Abstract
The human microbiota, a community of microorganisms in our bodies, is crucial for our health. This paper explores its development from birth through old age, highlighting some of the unique roles at key life stages—infancy, adulthood, and in the elderly years. Understanding the significant health impacts and consequences of changes in the microbiota offers insights for both the public and clinicians.
Introduction
The human microbiome has been intensely studied over the last two decades, leading to some surprising findings. We have previously written on the topic summarizing known mechanisms by which microbes influence health in this journal.1 Here, we highlight recent findings which show that at each age, the microbiota contributes uniquely to our health. Our bodies are protected by barriers such as the skin, as well as mucosa that lines the mouth, airway, and the intestinal tracts. Each anatomical site has a defined community of bacterial and fungal species. The collection of (“omes”) genomes that are encoded by the microbiota is called the microbiome. The gut microbiome is the largest by mass, most diverse, and most well studied. The gut microbiome is affected by age, sex, and geography. In addition, it is influenced by diet, whether we live in urban or rural setting, ethnicity, socioeconomic status, and other aspects including medications and presence of household pets.2–5 Rather than focusing on the variation across populations, here we summarize recent data that reveals how the microbiome develops and effects our health (Figure 1). We provide examples of functions encoded by the bacterial genomes and how gain or loss of these functions impacts health.
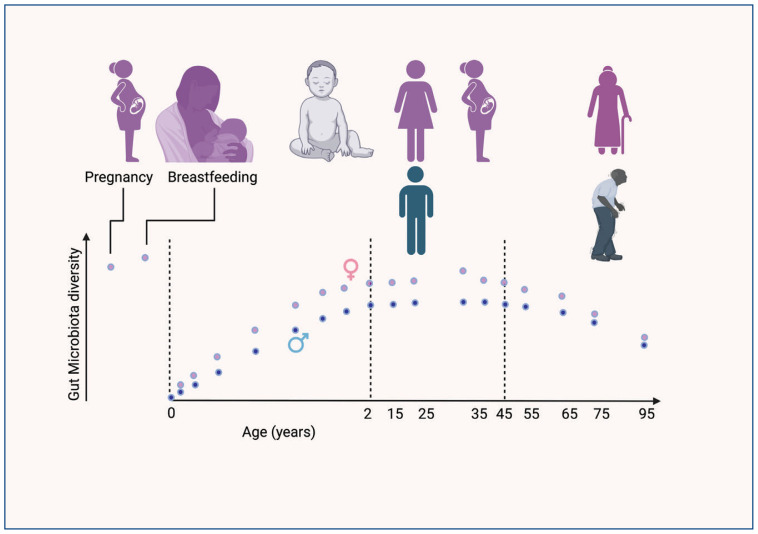
Figure 1.
Changes in gut microbiome diversity across age: Multiple recent studies show that the gut microbiome is established by age of two years. A majority of the gut microbiota is inherited from the mother via breastmilk, that remains stable into adulthood. The diversity and abundance of the taxa vary with sex, geography, pets, hygiene, and urban vs. rural environments. Antibiotic use also has a significant impact. As individuals age, the microbiota starts to change, coinciding with reproductive senescence (menopause and loss of testosterone). Diet, medications, and lifestyle influences the rate of change that impacts human physiology. The microbiota has both a negative and positive influence of risk diseases, strongly suggesting that early events may set the health trajectory during aging.
Source: This Figure was produced using Biorender.
Bacterial Nomenclature
A short paragraph on nomenclature may be helpful here. In the context of microbiology and clinical sciences, taxon refers to the groups or categories into which organisms are classified based on shared characteristics and genetic similarities. This classification system, known as taxonomy, is fundamental in understanding the diversity and relationships among different organisms, including bacteria, viruses, fungi, and other microorganisms commonly encountered in clinical settings. Taxa are arranged hierarchically, ranging from broad categories like kingdoms and phyla to more specific ones such as genus and species. For example, in the human microbiome, identifying taxa at various levels provides an understanding of the composition and functions of the communities present in different parts of the body. While clinically, bacterial classification uses gram staining positivity and growth in selective media (e.g., Bergey’s classification), molecular techniques such as 16S ribosomal RNA sequencing has given rise to classification based on class, order, family, and genus. Sequencing of amplicons identifies orthogonal taxonomic units (OTU) that correspond approximately to species level of taxonomy. In this review, we use taxa to specify organisms, typically at the genus level.
Microbiota Effect on Traits
A central tenet of biology is that all traits or phenotypes (P) arise from the interaction between the genetic material (G) inherited from the parents, and the environment (E), often written in shorthand as P = G × E. The microbiome is inherited and is also a consequence of the environment. As it is inherited the microbiome has been referred to as the second genome. Further, as it is both inherited and influenced by environment, the microbiome has a strong influence on development in early life. It has been shown to impact metabolism, education of the immune system, and cognitive development.6,7 One reason to focus on early events is because they set the foundation and determine the trajectory, or arc, of health and disease risk with age.
Maternal Microbiomes Seed the Infant’s Gut Microbiome at Birth
At birth, the infant’s gut microbiome is primarily seeded by the maternal microbiome. During the passage through the birth canal, the baby is exposed to a diverse array of bacteria, marking the first significant microbial contact.8 In the third trimester of pregnancy, the vaginal microbiome undergoes a transformation, increasing in microbial diversity and becoming rich in Lactobacilli species. These species are known to lower vaginal pH and enhance vaginal secretions, contributing to vaginal health. Concurrently, changes also occur in the maternal gut microbiome during pregnancy.
Infants born vaginally typically inherit a microbiome that reflects the maternal vaginal flora. This flora is abundant in Lactobacillus, Prevotella, and Sneathia species, which are instrumental in early gut colonization. These taxa are needed to digest and extract nutrient from milk. In contrast, infants delivered via Cesarean section are more likely to be colonized by skin and environmental microbes. This initial difference in microbial exposure can have enduring effects on the infant’s health, potentially influencing their susceptibility to various conditions and diseases.9 For instance, in premature infants admitted to neonatal intensive care units, prolonged exposure to antibiotics results in gut dysbiosis has been associated with adverse neonatal outcomes e.g., necrotizing enterocolitis a condition that is associated with high mortality and life-long morbidity in survivors.10,11
Interestingly, the composition of the vaginal microbiome varies based on factors such as ethnicity, maternal age, and parity.12,13 Alterations from the normal vaginal microbiota, known as dysbiosis, have been linked with pregnancy complications, with preterm labor being the most extensively studied condition.9 Thus, the mother’s microbiome, encompassing vaginal, gut, and skin microbiota, serves as a critical source for the newborn’s initial microbial colonization, setting the foundation for the development of the infant’s own microbiome.
Breastfeeding and Microbiome of the Infant
Exclusive human milk feeding for the first six months of life, with continued breastfeeding for one to two years of life or longer, remains the normative standard and is recommended by the American Academy of Pediatrics for infant feeding.14,15 Mother’s own milk is uniquely suited to infants, both in its nutritional composition and in the non-nutritive bioactive factors that promote survival and healthy development.16 Milk is not only a nutrient source for the infant, but also contains oligosaccharides and lipids that promote growth of specific gut microbiota (prebiotics).17–21 There are roughly 4,000 different species of (~1014 cells) bacteria that inhabit the gut. Remarkably, mothers sense their baby’s health and development and alter the composition of the milk within hours.22 Saliva during suckling is allowed to flow back into the breast, and the interaction between the saliva and the milk is sensed to alter the breastmilk composition.23–25
Recent studies on breastmilk microbiome have shown that various bacterial species contain genes for bile salt hydrolases (BSHs).26 The existence of BSHs in these bacteria is noteworthy. These enzymes split the peptide bond in bile acids, separating the amino acid group from the steroid core.27 Human milk, with a fat content of 5%, requires bile salts for fat solubilization. These salts are synthesized by the infant’s liver and are stored in the gall bladder. Normally, bile acids are effectively recycled through a process called enterohepatic recirculation. Both conjugated and unconjugated bile acids are absorbed throughout the gut passively and actively in the terminal ileum. BSH-produced bile acids and their metabolites act as ligands for the farnesoid X receptor (FXR), found in the liver. The metabolic derivatives of these reactions have diverse effects on human health.28 Some lead to weight gain, fatty liver disease, and other metabolic disorders.29 Other derivatives are implicated in cognitive and inflammatory diseases.30,31
The infant’s gut microbiota is colonized from both breastmilk and the skin. As the communities that colonize the skin are distinct from those in breastmilk, the infant’s fecal microbiome shows that about 70% is derived from breastmilk and remainder comes from the areole.32,33
Next, we summarize evidence that the microbiome can have a positive and negative influence in commonly observed pathologies like atherosclerosis, metabolic diseases, and bone fracture healing in adults.
Changes in the Microbiome From Two Years Old to Adulthood
Puberty is a critical milestone that is associated with multiple physiological changes driven by sex hormones during the transition towards adulthood. Therefore, this dynamic period could have significant impact on the gut microbiota. Several studies have examined the dynamics of the microbiome between the ages of two and 15 years of age. These studies show that by the age of five years there are clear differences in the microbiome between boys and girls. Surprisingly, puberty (i.e., sex hormones) does not appear to have a significant impact on the gut or oral microbiome.34–36 The overwhelming conclusion from these studies is that by age two years the microbiome is established and then remains stable over time.37–39
As the microbiota is being established at this early age, broad-spectrum antibiotics can have a lasting impact on the disease risk in the infant and into adulthood. In addition to having concerns for developing drug resistance, the benefits of antibiotics should be carefully balanced against the risks on the infants’ health. Probiotics are live bacteria that provide benefits. The use of appropriate probiotics should be considered post-antibiotic therapy, but clinical trials are needed to determine the use of standardized probiotics.
Impact of the Adult Microbiome on Disease Risk
The adult human microbiome is a complex ecosystem, comprised of approximately 5,000 bacterial species. A meta-analysis representing 545 microbiomes collected from around the world showed that Bacteroides, Ruminococcus, Blautia, Clostridium, and Coprococcus are prevalent in all human populations studied.40 This research underscored the crucial role of the microbiome in maintaining systemic homeostasis, influencing a range of physiological processes including metabolism, epithelial barrier integrity, immune and inflammatory responses, neuroendocrine functions, and hematopoiesis. The gut microbiome functions akin to an autonomous organ within the host, orchestrating a bidirectional communication network with various body systems through neural, endocrine, immune, and metabolic pathways. While stable from the age of two years, the adult microbiome’s composition can be modulated by dietary habits, cultural factors, use of prebiotics, probiotics, medications like proton pump inhibitors (PPIs) and antibiotics. Alterations leading to a detrimental imbalance in the microbiome, termed dysbiosis, have been implicated in various disease states.41,42
Interplay Between Gut Microbiota and the Immune System
The gut microbiota has a complex relationship with the immune system that is multifaceted and impacts host physiology. The gastrointestinal tract, beyond its roles in digestion and nutrient absorption, harbors a diverse microbial community that engages in a symbiotic relationship with the host. Many gut bacteria, such as commensal Escherichia coli providing essential nutrients derived from metabolic byproducts in exchange for a habitat and steady nutrient supply. The immune system, particularly within the gut mucosa, is instrumental in distinguishing pathogenic microbes from commensals, maintaining a balance between immune tolerance and activation. Interestingly, the same E. coli strain can elicit distinct immune responses depending on their location within the body. T cells, for instance, demonstrate both tolerance to commensal E. coli in the gut and robust inflammatory responses to these bacteria in other tissues, such as the bladder via a contaminated catheter, or lungs post intubation. This suggests a sophisticated level of immune education by the microbiome that is both microbial species and tissue specific. This implies that the gut microbiome is pivotal in shaping the resident T cell memory population.43–45
Gut-Bone Crosstalk in Osteoimmunology
Recent advancements in osteoimmunology have elucidated the intricate relationship between the gut microbiome and bone health. Notably, research in murine models has identified key pathways linking the microbiome to bone physiology, encompassing the immune system, metabolic byproducts, endocrine signals, and extracellular vesicles. The microbiome’s influence on T cell differentiation, particularly in the context of bone healing, has garnered attention. The work of Dar et al. highlights the microbiome’s role in bone repair processes.46 They observed that gut microbiota influences the production of TH17 cells in the intestines. Post-fracture, sphingosine-1-phosphate, a signal molecule released by bone tissue, attracts these TH17 cells from the gut. These cells are crucial in promoting bone healing. Colonization of segmented filamentous bacteria (SFB) increased TH17 cell populations in the gastrointestinal tract, correlated with increased TH17 presence at fracture sites in the mouse model. This was further confirmed using a UV reporter mouse strain to track the migration of TH17 cells from the gut to the healing bones, underscoring the gut microbiome’s impact on bone healing. These findings suggest the gut microbiome’s potential as a therapeutic target, especially considering how disruptions like antibiotic use could impact bone healing.46,47
The gut microbiome has also been shown to contribute to osteoporosis. Das et al. analyzed the fecal microbiomes from 181 men and women over the age of 55 with either normal bone mineral density (BMD), osteopenia, or osteoporosis.48,49 They corrected for several confounding factors such as dietary habits, prescribed drugs, leisure time, and physical activity using statistical models to partition the taxa and assign them to each of these factors. They identified six genera that were significantly altered in abundance in the osteoporosis or osteopenic groups compared with age- and gender-matched controls. Despite controlling for biological confounders like BMI, health status, diet, and medication, which explained 15–17% of the variance within the microbiota dataset, these taxa remained significantly associated with bone mineral density. The cohort with osteoporosis had lowest microbial diversity with lowest levels of these taxa. Unexpectedly, the cohort with osteopenia had the highest diversity and higher abundance the six taxa relative to the cohort with normal BMD. Consistent with prior studies, the study finds that proton pump inhibitors (PPI) had a large impact on reducing the diversity of microbiome taxa, presumably because it alters the gut pH and reduction of these taxa also affected BMD. Together, these studies indicate that either an increase or decrease in diversity impacts the immune response and bone health.
Changes with Aging
Aging has been an area of intense study over the last five decades in hopes of understanding the biology and regulation of the process. Recently scientists have proposed twelve hallmarks of aging. Broadly, these hallmarks are interconnected among each other and include dysbiosis of the host microbiome. It is now recognized that aging is influenced by the interaction of the host and the gut microbiome. The microbiome itself has mechanistic pathways that have been associated with aging.
Recent research of individuals 65 years and older has revealed important connections between the composition of the gut microbiome and various health indicators, such as physical fitness, frailty, and dietary habits.50 This research builds on earlier findings that demonstrated a change in the microbiome from adulthood into older age. This change is characterized by a decrease in microbiome diversity, lower levels of Bifidobacterium, and increased populations of bacteria such as Clostridium, Lactobacillus, Enterobacteriaceae, and Enterococcus. A significant 2021 study, examining the gut microbiomes and metabolic products of 9,000 people across three cohorts, found that microbiome diversity increases with age.51 Despite the variations in microbiome composition, metabolic functions remained relatively consistent. The study highlighted two metabolites—tryptophan-derived indole and phenylacetylglutamine—which are linked to longer lifespans in mice and found in high concentrations in human centenarians. Notably, this unique microbiome evolution was mainly seen in healthy individuals aged 80 and above, while it was less evident in their less healthy peers. These insights suggest an ongoing development and specialization of the gut microbiome in the context of healthy aging.52–54
Is it possible to slow aging by altering the gut microbiome? Metformin, a diabetes treatment used for over 60 years, offers an interesting case.55 Not only does metformin extend life expectancy in humans and animals, but it also influences various aging-related factors, including the gut microbiome.56 Its concentration in the gut is significantly higher than in the blood.57 Metformin’s impact on the gut microbiome includes inhibiting the growth of pathogenic bacteria in older individuals. This action helps reduce inflammation and counteract the immune system weakening caused by dysbiosis.58,59
Conclusion
Summarizing, the gut microbiome changes dynamically in the first two years of life and then is highly stable until age 40 (Figure 1). The microbiome is inherited primarily from the mother in these first years. After the age of 45 years, the microbiome changes, which coincides with reproductive senescence. In infants the gut microbiomes vary by sex, ethnicity, and geography. All data strongly suggest that the microbiota have a significant impact early in life on neural/cognitive development, and on metabolism setting the health trajectory to develop, for instance obesity, cardiovascular disease, and other comorbidities of aging.
A great achievement of modern medicine has been to increase lifespans. Recognizing the key contributions of microbiome starting at infancy and its relationship to the hallmarks of aging may allow us to develop new interventions that reduce the comorbidities of aging.
Footnotes
Rajeev Aurora, PhD, is in the Department of Molecular Microbiology and Immunology and Thomas Sanford, MD, is in the Department of Otolaryngology; both are at the Saint Louis University School of Medicine, St. Louis, Missouri.
Disclosure: No financial disclosures reported. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.Aurora R, Sanford T.Host Microbiota Contributes to Health and Response to Disease Mo Med Jul–Aug20151124317–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Fu X, Ou Z, et al. Environmental determinants and demographic influences on global urban microbiomes, antimicrobial resistance and pathogenicity. NPJ Biofilms Microbiomes. 2023 Dec 7;9(1):94. doi: 10.1038/s41522-023-00459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei M, Xu C, Xu X, Zhu C, Li J, Lv G. Characteristics of atmospheric bacterial and fungal communities in PM(2.5) following biomass burning disturbance in a rural area of North China Plain. Sci Total Environ. 2019 Feb 15;651(Pt 2):2727–2739. doi: 10.1016/j.scitotenv.2018.09.399. [DOI] [PubMed] [Google Scholar]
- 4.Hsu T, Joice R, Vallarino J, et al. Urban Transit System Microbial Communities Differ by Surface Type and Interaction with Humans and the Environment. mSystems. 2016 May–Jun;1(3) doi: 10.1128/mSystems.00018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Dalton KR, Lee M, et al. Metagenomics reveals novel microbial signatures of farm exposures in house dust. Frontiers in microbiology. 2023;14:1202194. doi: 10.3389/fmicb.2023.1202194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linehan K, Dempsey EM, Ryan CA, Ross RP, Stanton C. First encounters of the microbial kind: perinatal factors direct infant gut microbiome establishment. Microbiome Res Rep. 2022;1(2):10. doi: 10.20517/mrr.2021.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mesa MD, Loureiro B, Iglesia I, et al. The Evolving Microbiome from Pregnancy to Early Infancy: A Comprehensive Review. Nutrients. 2020 Jan 2;12(1) doi: 10.3390/nu12010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn AB, Jordan S, Baker BJ, Carlson NS.The Maternal Infant Microbiome: Considerations for Labor and Birth MCN Am J Matern Child Nurs Nov/Dec2017426318–325. 10.1097/NMC.0000000000000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahrodia T, Yodhaanjali JR, Das B. Vaginal microbiome dysbiosis in preterm birth. Prog Mol Biol Transl Sci. 2022;192(1):309–329. doi: 10.1016/bs.pmbts.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Cuna A, Morowitz MJ, Sampath V. Early antibiotics and risk for necrotizing enterocolitis in premature infants: A narrative review. Front Pediatr. 2023;11:1112812. doi: 10.3389/fped.2023.1112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dierikx TH, Deianova N, Groen J, et al. Association between duration of early empiric antibiotics and necrotizing enterocolitis and late-onset sepsis in preterm infants: a multicenter cohort study. Eur J Pediatr. 2022 Oct;181(10):3715–3724. doi: 10.1007/s00431-022-04579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotkani ZG, Ghaedmohammadi S, Mozdoori N. Meta-analysis of race and age influence on the vaginal microbiome in pregnant and nonpregnant healthy women. Future Microbiol. 2022 Sep;17:1147–1159. doi: 10.2217/fmb-2021-0209. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Theis KR, Gomez-Lopez N, et al. The Vaginal Microbiota of Pregnant Women Varies with Gestational Age, Maternal Age, and Parity. Microbiol Spectr. 2023 Aug 17;11(4):e0342922. doi: 10.1128/spectrum.03429-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comprehensive implementation plan on maternal, infant and young child nutrition. World Health Organization; 2014. pp. 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Editors. Breastfeeding and the use of human milk. Pediatrics. 2012 Mar;129(3):e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 16.Oftedal OT. The evolution of milk secretion and its ancient origins. Animal. 2012 Mar;6(3):355–68. doi: 10.1017/S1751731111001935. [DOI] [PubMed] [Google Scholar]
- 17.Kozak K, Charbonneau D, Sanozky-Dawes R, Klaenhammer T. Characterization of bacterial isolates from the microbiota of mothers’ breast milk and their infants. Gut microbes. 2015;6(6):341–51. doi: 10.1080/19490976.2015.1103425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rautava S. Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis. 2016 Feb;7(1):5–14. doi: 10.1017/S2040174415001233. [DOI] [PubMed] [Google Scholar]
- 19.Newburg DS, Morelli L. Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr Res. 2015 Jan;77(1–2):115–20. doi: 10.1038/pr.2014.178. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez E, Brereton NJB, Li C, et al. Distinct Changes Occur in the Human Breast Milk Microbiome Between Early and Established Lactation in Breastfeeding Guatemalan Mothers. Frontiers in microbiology. 2021;12:557180. doi: 10.3389/fmicb.2021.557180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012 Sep;96(3):544–51. doi: 10.3945/ajcn.112.037382. [DOI] [PubMed] [Google Scholar]
- 22.Tomaszewska A, Jeleniewska A, Porebska K, et al. Immunomodulatory Effect of Infectious Disease of a Breastfed Child on the Cellular Composition of Breast Milk. Nutrients. 2023 Sep 3;15(17) doi: 10.3390/nu15173844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Shehri SS, Knox CL, Liley HG, et al. Breastmilk-Saliva Interactions Boost Innate Immunity by Regulating the Oral Microbiome in Early Infancy. PLoS One. 2015;10(9):e0135047. doi: 10.1371/journal.pone.0135047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gothefors L, Marklund S. Lactoperoxidase activity in human milk and in saliva of newborn infants. Infect Immun. 1975 Jun;11(6):1210–5. doi: 10.1128/iai.11.6.1210-1215.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweeney EL, Al-Shehri SS, Cowley DM, et al. The effect of breastmilk and saliva combinations on the in vitro growth of oral pathogenic and commensal microorganisms. Sci Rep. 2018 Oct 11;8(1):15112. doi: 10.1038/s41598-018-33519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia B, Park D, Hahn Y, Jeon CO. Metagenomic analysis of the human microbiome reveals the association between the abundance of gut bile salt hydrolases and host health. Gut microbes. 2020 Sep 2;11(5):1300–1313. doi: 10.1080/19490976.2020.1748261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begley M, Hill C, Gahan CG. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol. 2006 Mar;72(3):1729–38. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourgin M, Kriaa A, Mkaouar H, et al. Bile Salt Hydrolases: At the Crossroads of Microbiota and Human Health. Microorganisms. 2021 May 22;9(6) doi: 10.3390/microorganisms9061122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang JYL, Ferrell JM. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am J Physiol Gastrointest Liver Physiol. 2020 Mar 1;318(3):G554–G573. doi: 10.1152/ajpgi.00223.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dilmore AH, Martino C, Neth BJ, et al. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer’s disease. Alzheimers Dement. 2023 Nov;19(11):4805–4816. doi: 10.1002/alz.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farooqui N, Elhence A, Shalimar A Current Understanding of Bile Acids in Chronic Liver Disease. J Clin Exp Hepatol. 2022 Jan–Feb;12(1):155–173. doi: 10.1016/j.jceh.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JE, Carrothers JM, Lackey KA, et al. Strong Multivariate Relations Exist Among Milk, Oral, and Fecal Microbiomes in Mother-Infant Dyads During the First Six Months Postpartum. J Nutr. 2019 Jun 1;149(6):902–914. doi: 10.1093/jn/nxy299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pannaraj PS, Li F, Cerini C, et al. Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome. JAMA Pediatr. 2017 Jul 1;171(7):647–654. doi: 10.1001/jamapediatrics.2017.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Gut microbiota: effect of pubertal status. BMC Microbiol. 2020 Nov 3;20(1):334. doi: 10.1186/s12866-020-02021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Sexual dimorphism of gut microbiota at different pubertal status. Microb Cell Fact. 2020 Jul 28;19(1):152. doi: 10.1186/s12934-020-01412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korpela K, Kallio S, Salonen A, et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci Rep. 2021 Dec 2;11(1):23297. doi: 10.1038/s41598-021-02375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM.Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome Res Microbiol Jun–Aug20191704–5192–201. 10.1016/j.resmic.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zhong H, Li Y, et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat Aging. 2021 Jan;1(1):87–100. doi: 10.1038/s43587-020-00014-2. [DOI] [PubMed] [Google Scholar]
- 39.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012 Jun 14;486(7402):222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piquer-Esteban S, Ruiz-Ruiz S, Arnau V, Diaz W, Moya A. Exploring the universal healthy human gut microbiota around the World. Comput Struct Biotechnol J. 2022;20:421–433. doi: 10.1016/j.csbj.2021.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012 Jun 13;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lloyd-Price J, Abu-Ali G, Huttenhower C. The healthy human microbiome. Genome Med. 2016 Apr 27;8(1):51. doi: 10.1186/s13073-016-0307-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Littman DR, Pamer EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011 Oct 20;10(4):311–23. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016 Jul 7;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 45.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017 May 16;474(11):1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dar HY, Perrien DS, Pal S, et al. Callus gammadelta T cells and microbe-induced intestinal Th17 cells improve fracture healing in mice. J Clin Invest. 2023 Apr 17;133(8) doi: 10.1172/JCI166577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aurora R, Silva MJ. T cells heal bone fractures with help from the gut microbiome. J Clin Invest. 2023 Apr 17;133(8) doi: 10.1172/JCI167311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aurora R. Confounding factors in the effect of gut microbiota on bone density. Rheumatology (Oxford) 2019 Dec 1;58(12):2089–2090. doi: 10.1093/rheumatology/kez347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Das M, Cronin O, Keohane DM, et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford) 2019 Dec 1;58(12):2295–2304. doi: 10.1093/rheumatology/kez302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bana B, Cabreiro F. The Microbiome and Aging. Annu Rev Genet. 2019 Dec 3;53:239–261. doi: 10.1146/annurev-genet-112618-043650. [DOI] [PubMed] [Google Scholar]
- 51.Wilmanski T, Diener C, Rappaport N, et al. Author Correction: Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. 2021 Apr;3(4):586. doi: 10.1038/s42255-021-00377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021 Oct;22(5–6):289–303. doi: 10.1038/s41435-021-00126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Jazwinski SM. The Gut Microbiota and Healthy Aging: A Mini-Review. Gerontology. 2018;64(6):513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016 Dec 15;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 55.Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017 Sep;60(9):1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 56.Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne) 2021;12:718942. doi: 10.3389/fendo.2021.718942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buse JB, DeFronzo RA, Rosenstock J, et al. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care. 2016 Feb;39(2):198–205. doi: 10.2337/dc15-0488. [DOI] [PubMed] [Google Scholar]
- 58.Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018 Dec;24(12):1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang W, Xu JH, Yu T, Chen QK. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother. 2019 Oct;118:109131. doi: 10.1016/j.biopha.2019.109131. [DOI] [PubMed] [Google Scholar]