Abstract
Oral Squamous Cell Carcinoma (OSCC) it was reported to be the 6th on the list of human malignant neoplasms responsible for high morbidity and mortality worldwide. We conducted a retrospective study between 2009-2019, investigating 50 such cancers hospitalized and diagnosed during this period in our institution. The purpose of the study was to establish a clinical-morphological profile of this type of cancer developed in the geographical area served by our institution. The epidemiological study highlighted the predominance of cases in men over 50 years old, mainly affecting the tongue, followed by the lips and oral floor. The histopathological study showed the prevalence of conventional cases of OSCC (70%) and the rest of the cases belonging to rarer forms (acantholytic-18%, verrucous-6%, basaloid-4% and sarcomatoid-2%). In terms of the degree of differentiation, the moderately differentiated cases prevailed (64%) and according to the TNM clinical stage, most cases were diagnosed in stage II (36%) and IV (26%). 70% of investigated cases presented muscle invasion and 38% perineural invasion. Our investigation highlighted the existence of particular morpho-clinical profiles depending on the tumor topography. Thus, tumors developed at the tongue level reached the maximum frequency in the 6th decade of life, being absent in the 8th decade and most often associated muscle invasion and perineural invasion, being diagnosed in advanced pTNM stages.
Keywords: Epidemiology , Histopathology , Oral cavity , Oral squamous cell carcinomas
Introduction
In the European demographic space, malignant neoplasms of the head and neck amount to 4% of all cancers. In 2012, there were approximately 140,000 new cases of oral squamous cell carcinoma and around 63,500 deaths due to this form of oral malignancy [1].
According to the latest statistics provided by GLOBOCAN regarding malignant neoplasms of the oral cavity and lips, this form of cancer is the sixth most common worldwide, reporting for the year 2020, approximately 377,713 new cases of oral squamous cell carcinoma and around 177,757 deaths due to them [2].
Squamous cell carcinoma of the mucosa of the oral cavity has an exceptional morbidity and mortality considering the many functions in which this mucosa participates. Thus, the 5-year survival in localized clinical forms of the disease is between 75-84%. In advanced clinical forms accompanied by metastasis, the survival over the same period of time is 20% (location: floor of the oral cavity) and 36% (location: tongue) [3].
Despite the progress achieved in the last period of time, the showing up of improved diagnostic techniques, the discovery of new therapies, as well as new management protocols for head and neck cancer, the prognosis of these patients still remains reserved, not changing significantly in the last three decades, the 5-year survival rate remains around 50-60% [4].
Over time, numerous studies have been carried out regarding the identification of the most feasible prognostic, clinical and histopathological parameters of oral squamous cell carcinomas, highlighting as more important: tumor size, lymph nodal and distant dissemination status (pTNM clinical staging), along with the histopathological evaluation of the perineural/ lymphovascular invasion and, respectively, of the status of the resection margins.
Considering the aforementioned, the main aim of the study is to outline a particular epidemiological and histopathological profile of oral squamous cell carcinomas diagnosed and treated in the County Emergency Clinical Hospital of Craiova No. 1, in the last decade.
Materials and Methods
The present study was carried out by analyzing 50 cases of oral squamous cell carcinoma, between 2009-2019, of some patients hospitalized in the Oro-Maxillo-Facial Surgery Clinic and diagnosed histopathologically in the Pathology Laboratory of the County Emergency Clinical Hospital No. 1 of Craiova.
The study of the medical records of the targeted patients allowed us to collect epidemiological data, among which we retained: age, gender, topography of tumor lesions (jugal mucosa, oral floor, tongue, gingival mucosa, palatal fibromucosa, oropharynx), pTNM staging, dominant clinical appearance of the tumor lesions, the presence or absence of clinically detectable nodal metastases, as well as data related to the local and regional extension of the tumor. Last but not least, the detailed study of the medical records allowed us to extract some precious data regarding certain essential risk and promoters factors of this oral pathology: the status of oral hygiene, inadequate working conditions and vicious habits.
We collected from the records of the Pathology Laboratory of the same hospital the corresponding histopathological data of these patients, such as: the histopathological subtype of oral squamous cell carcinoma according to the WHO criteria [5], the degree of malignancy, the presence and absence of bone invasion, the presence and absence of muscular invasion and perineural invasion, the presence and absence of lymph node metastases, the pattern of tumor invasion, histological grading systems [6, 7], pTNM staging, the presence and absence of areas of necrosis and inflammatory infiltrate, the presence and absence of vascular invasion and areas of ulceration, association or not of some precancerous lesions and last but not least the status of the resection margins.
The collected data were entered into an Excel and we analyzed them statistically using SPSS software version 12 and descriptive statistical methods that present the quantitative variables in the form of absolute and relative frequencies. To compare the variables, the Chi square test was used, with p<0.05.
The study was approved by the Ethics and Deontology Committee of the University of Medicine and Pharmacy of Craiova, (no. 198/18.10.2022) and all patients signed a written informed consent regarding their participation in the study.
Results
Based on our research, from the histopathological perspective, the conventional well-differentiated form of oral squamous cell carcinomas (OSCC) most often appeared like invasive islands of malignant Malpighian cells.
In our casuistry the conventional well-differentiated squamous cell carcinoma developed to the following locations: lip, oral floor, tongue, alveolar ridge and intermaxillary commissure. In addition, the conventional moderate differentiated squamous carcinomas, beside the mentioned locations, it appeared on the gingival mucosa and on the palate.
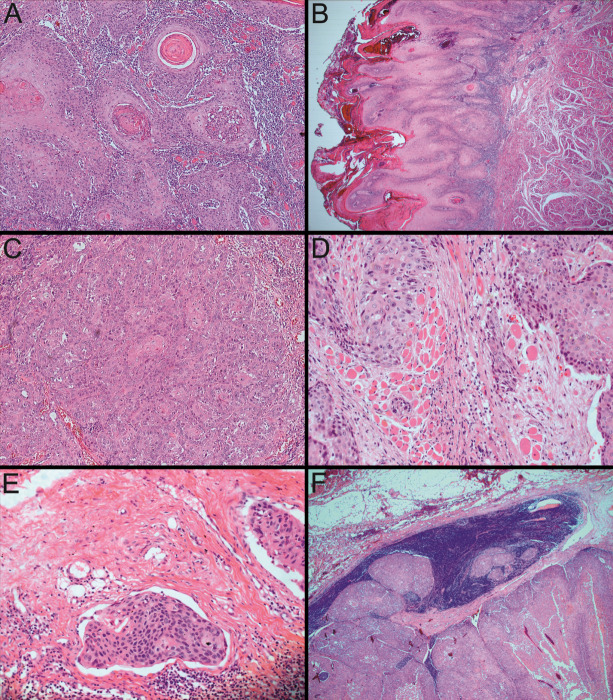
The definitory characteristic of the well-differentiated form is that the tumor proliferation achieves nearly completely the cytoarchitecture of normal Malpighian epithelium, but what is still remaining as obvious as that, are the attributes of malignancy such as: dyskeratosis of the tumor cells, keratin pearls development, invasion to such an extent in the superficial underlying stroma, the polarity of the epithelial cells tends to get lost, the basement membrane of the lip mucosa broke apart and this phenomenon contributed to the extent of invasion, atypical cells are minimal and the nuclear-cytoplasmic ratio is in favor of the nucleoli (Figure 1A).
Figure 1.
Oral Squamous Cell Carcinoma (OSCC).
A. Lip with conventional well-differentiated SCC, neoplastic proliferations with squamous epithelial architecture and dyskeratosis with keratin pearls formation. HE staining, 100x;
B. Lip with verrucous SCC, neoplastic proliferation with abundant keratosis and parakeratosis in an acanthotic squamous epithelium accomplishing the “church spires” appearance. HE staining, 25x;
C. Tongue with conventional moderate-differentiated SCC, neoplastic proliferation with obvious malignancy appearances and few keratin pearls formation. HE staining, 100x;
D. Tongue with conventional moderate-differentiated SCC, invading the underlying striated muscle fiber bundles. HE staining, 200x;
E. Oral floor with conventional moderate-differentiated SCC, with vascular invasion. HE staining, 200x;
F. Oral floor with conventional moderate-differentiated SCC, developing locoregional lymph node metastases. HE staining, 25x.
Furthermore, the form of conventional well-differentiated lip squamous cell carcinoma from our case history, developed exclusively to males (4 cases) with ages between 72 and 86 years-old, from rural area, who had as vicious habits, risk and promoter factors smoking, long-term exposure to UV rays and bad oral hygiene. The other 28 cases of conventional carcinomas in male patients had other topography. As medical history, only one of them was diagnosed with diabetes type II and high blood pressure, the other two did not mention any known medical history. The tumors’ diameters were between 2-5cm. The stages pTNM they were diagnosed in were IVA for two of them and stage III and II for the remaining two male patients with the same form and location. Concerning the tumors’ invasive behavior, they all invaded the muscle in the forementioned cases. With regard to female patients with conventional oral squamous cell carcinoma, their number was of 3 cases and the locations were the lip one case, the tongue and palate, each with one case. Their ages ranged between 48 and 74 years and they had as risk and promoter factors: smoking, alcohol consumption and bad oral hygiene. From their medical history we retained a few pathologies, not primary correlated with the malignant lesions, such as: otomastoiditis, treated tuberculosis, hyperlipidemia, high blood pressure grade III.
The variant of verrucous OSCC was diagnosed in three cases, all male patients with ages ranging from 52 years to 67 years, all located on the lips. These patients had as risk and promoter factors the consumption of alcohol, smoking and bad oral hygiene. Through one of the patients’ medical history, we found the following pathologies, non-related directly with the malignancy: ischemic stroke, high blood pressure, operated gastric ulcer and aortic insufficiency. The diameters of the neoplasms were between 2 and 4cm and the pTNM stages of these cases were III for one of them and respectively, I for the other two. From the histopathological perspective, we noticed that the neoplastic proliferation was ample in keratosis and parakeratosis, with an acanthotic squamous epithelium accomplishing the so called “church spires” aspect (Figure 1B).
The features of Malpighian epithelium in conventional moderate differentiated carcinomas are not so evident because the attributes of malignancy become more and more obvious: there are few keratin pearls formation or singular cell keratinizations, the nuclear pleiomorphism is extending and the number of mitosis is increasing (Figure 1C). The lesions placed and developed on the tongue almost constantly presented the invasion of underlying striated muscle fiber bundles (Figure 1D) and in one case located on the oral floor it presented vascular invasion (Figure 1E), these features marking the changing in aggressiveness. As another marker of aggressiveness, we also spotted locoregional lymph node metastases on a case located on the oral floor, too (Figure 1F).
The acantholytic form of oral squamous cell carcinomas, as a particular form of moderate differentiated squamous carcinoma, were found in a total number of 9 cases, from which 6 cases in male patients and 3 cases in female patients, with their ages in the interval of 40 and 80 years. Four of the six male patients developed this form on the oral floor, respectively the remaining cases, 1 on the tongue and 1 on the lip. By approaching the same trait in female cases, we observed that two of them formed these carcinomas on the tongue and one on the lip. In these patients’ instance, we can remember as risk factors smoking, alcohol consumption and bad oral hygiene. One of the forementioned patients of 69 years, had multiple pathologies, including: coronary cardiopathy, virus C hepatitis, obstructive hypertrophic cardiopathy and angina pectoris. The diameters of the tumor masses were between 1-7cm.
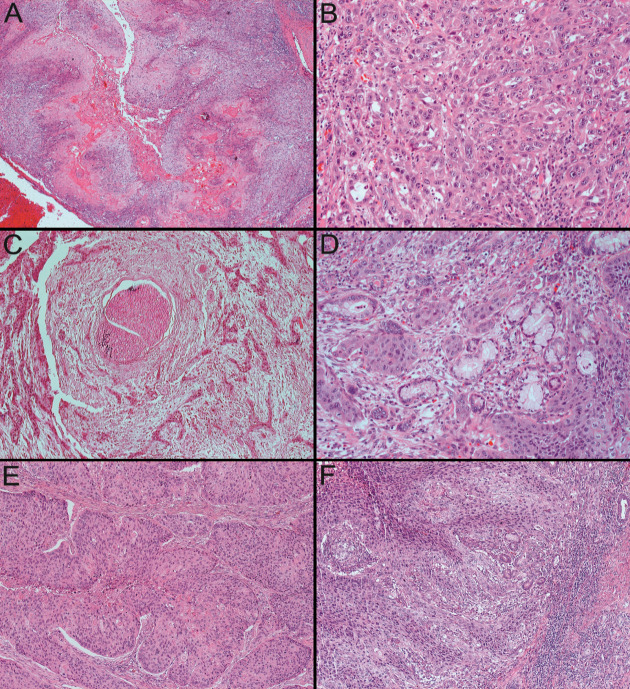
Histopathologically, we observed the insular aspect of the neoplastic proliferation which exhibited the pseudoglandular spaces in the central part as a consequence of the acantholysis process (Figure 2A).
Figure 2.
Oral Squamous Cell Carcinoma (OSCC)
A. Alveolar ridge with acantholytic SCC, characteristically having the presence of pseudoglandular spaces in the central part of insular neoplastic proliferations created by acantholysis process. HE staining, 25x;
B. Tongue with conventional poor-differentiated SCC, the neoplastic proliferations do no longer resembles squamous epithelium, keratinization is minimal and nuclear atypia are evident. HE staining, 200x;
C. Tongue with conventional poor-differentiated SCC, with perineural invasion. HE staining, 100x;
D. Tongue with conventional poor-differentiated SCC, invading minor salivary glands. HE staining, 200x:
E. Oral floor with basaloid SCC, in which the neoplastic cells at the periphery of the proliferations have a basaloid morphology (cubic-cylindrical cells with little cytoplasm and tachychromatic nuclei) and have a "palisaded" arrangement. HE staining, 100x;
F. Oral floor with sarcomatoid SCC, in which there were areas of conventional squamous carcinoma mixed with foci of malignant neoplastic proliferation with spindle cell morphology. HE staining, 100x
Concerning the attributes of conventional poor differentiated carcinomas, we can say that the cytoarchitecture of the lesion no longer resemblance to the normal squamous epithelium, in the microenvironment of the tumor the immature cells are predominant, there is minimal or even absent keratinization and the mitoses are abundant, atypical and typical (Figure 2B). Also, these forms tend to be more aggressive beginning to invade the perineural area and minor salivary glands (Figure 2C, Figure 2D).
From these cases of poor differentiated squamous carcinomas, arose as a particular pattern of malignancy, the basaloid form which appeared to two male patients, one of 58 years old and respectively 69 years old the other one. Both of these cases occurred to the oral floor mucosa. These patients did not mention having any of the risk and promoter factors, aside the bad oral hygiene, and the same situation goes for the medical history where they did not relate any. The diameters of the neoplasms were 4cm for the first case, with pTNM stage IVA and 3cm for the second one, with pTNM stage II.
Histopathologically speaking, the neoplastic proliferations were consisted of cubic and cylindrical cells, which had tachychromatic nuclei and “palisaded” array (Figure 2E).
Also, as a particular form of poor differentiated carcinoma there was one case of sarcomatoid pattern, diagnosed to a male patient of 65 years of age, located alike basaloid forms on the oral floor mucosa. The case belongs to pTNM stage III and had 2/3cm in diameter.
From the histopathological perspective there were blended areas of conventional squamous carcinoma with malignant neoplastic proliferation with spindle cell morphology (Figure 2F).
Our study included the analysis of 50 patients with oral squamous cell carcinoma (OSCC), 35 (70%) from rural areas and 15 (30%) from urban areas, with an average age of diagnosis of 60.82±11.61 years, with a variation between 39 and 86 years old. Most cases of oral squamous cell carcinomas were diagnosed in male patients, 44 of the cases (88%) and the remaining 6 cases were diagnosed in female patients (12%), resulting a gender ratio of 7.33:1 in favor of male patients (Table 1). Their most frequent locations are on the tongue 15 cases (30%), 14 cases on the lip (28%), 14 cases on the oral floor (28%), on the alveolar ridge 3 cases (6%), at the level of the palate 2 cases (4%), at the level of the gingival mucosa and intermaxillary commissure 1 case each (2% each) (Table 1). The tumor formations presented the following macroscopic clinical aspects: ulcerative in 27 cases (54%), ulcerative and exophytic lesions in 15 cases (30%) and exophytic lesions in 8 cases (16%) (Table 1). The sizes of the tumor lesions were between 1and 7cm, with an average of 2.87±1.25cm. The most frequently encountered histopathological subtypes were: conventional 35 cases (70%), acantholytic 9 cases (18%), verrucous 3 cases (6%), basaloid 2 cases (4%) and sarcomatoid 1 case (2%) (Table 1, Figure 1, Figure 2). Among the analysed cases, 15 cases (30%) were well differentiated (G1), 32 cases (64%) were moderately differentiated (G2) and 3 cases (6%) were poorly differentiated (G3) (Table 1, Figure 1, Figure 2). The most prevalent pTNM stage was stage II, 18 cases (36%) (Table 1). At the time of diagnosis, 6 patients (12%) presented detectable metastases on clinical examination, in 41 patients (82%) they were absent, and 3 patients (6%) presented reactive lymph nodal hypertrophies. Muscular invasion was present in 35 cases (70%) and absent in 15 cases (30%), perineural invasion present in 19 cases (38%) and absent in 31 cases (62%) and lymphatic invasion present in 6 cases (12%) and absent in 44 cases (88%) (Table 1, Figure 2). The resection margins of the tumor fragments were invaded in 23 cases (46%) and not invaded in 27 cases (54%) (Table 1). The histopathological analysis of the 50 cases of OSCC showed that more than half of them were classified in the Bryne grade II (38 cases=76%), and the rest in the Bryne grade I (8 cases=16%) and in the Bryne grade III (4 cases=8%) (Table 1).
Table 1.
Clinical and histopathological parameters of OSCC
|
Features |
Parameters |
No. of cases |
Percentage % |
|
Gender |
Females Males |
6 44 |
12% 88% |
|
Locations |
Lip Tongue Oral floor Alveolar ridge Gingival mucosa Intermaxillary commissure Palate |
14 15 14 3 1 1 2 |
28% 30% 28% 6% 2% 2% 4% |
|
Macroscopic aspect |
Ulcerative Ulcerative and exophytic Exophytic |
27 15 8 |
54% 30% 16% |
|
Resection margins |
Invaded Uninvaded |
23 27 |
46% 54% |
|
Degree of differentiation |
G1 G2 G3 |
15 32 3 |
30% 64% 6% |
|
pTNM staging |
I II III IVA |
9 18 10 13 |
18% 36% 20% 26% |
|
Histopathological subtype |
Conventional Acantholytic Verrucous Basaloid Sarcomatoid |
35 9 3 2 1 |
70% 18% 6% 4% 2% |
|
Muscular invasion |
Present Absent |
35 15 |
70% 30% |
|
Perineural invasion |
Present Absent |
19 31 |
38% 62% |
|
Lymphatic invasion |
Present Absent |
6 44 |
12% 88% |
|
Bryne grade |
I II III |
8 38 4 |
16% 76% 8% |
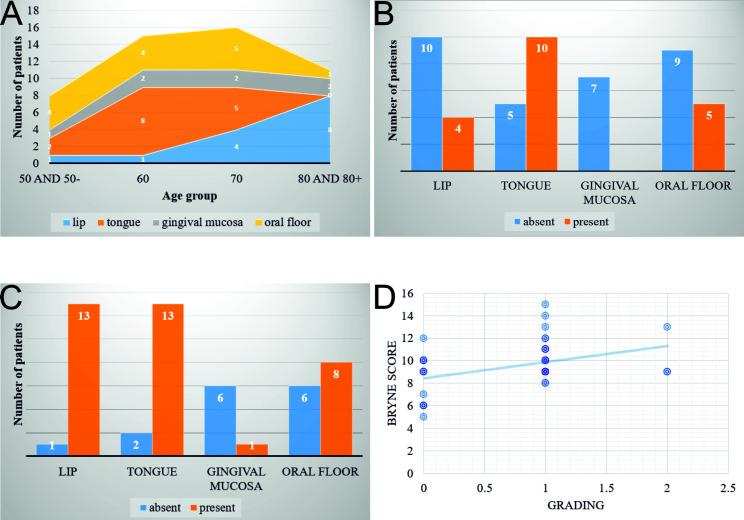
The statistical analysis highlighted that the distribution of cases according to age and location shows the following trends: cases located at the level of the tongue reached the maximum frequency in the 6th decade of life, being absent in the 8th decade; cases located on the lip showed an increase in incidence with aging, with a peak in the 8th decade; cases developed at the level of the oral floor had a linear distribution, with a sharp decrease in the 8th decade; in the case of gingival mucosa, after the age of 50 years, the distribution of cases was also linear. The differences between these distributions are statistically significant according to Fisher's Exact test, which indicated a p value of 0.0131 (Figure 3A).
Figure 3.
Oral Squamous Cell Carcinoma (OSCC)-statistical analysis
A. The distribution of cases stratified by age and location;
B. The distribution of perineural invasion stratified by location;
C. The distribution of muscular invasion stratified by location;
D. Linear regression analysis showing the influence of tumor grading on Bryne score of the investigated cases.
Regarding perineural invasion, we observed a greater tendency for tumors located on the tongue to be associated with this parameter compared to those located on the lips (χ2=4.2086, p <0.05) (Figure 3B). In addition, tumors located on the tongue and lips more frequently showed muscular invasion compared to those in other locations, with high statistical significance (χ2=16.9161, p<0.01) (Figure 3C).
Regarding the association with the Bryne grade, we observed that the second Bryne grade occurs much more frequently in the investigated cases, regardless of location. Also, this grade was found more frequently in young people (≤50 years), while Bryne grade I was seen more often in older people, but the difference was not statistically significant.
In addition, multiple regression analysis showed that the degree of differentiation is the only variable that significantly influences the Bryne grade (p=0.007). This significance was also confirmed by linear regression, which had a p=0.004, with an intercept of 8.46 and a coefficient of 1.42 (Figure 3D).
Although we can not speak of statistical significance between the stage and lymphatic invasion, we noticed the tendency of the presence of invasion exclusively in the advanced pathological stages (III and IVA).
Otherwise, we could not establish statistically significant correlations between the main morphoclinical investigated parameters.
Discussion
Regarding the topographic point of reference, carcinomas of the oral cavity were ranked globally as the 18th to the 26th most common form of human cancer [8].
According to some studies, the highest rate of cases of oral squamous cell carcinoma was observed in France, Croatia, Hungary, Sri Lanka and Melanesia. Over the years, there has been an increased incidence of oral cancer in South Asia, as well as oral and nasopharyngeal cancer in Southeast Asia [9].
From the point of view of the topography of cancerous lesions, India, Bangladesh, Pakistan and Sri Lanka presented about 1/3 of the malignant neoplasms developed in the oral cavity [10].
In accordance with other researches, in the United States of America there was a stabilization, even a decrease in the cases of oral and pharyngeal cancer for both genders in most decades of life, between the years 1973-2003 [9].
In addition, according to another study, an upward trend was noticed for lip cancers in Caucasian individuals living in the equatorial region, with long-term exposure to an increased proportion of ultraviolet rays being the main contributing factor [11].
Based on extensive studies, a mortality peak was remarked among men of Italian and French nationality during the 1980s, which began to decline only after 1990 [12].
However, there was a constant increase in the parameter previously mentioned in Belgium, Greece, Denmark, Scotland and Portugal [13].
According to our research, OSCC predominantly affected men, 88% of the analysed cases and only 12% were diagnosed in women, an aspect also supported by other studies [1, 14, 15].
The demographic distribution by place of origin highlighted that 35 (70%) of the diagnosed patients came from the rural area and the difference of 15 cases (30%) from the urban area. Harris JA et al. [16], in a similar study published in 2020, highlighted a similar aspect regarding this epidemiological parameter, demonstrating that over 80% of the patients came from rural areas. The average age of diagnosis in our study was 60.82 years, an aspect supported by a statistical study carried out in the USA between 2003-2007, by Altekruse SF et al. [17], which claims that the average age of diagnosis of cancer of the oral cavity and pharynx is 62 years.
Daroit NB et al. [18], according to a research carried out in 2023 on a large group of patients, found that from a topographical point of view, oral malignant neoplasms developed and affected in most cases the tongue, following them in second place the oral floor. Following the analysis carried out in our research, we reached the same results, the most frequent localization of cases of oral squamous cell carcinomas being at the level of the tongue, 30% of the cases, being followed in second place by the oral floor and the lip, each of these two locations statistically contributing 28% of cases.
Regarding the macroscopic clinical aspect of the lesions, ulcerative lesions were predominant, 54% of the cases analyzed and diagnosed, the same thing was highlighted by Daroit NB et al. [18] in the study they realized, where 484 (49%) of the cases had the same macroscopic appearance.
The histopathological analysis of the cases studied by us highlighted that the most common morphopathological subtype of oral squamous cell carcinomas was represented by the conventional subtype, with a number of 35 cases (70%) from the total case history. Apart from this, we also identified the following histopathological subtypes: acantholytic (18%), verrucous (6%), basaloid (4%) and sarcomatoid (2%). This analysis of the morphoclinic parameter corresponds to data highlighted by Ciucă FI et al. [19] who identified more than half of the cases (53.7%) as belonging to the conventional subtype and Inaut AF et al. [20] who identified over 90% of the cases as belonging to the same conventional histopathological subtype.
The degree of differentiation of oral squamous cell carcinomas most common in our case series is represented by the moderately differentiated forms, totaling 32 cases (64%), being followed by the well-differentiated forms (30%) and finally by the poorly differentiated ones (6%). At the same time, our study highlighted the fact that most of the resection margins were not invaded (54%) by the carcinomas, the percentage difference being represented by the invaded resection margins. Studies and specialized literature highlight similar aspects as follows: according to a study conducted in 2024, the majority were moderately differentiated forms of oral squamous cell carcinomas (52.5%) [20] and at the same time, in accordance with a study conducted in 2018, most of the resection margins were uninvaded (76%) [19].
Our study, regarding pTNM staging, highlights that the predominant stage is represented by stage II (36%), followed in descending order by stage IVA (26%), stage III (20%) and stage I (18%). By comparing with the specially designed literature, there is a slight change in the trend of this parameter over time, the parallel being made with the study carried out by Ciucă FI et al. in 2018 [19], where the most frequent pTNM stage was represented by stage III (37.04%), with a small percentage difference followed by stage II (33.33%), and on the last two places ranking stage IV (16.67%) and stage I (12.96%).
By following the statistical analysis carried out in our study, we observed the following aspects: oral squamous cell carcinomas developed on the tongue reached a maximum frequency in the 6th decade of life, and in the 8th decade they were absent; regarding the cases developed at the level of the lips, they presented a maximum incidence in the 8th decade of life; carcinomas developed from the mucosa of the oral floor showed a sudden decrease in incidence in the 8th decade of life, otherwise having a linear distribution, similar to carcinomas developed from the gingival mucosa which after the age of 50 years showed the same linear distribution. After carrying out the Exact Fisher test, it emerged that the differences between the previously mentioned distributions are statistically significant (p=0.0131). In general, it is considered that tongue cancer predominates in men over 50 years old [21], but studies of the last decade indicate an increase in the incidence among young people and especially among women [22].
Regarding the invasive nature of OSCC, we observed a greater tendency for tumors developed on the tongue to be associated with perineural invasion, compared to those developed on the lips (χ2=4.2086, p<0.05). The vast majority of studies highlighted the fact that in oral cancer, perineural invasion is associated with a poor prognosis and its presence is considered a clinical indication for radiotherapy and systemic treatment [23, 24, 25].
Recently, Huang Q et al., showed that lymphovascular and perineural invasions are independent negative prognostic factors for tongue cancer, patients with lymphovascular and/or perineural invasion may have significantly poorer overall survival [26].
In addition, we noticed that muscular invasion appeared with a much higher frequency in tumors from the tongue and lips, compared to tumors from other locations, an aspect that had a high statistical significance (χ2=16.9161, p<0.01). Literature studies indicate that in OSCC the muscle invasion can be used as a prognostic parameter, cases with skeletal muscle invasion have a poor prognosis and the degree of muscle invasion increases as the degree of tumor differentiation decreases [27].
For tongue OSCC it seems that the pattern of muscle invasion plays an important role in the progression of the disease, the local recurrence being higher in patients with muscle invasion [28].
After performing the multiple regression analysis, we noticed that the only variable that significantly influences the Bryne grade is represented by the degree of tumor differentiation (p=0.007). The previously mentioned significance was also confirmed by the linear regression, which had a p=0.004, with an intercept of 8.46 and a coefficient of 1.42. The literature indicates Bryne's grading system as the best in in predicting the outcome of OSCC and this is mainly due to the fact that it takes into account the tumor morphology from the invasion front [29, 30].
Conclusion
The results of our investigation indicate the prevalence of casuistry among men, with an average age of diagnosis around 61 years, more frequent developed in the tongue, followed by lip and oral floor. Histopathologically prevailed the conventional type of OSCC, moderately differentiated (G2), much more frequently with pTNM stage II, almost two thirds showing muscle invasion and 38% perineural invasion. Particularly for cases with tongue localization, we found a maximum incidence in the 6th decade of life, most cases presenting muscle invasion and perineural invasion and advanced clinical stages at the time of diagnosis. It seems that for OSCC, tumor topography influences the clinical behavior and the morphological profile, dictating different therapeutic strategies to be taken in such patients.
Conflict of interests
The authors have no conflict of interest to declare.
References
- 1.Capote-Moreno A, Brabyn P, Munoz-Guerra MF, Sastre-Perez J, Escorial-Hernandez V, Rodriguez-Campo FJ, Garcia T, Naval-Gias L. Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int. J. Oral Maxillofac. Surg. 2020;49:1525–1534. doi: 10.1016/j.ijom.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Alexandra C, Andrei N, Mihaela M, Eugenia P. Predictive Factors in the Appearance and Evolution of Squamous Cell Carcinomas of the Oral Cavity. Medicina. 2022;58(5):570–570. doi: 10.3390/medicina58050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christina McC, Alex K, Marco AM, Iona TL, Tanya J, Grace B. Oral Squamous Cell Carcinoma Associated with Precursor Lesions. Cancer Prev Res. 2021;14(9):873–883. doi: 10.1158/1940-6207.CAPR-21-0047. [DOI] [PubMed] [Google Scholar]
- 4.Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA. year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol. 2014;50:651–656. doi: 10.1016/j.oraloncology.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 5. Johnson N , Franceschi S , et al. In: World Health Organization classification of tumours: pathology and genetics of head and neck tumours . Barnes L , Eveson JW , et al., editors. Lyon : IARC ; 2005 . Squamous cell carcinoma ; pp. 168 – 175 . [Google Scholar]
- 6.Bryne M, Koppang HS, Lilleng R, Kjærheim A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. The Journal of pathology. 1992;166(4):375–381. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 7.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. European Journal of Oral Sciences. 1987;95(3):229–249. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 8.PĂtru A, Şurlin V, MĂrgĂritescu C, CiucĂ E, MĂrgĂritescu OC, Camen A. Palate Squamous Cell Carcinomas:A Ten-Year Single Institute Experience. Curr Health Sci J. 2020;46(4):358–370. doi: 10.12865/CHSJ.46.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newell Johnson, Prasanna J, Hemaltha AA, Amarasinghe K. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 2000. 2011;57(1):19–37. doi: 10.1111/j.1600-0757.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R. Oral cancer in India: an epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol. 1990;69:325–330. doi: 10.1016/0030-4220(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore SR, Allister J, Roder D, Pierce AM, Willson DF. Lip cancer in South Australia, 1977-1996. Pathology. 2001;33:167–171. [PubMed] [Google Scholar]
- 12.Tanaka S, Sobue T. Comparison of oral and pharyngeal cancer mortality in five countries: France, Italy, Japan, UK and USA from the WHO Mortality Database (1960-2000) Jpn J Clin Oncol. 2005;35:488–491. doi: 10.1093/jjco/hyi133. [DOI] [PubMed] [Google Scholar]
- 13.Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia. Cancer mortality in the European Union 1970-2003, with a joinpoint analysis. Ann Oncol. 2008;19:631–40. doi: 10.1093/annonc/mdm597. [DOI] [PubMed] [Google Scholar]
- 14.Leite AA, Leonel ACLS, Castro JFl, Carvalho EJA, Vargas PA, Kowalski LP, Perez DEC. Oral squamous cell carcinoma: a clinicopathological study on 194 cases in northeastern Brazil. A cross-sectional retrospective study. Sao Paulo Med J. 2018;136(2):165–169. doi: 10.1590/1516-3180.2017.0293061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alves AM, Correa MB, Karine DS, Maria LAA, Ana CUV, Ana PNG, Adriana E, Sandra BCT. Demographic and Clinical Profile of Oral Squamous Cell Carcinoma from a Service-Base Population. Brazilian Dental Journal. 2017;28(3):301–306. doi: 10.1590/0103-6440201601257. [DOI] [PubMed] [Google Scholar]
- 16.Harris JA, Hunter WP, Hanna GJ, Treister NS, Menon RS. Rural pacients with oral squamous cell carcinoma experience better prognosis and long-term survival. Oral Oncology. 2020;111:1–7. doi: 10.1016/j.oraloncology.2020.105037. [DOI] [PubMed] [Google Scholar]
- 17. Altekruse SF , Kosary CL , et al. SEER Cancer Statistics Review, 1975-2007 . Bethesda MD : National Cancer Institute ; 2010 . pp. 423 – 431 . [Google Scholar]
- 18.Daroit NB, Martins LN, Garcia AB, Haas AN, Dal Moro, Rados PV. Oral cancer over six decades: a multivariable analysis of a clinicopathologic retrospective study. Brazilian Dental Journal. 2023;34(5):115–124. doi: 10.1590/0103-6440202305264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciucă FI, Mărăşescu PC, Matei M, Florescu AM, Mărgăritescu C, Petrescu SMS, Dumitrescu CI. Epidemiological and Histopathological Aspects of Tongue Squamous Cell Carcinomas- Retrospective Study. Current Health Science Journal. 2018;44(3):211–224. doi: 10.12865/CHSJ.44.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inaut AF, Jose MAU, Jose MSP, Cintia CP, Irene LIM, Xabier MM, Andres BC, Jose AL, Abel GG. Epidemiological, clinical and prognostic analysis of oral squamous cell carcinoma diagnosed and treated in a single hospital in Galicia (Spain): a retrospective study with 5-year follow-up. Med Oral Patol Oral Cir Bucal. 2024;29(1):36–43. doi: 10.4317/medoral.26047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4-5):309–316. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Patel SC, Carpenter WR, Tyree S, Couch ME, Weissler M, Hackman T, Hayes DN, Shores C, Chera BS. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488–1494. doi: 10.1200/JCO.2010.31.7883. [DOI] [PubMed] [Google Scholar]
- 23.Binmadi N, Alsharif M, Almazrooa S, Aljohani S, Akeel S, Osailan S, Shahzad M, Elias W, Mair Y. Perineural Invasion Is a Significant Prognostic Factor in Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics. 2023;13:3339–3339. doi: 10.3390/diagnostics13213339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper JS, Zhang Q, Pajak TF, Forastiere AA, Jacobs J, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, Lustig R, Ensley JF, Thorstad W, Schultz CJ, Yom SS, Ang KK. Long-term follow-up of the RTOG 9501/intergroup phase III trial: postoperative concurrent radiation therapy and chemotherapy in high-risk squamous cell carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 2012;84(5):1198–1205. doi: 10.1016/j.ijrobp.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sher DJ, Adelstein DJ, Bajaj GK, Brizel DM, Cohen EEW, Halthore A, Harrison LB, Lu C, Moeller BJ, Quon H, Rocco JW, Sturgis EM, Tishler RB, Trotti A, Waldron J, Eisbruch A. Radiation therapy for oropharyngeal squamous cell carcinoma: Executive summary of an ASTRO Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol. 2017;7(4):246–253. doi: 10.1016/j.prro.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, Huang Y, Chen C, Zhang Y, Zhou J, Xie C, Lu M, Xiong Y, Fang D, Yang Y, Hu W, Zheng F, Zheng C. Prognostic impact of lymphovascular and perineural invasion in squamous cell carcinoma of the tongue. Sci Rep. 2023;13(1):3828–3828. doi: 10.1038/s41598-023-30939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatterjee S, Devi A, Kamboj M, Narwal A, Anand R, Bhola R. The road less travelled: Skeletal muscle invasion in oral squamous cell carcinoma. J Oral Biol Craniofac Res. 2022;12(5):516–521. doi: 10.1016/j.jobcr.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandler K, Vance C, Budnick S, Muller S. Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: just as effective as depth of invasion. Head Neck Pathol. 2011;5(4):359–363. doi: 10.1007/s12105-011-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thamilselvan S, Pandiar D, Krishnan RP, Ramalingam K, Pavithran P. Comparison of Broder's and Bryne's Grading System for Oral Squamous Cell Carcinoma with Lymph Node Metastases and Prognosis: A Scoping Review. Cureus. 2024;16(1):1–1. doi: 10.7759/cureus.51713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner VP, Webber LP, Curra M, Klein IP, Meurer L, Carrad VC, Martins MD. Bryne's grading system predicts poor disease-specific survival of oral squamous cell carcinoma: a comparative study among different histologic grading systems. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(6):688–696. doi: 10.1016/j.oooo.2017.02.012. [DOI] [PubMed] [Google Scholar]