorded using the microneurography technique in human subjects. 2. In a sample of fifty-five mechanoreceptive units with fast-conducting nerve fibres, five types were identified, i.e. SAI (slowly adapting type I, Merkel), SAII (slowly adapting type II, Ruffini), hair units, field units and Pacinian-type units. The latter three unit types were all rapidly adapting. 3. The detailed structure of thirty-five receptive fields of SAI, SAII, hair and field units was explored with a method which was objective and independent of the experimenter's skill and experience. A lightweight probe was used to scan the receptive field area in a series of tracks 0.23 mm apart while single-unit activity was recorded. 4. SAI fields were small and composed of two to four well-separated high-sensitivity spots and often, in addition, one minor spot of lower sensitivity. SAII units typically fired spontaneously at a low and regular rate. Most fields consisted of one single spot of high sensitivity with diffuse borders. The hair units innervated ten to thirty-three (or more) hairs, which were evenly distributed over a large area. The field units were characterized by a number of small and closely packed high-sensitivity spots with diffuse borders. A conservative estimate indicated eleven spots per unit. 5. The findings indicate that the sheet of mechanoreceptors on the skin of the forearm is distinctly different from that on the dorsum of the hand and in the face. It seems reasonable to assume that the former is more representative for the hairy skin covering the main parts of the body.
Full text
PDF
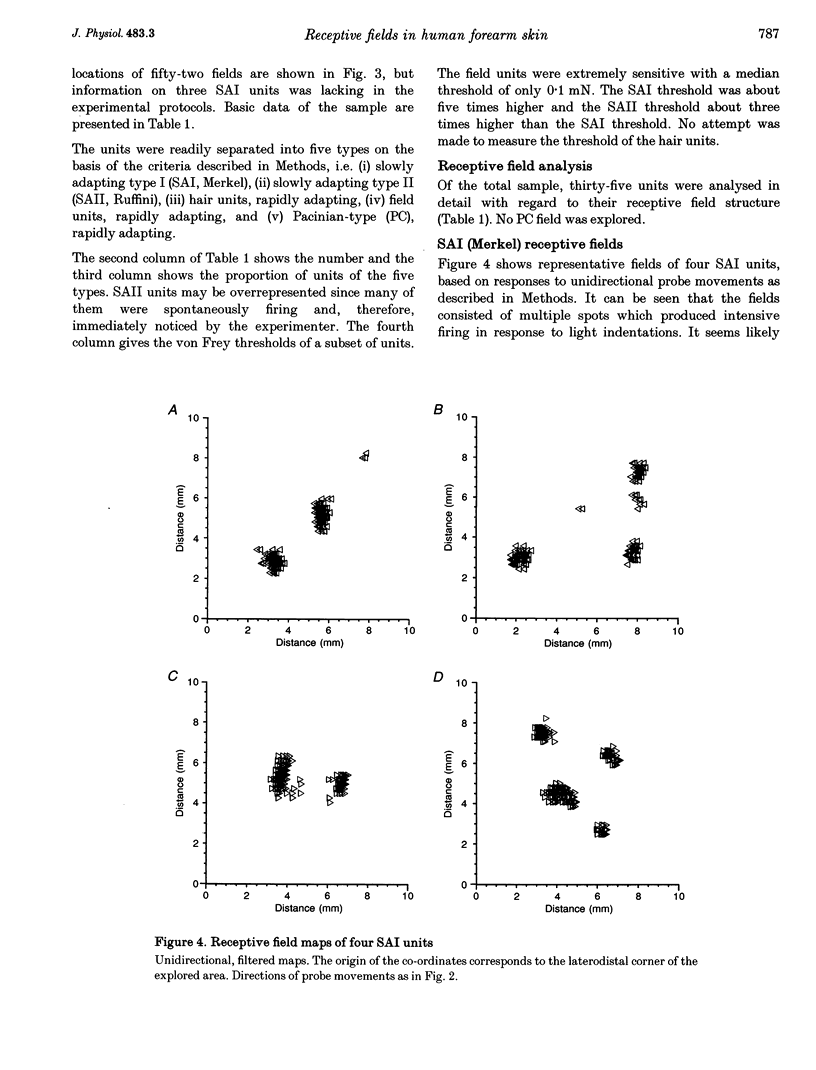
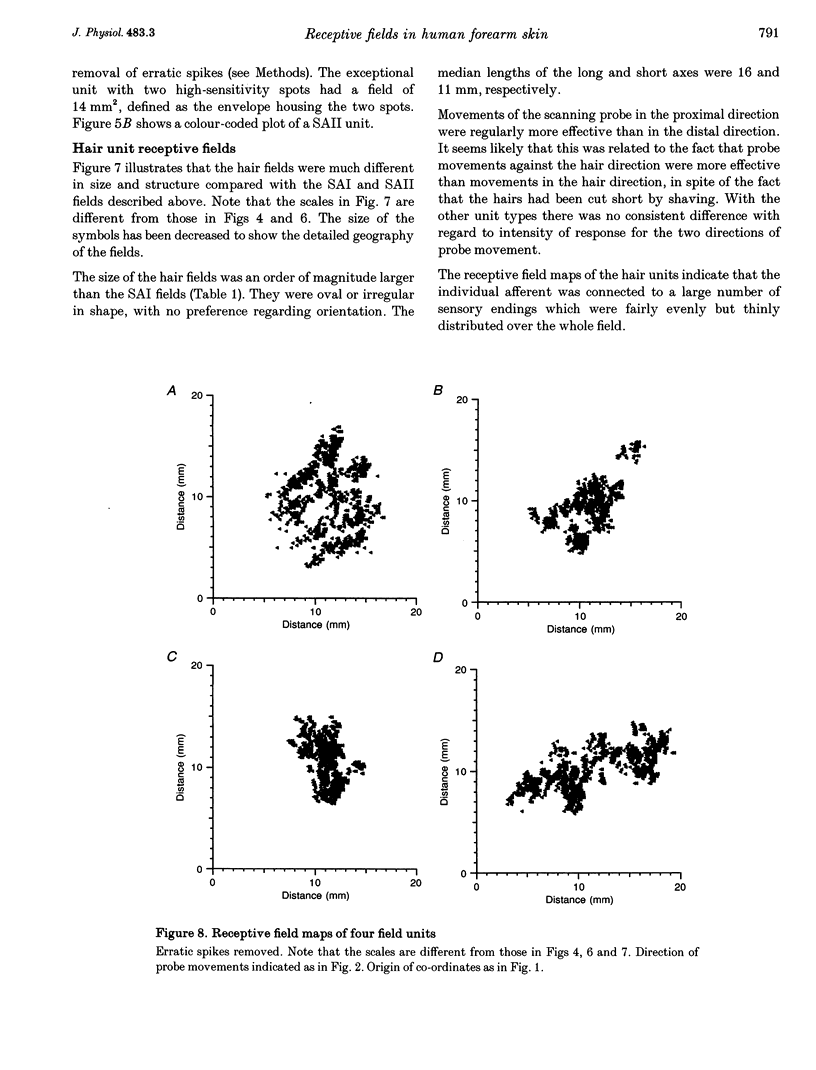




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. B., Stacy C., Cohen J. Agraphesthesia. A disorder of directional cutaneous kinesthesia or a disorientation in cutaneous space. J Neurol Sci. 1982 Mar;53(3):531–555. doi: 10.1016/0022-510x(82)90249-0. [DOI] [PubMed] [Google Scholar]
- Bessou P., Burgess P. R., Perl E. R., Taylor C. B. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J Neurophysiol. 1971 Jan;34(1):116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., RITCHIE J. M. Nonmedullated fibres in the saphenous nerve which signal touch. J Physiol. 1957 Dec 31;139(3):385–399. doi: 10.1113/jphysiol.1957.sp005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. B., Abbs J. H. Finger movement responses of cutaneous mechanoreceptors in the dorsal skin of the human hand. J Neurophysiol. 1991 Mar;65(3):657–670. doi: 10.1152/jn.1991.65.3.657. [DOI] [PubMed] [Google Scholar]
- Edin B. B. Quantitative analysis of static strain sensitivity in human mechanoreceptors from hairy skin. J Neurophysiol. 1992 May;67(5):1105–1113. doi: 10.1152/jn.1992.67.5.1105. [DOI] [PubMed] [Google Scholar]
- Hankey G. J., Edis R. H. The utility of testing tactile perception of direction of scratch as a sensitive clinical sign of posterior column dysfunction in spinal cord disorders. J Neurol Neurosurg Psychiatry. 1989 Mar;52(3):395–398. doi: 10.1136/jnnp.52.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGGO A. Cutaneous mechanoreceptors with afferent C fibres. J Physiol. 1960 Jul;152:337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S. Tactile sensibility in the human hand: receptive field characteristics of mechanoreceptive units in the glabrous skin area. J Physiol. 1978 Aug;281:101–125. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson R. S., Trulsson M., Olsson K. A., Westberg K. G. Mechanoreceptor activity from the human face and oral mucosa. Exp Brain Res. 1988;72(1):204–208. doi: 10.1007/BF00248518. [DOI] [PubMed] [Google Scholar]
- Johansson R. S., Vallbo A. B. Spatial properties of the population of mechanoreceptive units in the glabrous skin of the human hand. Brain Res. 1980 Feb 24;184(2):353–366. doi: 10.1016/0006-8993(80)90804-5. [DOI] [PubMed] [Google Scholar]
- Johnson K. O., Phillips J. R. A rotating drum stimulator for scanning embossed patterns and textures across the skin. J Neurosci Methods. 1988 Jan;22(3):221–231. doi: 10.1016/0165-0270(88)90043-x. [DOI] [PubMed] [Google Scholar]
- Järvilehto T., Hämäläinen H., Laurinen P. Characteristics of single mechanoreceptive fibres innervating hairy skin of the human hand. Exp Brain Res. 1976 May 10;25(1):45–61. doi: 10.1007/BF00237325. [DOI] [PubMed] [Google Scholar]
- Järvilehto T., Hämäläinen H., Soininen K. Peripheral neural basis of tactile sensations in man: II. Characteristics of human mechanoreceptors in the hairy skin and correlations of their activity with tactile sensations. Brain Res. 1981 Aug 24;219(1):13–27. doi: 10.1016/0006-8993(81)90264-x. [DOI] [PubMed] [Google Scholar]
- Konietzny F., Hensel H. Response of rapidly and slowly adapting mechanoreceptors and vibratory sensitivity in human hairy skin. Pflugers Arch. 1977 Mar 11;368(1-2):39–44. doi: 10.1007/BF01063452. [DOI] [PubMed] [Google Scholar]
- Kumazawa T., Perl E. R. Primate cutaneous sensory units with unmyelinated (C) afferent fibers. J Neurophysiol. 1977 Nov;40(6):1325–1338. doi: 10.1152/jn.1977.40.6.1325. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Nordin M., Hagbarth K. E. Mechanoreceptive units in the human infra-orbital nerve. Acta Physiol Scand. 1989 Feb;135(2):149–161. doi: 10.1111/j.1748-1716.1989.tb08562.x. [DOI] [PubMed] [Google Scholar]
- Nordin M. Low-threshold mechanoreceptive and nociceptive units with unmyelinated (C) fibres in the human supraorbital nerve. J Physiol. 1990 Jul;426:229–240. doi: 10.1113/jphysiol.1990.sp018135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrsell U., Olausson H. Spatial cues serving the tactile directional sensibility of the human forearm. J Physiol. 1994 Aug 1;478(Pt 3):533–540. doi: 10.1113/jphysiol.1994.sp020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. R., Johansson R. S., Johnson K. O. Representation of braille characters in human nerve fibres. Exp Brain Res. 1990;81(3):589–592. doi: 10.1007/BF02423508. [DOI] [PubMed] [Google Scholar]
- Shea V. K., Perl E. R. Sensory receptors with unmyelinated (C) fibers innervating the skin of the rabbit's ear. J Neurophysiol. 1985 Sep;54(3):491–501. doi: 10.1152/jn.1985.54.3.491. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Johansson R. S. Properties of cutaneous mechanoreceptors in the human hand related to touch sensation. Hum Neurobiol. 1984;3(1):3–14. [PubMed] [Google Scholar]
- Vallbo A., Olausson H., Wessberg J., Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993 Nov 19;628(1-2):301–304. doi: 10.1016/0006-8993(93)90968-s. [DOI] [PubMed] [Google Scholar]
- Wall P. D., Noordenbos W. Sensory functions which remain in man after complete transection of dorsal columns. Brain. 1977 Dec;100(4):641–653. doi: 10.1093/brain/100.4.641. [DOI] [PubMed] [Google Scholar]