Abstract
Moringa oleifera (MO) has been an important plant for food and traditional medicine in Asian countries, including Indonesia. The leaves of these plants are reported to be rich in antioxidants, vitamins, and micronutrients and have been proven to have nootropic properties. Therefore, we investigated whether MO could provide protective effects on SH-SY5Y neuroblastoma cells exposed to H2O2. In this study, we observed cotreating water-extracted MO leaves on the inhibition of reactive oxygen species (ROS). We found that this treatment enhanced the activities of glutathione peroxidase, catalase, and superoxide dismutase. In addition, it suppressed the mRNA expression levels of apoptotic gene-related genes, specifically Bcl-2 associated protein X (BAX) and caspase 3. Furthermore, it promoted neuroplasticity by increasing the brain-derived neurotropic factor (BDNF) mRNA expression in SH-SY5Y cells. The protein expression of phosphorylated-Akt and phosphorylated-CREB, essential genes in neuroplasticity, was also increased in cells treated with H2O2 and MO. Therefore, the neuroprotective effects of MO against oxidative stress are attributed to its antioxidant and antiapoptotic properties, as well as its ability to modify the neuronal signaling pathway.
Keywords: antioxidant, apoptotic, Moringa oleifera, neuroplasticity, SH-SY5Y cells
1. Introduction
Oxidative stress arises from an inequilibrium between the generation of reactive oxygen species (ROS) and the ability of biological systems to detoxify reactive intermediates [1]. Oxidative stress is associated with the progression of many neurodegenerative disorders, including Alzheimer's disease (AD), Parkinson's disease (PD), and other neurodegenerative diseases. Oxidative stress, which results in the damaging effect of free radicals on neurons, plays a detrimental role in the process of neurodegeneration [2]. ROS toxicity is responsible for causing protein misfolding, activation of glial cells, dysfunction of mitochondria, and ultimately leading to cell apoptosis [3]. Other evidence also showed that oxidative stress overproduction is the main cause of neuronal apoptosis in AD [4, 5]. The brain is vulnerable to oxidative stress due to its high level of activity. The brain also has a high oxygen demand, consuming 20% more oxygen than the remainder of the body. The brain is also rich in redox-active metals (copper and iron) actively involved in ROS generation. Brain cell membranes are also enriched with polyunsaturated fatty acids (PUFAs), making them susceptible to lipid peroxidation [6]. Moreover, neurons are thought to be more susceptible to ROS due to increased oxidative metabolism and a lower number of antioxidative enzymes [7]. Therefore, suppression of ROS generation and inhibition of apoptosis can potentially prevent neurodegeneration [8].
Brain-derived neurotrophic factor (BDNF) is an important neurotrophic factor for protecting neurodegenerative diseases. BDNF may allow AKT (also known as protein kinase B) and CREB (cAMP response element-binding protein) activation to modulate neuroplasticity [9]. Several studies have indicated a correlation between oxidative stress and BDNF in the central nervous system [10]. Antioxidant treatment has the potential to enhance the level of BDNF and neurosynaptic function, thereby promoting cognitive preservation. These data suggest that ROS may affect the BDNF signaling pathway in neurodegenerative diseases [11].
Moringa oleifera (MO), a plant belongs to the Moringa family, has been consumed and utilized for both culinary and medicinal purposes in various Asian countries, such as Indonesia, where it is commonly referred to as “kelor.” [12]. The leaves of this plant are reported to be a rich source of antioxidants, vitamins, and micronutrients such as potassium, calcium, phosphorus, iron, vitamins A and D, essential amino acids, beta-carotene, vitamin C, and flavonoids [13]. Several studies have shown that MO has numerous benefits, including analgesic, antipyretic, anti-inflammatory, antiallergic, hepatoprotective, gastroprotectives, anticancer, immunostimulant, and the ability to prevent cardiovascular and metabolic disorder [14]. Notably, the leaf extract also exhibits potential as a cognitive enhancer and neuroprotectant in an animal model of dementia. This effect is most likely achieved by reducing oxidative stress and enhancing cholinergic function [15].
Neuroblastoma cell line SH-SY5Y is frequently used to investigate the molecular and cellular mechanisms involving the effects of PD-associated toxins, perform functional studies of familial PD genes, and evaluate potential protective compounds for PD treatment. This cell line has been a valuable asset for elucidating the molecular complexity of PD [16]. This study is the first report on the investigation of MO water extraction on its antioxidant activity, along with antiapoptotic and neuroplasticity-related genes in the SH-SY5Y cell line. A recent study of MO methanol extraction in 2021 on the SH-SY5Y cell line showed promising results in antioxidant activity. However, it did not analyze antiapoptotic and neuroplasticity-related genes [17].
This study is also the first to examine phosphorylated CREB, a neuroplasticity essential gene, in the leaf extract of Moringa. The result of this study may pave the way for further in vivo investigation of the beneficial effect of MO for neuroprotective agents, especially for neurodegenerative diseases caused by oxidative stress.
2. Materials and Methods
2.1. MO Extract (MOE) Leaves Materials
The MOE was purchased from PT Javaplant (Indonesia). The extract was produced using aqueous extraction and supplemented with maltodextrin. The details of the herb extraction process are provided in Supporting Figure 1, while Supporting Figure 2 includes the certificate of analysis for the MOE.
2.2. Cell Culture Study
SH-SY5Y cells, a human neuroblastoma cell line, were purchased from Elabscience. The cells were maintained in DMEM-F12 medium (Gibco) with 15% fetal bovine serum (FBS) (Gibco) and 1x antibiotic–antimycotic (ABAM, 100x Solution, Gibco) under 37°C and 5% CO2. Cells were plated in the previously coated dishes with the poly-D-lysine (Gibco).
The cells were initially cultured on a 10 cm dish until they reached 80% confluency and then passaged into a 96-well plate for cell viability assay, 12-well plate for mRNA analysis, and 6-well plates for immunoblotting analysis, respectively. Afterward, the SH-SY5Y cells were simultaneously exposed to vehicle, H2O2, or H2O2 plus MO for 24 h then further analyzed those cells. Phosphate buffered saline (PBS), pH 7.4 with 0.9% NaCl (9 g/L) (Gibco) and dimethyl sulfoxide (DMSO) < 0.1% (Sigma-Aldrich) were used as the control for MOE and H2O2 treatment, respectively. In addition, a mixture of PBS and DMSO < 0.1% was used for the vehicle.
2.3. Cell Viability Assay
A 96-well plate was used for this assay, and 5000 cells were maintained for each well [18]. MOE or H2O2 was given at various concentrations from 1 to 100 μg/mL and 0.1 to 50 mM, respectively. In brief, eight wells in each group were treated with a certain dose for 24 h and then the cell viability was analyzed using the Vybrant MTT Cell Proliferation Assay Kit (V-13154) (Thermo Fisher, USA), and the proliferation phenotype was detected by analyzing the absorbance at 570 nm with microplate reader.
2.4. Quantitative PCR
SH-SY5Y cells were lyzed and extracted into the RNA sample using a Direct-zolTM RNA Miniprep Plus (Zym Research). The cDNA samples were made from roughly 0.5 μg of total RNA using ReverTra qPCR RT Mastermix/gDNA remover kit (Toyobo). Moreover, cDNA was used with the primers and Thunderbird Sybr qPCR mix (Toyobo) to perform quantitative real time-PCR (qRT-PCR) analysis as previously described [18]. The primers used are shown in Table 1. The samples underwent incubation under the specified conditions: cDNA synthesis was conducted for a duration of 10 min at 50°C, iScript reverse transcriptase inactivation was performed for 5 min at 95°C, and PCR cycling for 40 cycles. During the denaturation phase of PCR cycling, the samples were subjected to a temperature of 95°C for 10 s. In the annealing and extension phase, a temperature of 58°C was maintained for 30 s.
Table 1.
Primers for quantitative real-time PCR.
| Gene | Primer | Sequence |
|---|---|---|
| β-actin | Forward | TTGCGCTCAGGAGGAGCAAT |
| Reverse | TTCCAGCCTTCCTTCCTGG | |
|
| ||
| Caspase 3 | Forward | GGTTAACCCGGGTAAGAATGTGCA |
| Reverse | TCGGTCTGGTACAGATGTCGAT | |
|
| ||
| Bax | Forward | CATGTTTTCTGACGGCAACTTC |
| Reverse | AGGGCCTTGAGCACCAGTTT | |
|
| ||
| SOD1 | Forward | CCACACCTTCACTGGTCCAT |
| Reverse | CTAGCGAGTTATGGCGACG | |
|
| ||
| GPx1 | Forward | TCGGCTTCCCGTGCAACCAG |
| Reverse | CGCACCGTTCACCTCGCACTT | |
|
| ||
| Catalase | Forward | TGGTAAACTGGTCTTAAACCGGAATC |
| Reverse | GGCGGTGAGTGTCAGGATAGG | |
|
| ||
| BDNF | Forward | CATCCGAGGACAAGGTGGCTTG |
| Reverse | GCCGAACTTTCTGGTCCTCATC | |
2.5. Immunoblotting
Immunoblotting was performed as previously explained with minor adjustment [19]. Protein isolated from SH-SY5Y cells was lyzed in radio-immunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, R0278) containing protease (Sigma-Aldrich, P0044) and phosphatase inhibitors (Sigma-Aldrich, P8340). The protein concentration was equalized with the Bradford method before heating in the sample buffer. Afterward, the samples that contained 40 ng of protein concentration were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto a nitrocellulose membrane. The membranes were blocked with 5% skim milk in TBS-T for 30 min and then probed with the first antibody diluted in blocking buffer overnight at 4°C, followed by an incubation process in secondary antibody diluting in blocking buffer. The targeted signals were visualized with enhanced chemiluminescence (ECL) substrate (BioRad) and detected using Chemiluminescence Alliance 4.7 (Uvitec). The data were then presented in arbitrary units after the signals were quantified by normalizing the phosphorylated with the total protein bands. All antibodies for immunoblotting analysis, such as phosphorylated CREB (CST, #9198), total CREB (CST, #9197), phosphorylated Akt (CST, #9271), total Akt (Cell Signaling; #9272), and Anti Rabbit IgG horseradish peroxidase (HRP) Link-Antibody (CST #7074S), were purchased from Cell Signaling Technology (USA).
2.6. Statistical Analysis
All data were presented as the mean ± standard error of the mean (SEM). Two-tailed Student's t-test was used to investigate the differences between the two groups. One-way ANOVA followed by Tukey's test was used to identify the differences among more than two groups. A p value of less than 0.05 was considered statistically significant. GraphPad Prism 8 (GraphPad Software, Inc.; 2018) was used for all statistical analyses.
3. Results
3.1. The Effect of MOE on the Viability of H2O2-Treated SH-SY5Y Cells
The initial study was performed to investigate whether MO may induce cytotoxicity at a certain dose. We found that MO treatment did not affect cell viability until MOS at 100 μg/mL (Figure 1(a)). On the other hand, the proliferation phenotype was gradually reduced at H2O2 treatment in a dose-dependent manner. It was observed that a concentration of 1 mM H2O2 could inhibit the viability of half of cell population, which is referred to as lethal concentration 50 (LC50) (Figure 1(b)). MO can enhance cell viability that has been exposed to H2O2 in a dose-dependent manner. Among the different concentrations tested, 25 μg/mL of MO showed the highest percentage in cell viability (Figure 1(c)). Therefore, these doses were used for the subsequent studies. Likewise, cell treated with 1 mM H2O2 showed the shrinkage morphology phenotype (Figures 2(b) and 2(e)) compared with the control group (Figures 2(a) and 2(d)). In addition, cell treated with both MO 25 μg/mL and 1 mM H2O2 (Figures 2(c) and 2(f)) displayed minimal morphological changes, indicating the protective effect of MO against H2O2 exposure in SH-SY5Y cells.
Figure 1.
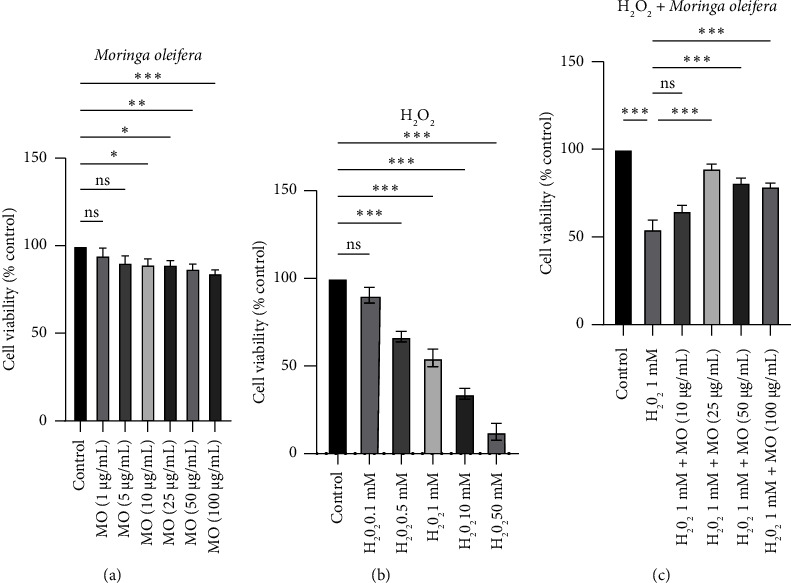
Cell viability assays in SH-SY5Y cells with Moringa oleifera (MO) extract and/or H2O2 treatment. (a) The effect of MO extract alone, ranging from 1 to 100 μg/mL, on cell viability in SH-SY5Y cells. (b) The effect of H2O2 alone, ranging from 0.1 to 50 mM, on cell viability in SH-SY5Y cells. (c) The effect of H2O2 concentration at 1 mM and MO extract from 1 to 100 μg/mL on cell viability in SH-SY5Y cells. ns: not significant, ∗: p < 0.05, ∗∗: p < 0.01, ∗∗∗: p < 0.001.
Figure 2.
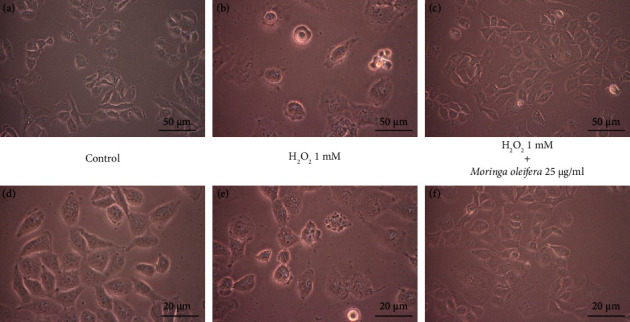
The effects of Moringa oleifera (MO) extract with/without H2O2 treatment on cell morphology in SH-SY5Y cells, with vehicle at 10x magnification (a) and 20x magnification (d), with H2O2 1 mM at 10x magnification (b) and 20x magnification (e), and with H2O2 1 mM and MO extract 25 μg/mL at 10x magnification (c) and 20x magnification (f).
3.2. The Effect of MOE on Endogenous Antioxidant Enzymes in H2O2-Treated SH-SY5Y Cells
We investigated the antioxidant properties of the MOE, which help maintain the viability phenotype in SH-SY5Y cells following H2O2 treatment. Several endogenous antioxidant enzymes, such as glutathione peroxidase 1 (GPx1), catalase, and superoxide dismutase (SOD1), were further analyzed [20]. GPx1 tended to be decreased in the H2O2 group, and MO significantly prevented the inhibition of this mRNA expression level in the H2O2+MO group (p < 0.05) (Figure 3(a)). Similarly, these phenomena were also observed in the catalase and SOD1 mRNA expression (Figures 3(b) and 3(c)).
Figure 3.
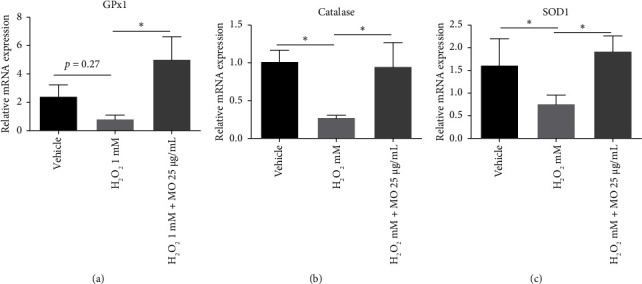
The effects of Moringa oleifera (MO) extract on the mRNA expressions of GPx1 (a), catalase (b), and SOD1 (c). Each bar represents the mean relative mRNA expression ± SEM of four samples. Data analysis was performed using one way ANOVA, followed by Tukey's multicomparison test. ns: not significant, ∗: p < 0.05.
3.3. The Effect of MOE on Apoptotic Markers at the mRNA Level in H2O2-Treated SH-SY5Y Cells
We investigated whether MOE extract prevents apoptosis markers in SH-SY5Y cells treated with H2O2. We found that the H2O2 group demonstrated the enhancement of Bax and caspase 3 mRNA expression levels compared with the vehicle group (p < 0.05). At the same time, MO treatment prevented the increase of these apoptotic markers at the mRNA level (p < 0.05) (Figures 4(a) and 4(b)). These data suggest that MOE inhibits Bax and caspase 3 apoptotic activity in SH-S5Y5 cells.
Figure 4.
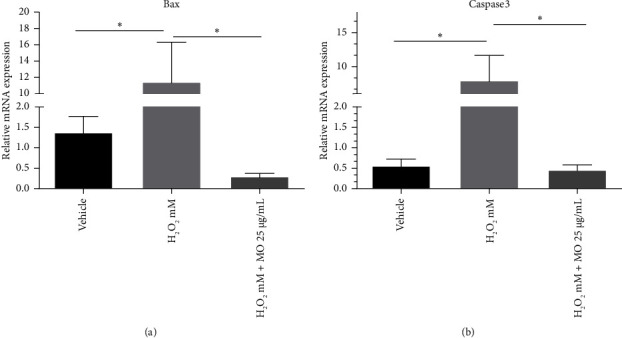
The effects of Moringa oleifera (MO) extract on the mRNA expressions of Bax (a) and caspase 3 (b). Each bar represents the mean relative mRNA expression ± SEM of four samples. Data analysis was performed using one way ANOVA, followed by Tukey's multicomparison test. ns: not significant, ∗: p < 0.05.
3.4. The Effect of MOE on Neuronal Signaling Pathways in H2O2-Treated SH-SY5Y Cells
To elucidate the effect of MOE on the gene involved in neuroplasticity, we analyzed BDNF expression, which plays a vital role in neurogenesis [21]. BDNF showed significantly low expression in the H2O2 group compared with the vehicle group, and the H2O2 + MO group was able to enhance the BDNF mRNA expression level (Figure 5(a)). The neurodegenerative diseases have been associated with the dysregulation of AKT and CREB that will impact BDNF gene expression [22]. Cells treated with H2O2 alone significantly reduced AKT and CREB protein expression. However, MOE treatment appeared to rescue the phosphorylation of the CREB (p-CREB) activity only but not phosphorylation of AKT (p-AKT) activity (Figures 5(b), 5(c), and 5(d)). These data strongly suggest that MO preserves the AKT activity in H2O2-treated SH-SY5Y cells.
Figure 5.
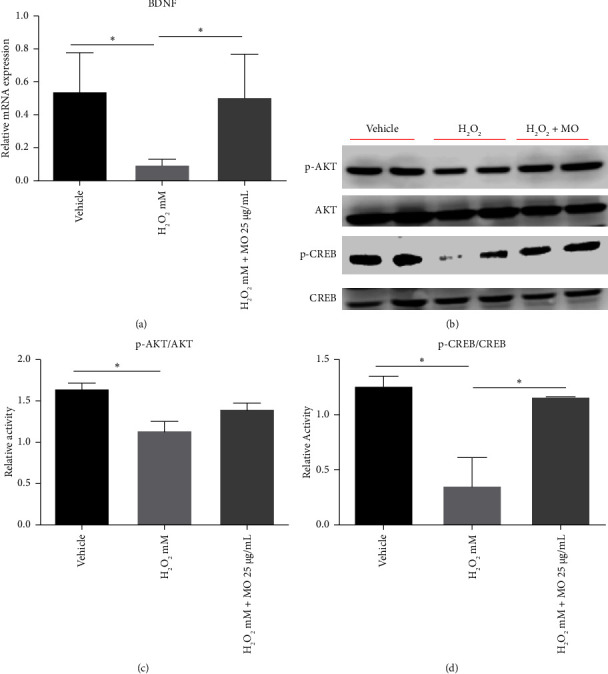
The effects of Moringa oleifera (MO) extract on the mRNA expressions of BDNF (a). The representative immunoblotting image in the SH-SY5Y cells of phosphorylated AKT (p-AKT), total AKT (AKT), phosphorylated CREB (p-CREB), and total CREB (CREB) (b). Quantified band analysis of p-AKT protein expression and normalized to AKT protein expression (c). Quantified band analysis of p-CREB protein expression and normalized to CREB protein expression (d). Each bar represents the mean relative mRNA expression ± SEM of four samples. Data analysis was performed using one way ANOVA, followed by Tukey's multicomparison test. ns: not significant, ∗: p < 0.05.
4. Discussion
MO is receiving significant attention as a potential treatment for neurodegenerative disease due to its antioxidant properties. Several neuroprotective phytochemicals, such as epigallocatechin gallate, quercetin, gallic acid, and genistein, have been isolated from MO, indicating its neuroprotective properties [23]. The compounds palmitic acid, oleic acid, stearic acid, stigmasterol, and β sitosterol exhibited the highest percentages in our previous gas chromatography/mass spectrometry (GC/MS) analysis with MOE from the same manufacturer [24]. This MOE has also been evaluated as a neuroprotective agent in mice with scopolamine-induced memory impairment, demonstrating promising outcomes [24]. Therefore, our study aimed to evaluate the neuroprotective pathway that potentially contributes to the nootropic effect of MO.
Our study found no decreased cell viability from 1 μg/mL to 100 μg/mL MOE concentration. This result is in accordance with another study that found that MOE did not impact cell viability until 100 μg/mL, with cell viability being compromised at 250 μg/mL and 500 μg/mL [17]. Another study showed that moringin, a bioactive compound found in MO, effectively upregulated the expression of p53, p21, and Bax proteins in SH-SY5Y, leading to cell cycle arrest [25]. Moringin also suppressed the activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), resulting in a decrease in its viability [25]. These concentration results can be a reference for further in vivo studies to maximize the therapeutic effect and minimize the adverse effect.
In our study, we observed that treating SH-SY5Y cells with a concentration of 25 μg/mL MOE resulted in the greatest improvement in cell viability when exposed to H2O2-induced cytotoxicity. However, a concentration of 10 μg/mL did not show any significant increase in cell viability. Interestingly, our findings contradict those of a prior investigation, which demonstrated that the viability of cells was enhanced at concentrations of 5, 10, and 25 μg/mL of MOE, with comparable values [17]. The difference in the MO extraction method can cause this contradiction. Our study utilized water extraction, whereas another study utilized methanol extraction. We use water extraction because it contains more alkaloids and saponins than methanol extraction, contributing to better antioxidant properties. However, water extraction has a lower phenol concentration [26, 27]. When choosing between water extraction, ethanol extraction, or other methods of MO extraction, it is important to carefully consider their chemical constituents.
Our study discovered that MOE improved the reduced expression of GPx, caspase, and SOD mRNA following H2O2 induction. Consistent with our research, a study conducted by Hashim et al. showed that MO leaves exhibited the most potent antioxidant activity compared to other plants examined. This effect was likely attributed to the high amount of polyphenolic compounds in MO leaves [28]. Extensive research has shown that a significant number of polyphenolic compounds can exert beneficial effects on neuroprotection by reducing inflammation, oxidative stress, and protein fibrillation [29]. Therefore, our study confirmed that MOE has capabilities to increase endogenous antioxidant enzymes.
MOE treatment significantly decreased Bax and caspase 3 mRNA expressions in H2O2-induced SH-SY5Y cells compared with the control group. In line with our study, concurrent treatment with MOE ameliorated oxidative stress, inflammation, and apoptosis in the brain cortex of rats exposed to lead acetate [30]. While an appropriate dose of MO reduces caspase expression, high dose would increase the expression of both caspases 3 and 9, indicating that MO could be toxic in high dose [25]. Caspase could induce chromatic condensation, DNA fragmentation, and blebbing, triggering an intrinsic apoptotic cascade [31]. Hence, MOE has the capacity to decrease the apoptotic markers in a neurotoxicity model with appropriate dosage, suggesting its role as a neuroprotective agent in neurons.
MOE cotreatment with H2O2 was observed to enhance the BDNF mRNA expression compared with cells treated with H2O2 alone. The activation of Akt and CREB may account for the increased BDNF expression, as shown by the increasing band density of the MOE group on the western blot. BDNF will activate signaling brain plasticity pathways such as phosphoinositide-3-kinase–protein kinase B/protein kinase B (PI3K/Akt), phospholipase C/inositol trisphosphate/Ca2+/calmodulin-dependent protein kinase II (PLC/IP3/CAMKII), and mitogen-activated protein kinase/extracellular signal-regulated kinase 1/2 (MAPK/Erk) pathways, which will increase synaptogenesis and neurogenesis [32, 33]. Our findings are consistent with those of a previous study indicating that MO can reverse the decline of CREB, albeit using a different MO preparation and cell model [34]. The effect of MO on the AKT pathway has been investigated in numerous studies employing various models with promising results [35–37].
Some limitations are needed to be addressed in this study. This study aims to investigate the efficacy of MOE in protecting SH-SY5Y cells after oxidative stress exposure. The herb company made the MOE and provided it to us. Therefore, we did not analyze the characterization or the chemical compositions of the extract. Further studies are necessary to be performed by using GC/MS or liquid chromatography mass spectrometry/mass spectrometry (LC-MS/MS) to analyze those issues. Moreover, future studies are also needed to perform endogenous antioxidant enzymes such as GPx, catalase, or SOD to further investigate those enzyme activities more in details. Another limitation of this study is the lack of experimental replicates, which could impact the reproducibility of the results. Future studies should address this by including multiple replicates to confirm the findings.
In conclusion, we revealed that the water extract of MO has neuroplasticity potential by upregulating BDNF expression via activating the Akt and CREB signaling pathways. We postulated that these effects were due to MOE's antioxidant (increasing GPx1, catalase, and SOD1 expressions) and antiapoptotic (decreasing Bax and caspase 3 expressions) properties.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
A.J.B., W.A., and H.J.L. conceived and designed the experiments. A.J.B., Y.R.D., and M.S.S. performed the experiments. A.J.B., W.A., and H.H. analyzed the data and wrote the manuscript. All the authors have checked and approved the final submitted version.
Funding
This research was supported by grants from Magister Research Grant, Directorate General of Research Enhancement and Development, Ministry of Research Technology and Higher Education (NKB-926/UN2.RST/HKP.05.00/2022), and the PUTI Q1 2022 Grant from Universitas Indonesia, under grant number NKB-444/UN2.RST/HKP.05.00/2022.
Supporting Information
Additional supporting information can be found online in the Supporting Information section.
Supporting Figure 1: Flowchart illustrating the water extracts of Moringa oleifera leaves process. (a) Extraction to evaporation process. (b) Drying to packing process. Supporting Figure 2: Water extracts of Moringa oleifera composition.
References
- 1.Schieber M., Chandel N. S. ROS Function in Redox Signaling and Oxidative Stress. Current Biology . 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., O W., Li W., Jiang Z.-G., Ghanbari H. Oxidative Stress and Neurodegenerative Disorders. International Journal of Molecular Sciences . 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson D. S. A., Oliver P. L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants . 2020;9:p. 743. doi: 10.3390/antiox9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan S., Wood M., Maher P. Oxidative Stress Induces a Form of Programmed Cell Death With Characteristics of Both Apoptosis and Necrosis in Neuronal Cells. Journal of Neurochemistry . 2002;71:95–105. doi: 10.1046/j.1471-4159.1998.71010095.x. [DOI] [PubMed] [Google Scholar]
- 5.Cao K., Dong Y.-T., Xiang J., et al. Reduced Expression of SIRT1 and SOD-1 and the Correlation Between These Levels in Various Regions of the Brains of Patients With Alzheimer’s Disease. Journal of Clinical Pathology . 2018;71:1090–1099. doi: 10.1136/jclinpath-2018-205320. [DOI] [PubMed] [Google Scholar]
- 6.Shichiri M. The Role of Lipid Peroxidation in Neurological Disorders. Journal of Clinical Biochemistry & Nutrition . 2014;54:151–160. doi: 10.3164/jcbn.14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim S.-Y., Kim H.-S. Oxidative Stress and the Antioxidant Enzyme System in the Developing Brain. Korean Journal of Pediatrics . 2013;56:p. 107. doi: 10.3345/kjp.2013.56.3.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A., Kukreti R., Saso L., Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules . 2019;24:p. 1583. doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. BDNF Function and Intracellular Signaling in Neurons. Histology & Histopathology . 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 10.Hacioglu G., Senturk A., Ince I., Alver A. Assessment of Oxidative Stress Parameters of Brain-Derived Neurotrophic Factor Heterozygous Mice in Acute Stress Model. Iranian Journal of Basic Medical Sciences . 2016;19:388–393. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu A., Ying Z., Gomez-Pinilla F. The Interplay Between Oxidative Stress and Brain-Derived Neurotrophic Factor Modulates the Outcome of a Saturated Fat Diet on Synaptic Plasticity and Cognition. European Journal of Neuroscience . 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 12.Fifi L., Reni S., Edi W., Taufiqurrahman T. Use of Local Kelor (Moringa oleifera) Leaves Powder From West Nusa Tenggara to Increase the Neutrophile Cell Phagocyte Index and Its Function on Rats With PEM Infected by Staphylococcus aureus. Linguistics and Culture Review . 2022;6:75–86. [Google Scholar]
- 13.Ma Z. F., Ahmad J., Zhang H., Khan I., Muhammad S. Evaluation of Phytochemical and Medicinal Properties of Moringa (Moringa oleifera) as a Potential Functional Food. South African Journal of Botany . 2020;129:40–46. [Google Scholar]
- 14.Bhattacharya A., Tiwari P., Sahu P., Kumar S. A Review of the Phytochemical and Pharmacological Characteristics of Moringa oleifera. Journal of Pharmacy and BioAllied Sciences . 2018;10:p. 181. doi: 10.4103/JPBS.JPBS_126_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutalangka C., Wattanathorn J., Muchimapura S., Thukham-mee W. Moringa oleifera Mitigates Memory Impairment and Neurodegeneration in Animal Model of Age-Related Dementia. Oxidative Medicine and Cellular Longevity . 2013;2013:1–9. doi: 10.1155/2013/695936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xicoy H., Wieringa B., Martens G. J. M. The SH-Sy5y Cell Line in Parkinson’s Disease Research: A Systematic Review. Molecular Neurodegeneration . 2017;12:p. 10. doi: 10.1186/s13024-017-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González-Burgos E., Ureña-Vacas I., Sánchez M., Gómez-Serranillos M. P. Nutritional Value of Moringa oleifera Lam. Leaf Powder Extracts and Their Neuroprotective Effects via Antioxidative and Mitochondrial Regulation. Nutrients . 2021;13:p. 2203. doi: 10.3390/nu13072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barinda A. J., Arozal W., Sandhiutami N. M. D., et al. Curcumin Prevents Epithelial-to Mesenchymal Transition-Mediated Ovarian Cancer Progression Through NRF2/ETBR/ET-1 Axis and Preserves Mitochondria Biogenesis in Kidney After Cisplatin Administration. Advanced Pharmaceutical Bulletin . 2020;12 doi: 10.34172/apb.2022.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arozal W., Monayo E. R., Barinda A. J., et al. Protective Effects of Silver Nanoparticles in Isoproterenol-Induced Myocardial Infarction in Rats. Frontiers of Medicine . 2022;9 doi: 10.3389/fmed.2022.867497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morén C., DeSouza R. M., Giraldo D. M., Uff C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. International Journal of Molecular Sciences . 2022;23:p. 9328. doi: 10.3390/ijms23169328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B., Nagappan G., Guan X., Nathan P. J., Wren P. BDNF-Based Synaptic Repair as a Disease-Modifying Strategy for Neurodegenerative Diseases. Nature Reviews Neuroscience . 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 22.Zarneshan S. N., Fakhri S., Khan H. Targeting Akt/CREB/BDNF Signaling Pathway by Ginsenosides in Neurodegenerative Diseases: A Mechanistic Approach. Pharmacological Research . 2022;177:p. 106099. doi: 10.1016/j.phrs.2022.106099. [DOI] [PubMed] [Google Scholar]
- 23.Ghimire S., Subedi L., Acharya N., Gaire B. P. Moringa Oleifera: A Tree of Life as a Promising Medicinal Plant for Neurodegenerative Diseases. Journal of Agricultural and Food Chemistry . 2021;69:14358–14371. doi: 10.1021/acs.jafc.1c04581. [DOI] [PubMed] [Google Scholar]
- 24.Arozal W., Purwoningsih E., Lee H. J., Barinda A. J., Munim A. Effects of Moringa oleifera in Two Independents Formulation and as Neuroprotective Agent Against Scopolamine-Induced Memory Impairment in Mice. Frontiers in Nutrition . 2022;9 doi: 10.3389/fnut.2022.799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cirmi S., Ferlazzo N., Gugliandolo A., et al. Moringin From Moringa oleifera Seeds Inhibits Growth, Arrests Cell-Cycle, and Induces Apoptosis of SH-Sy5y Human Neuroblastoma Cells Through the Modulation of NF-Κb and Apoptotic Related Factors. International Journal of Molecular Sciences . 2019;20:p. 1930. doi: 10.3390/ijms20081930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asghar N., Aziz A., Farooq Azhar M., et al. Assessment of Phytochemical Analysis, Nutritional Composition and Antimicrobial Activity of Moringa oleifera. Phyton . 2022;91:1817–1829. [Google Scholar]
- 27.Vongsak B., Sithisarn P., Mangmool S., Thongpraditchote S., Wongkrajang Y., Gritsanapan W. Maximizing Total Phenolics, Total Flavonoids Contents and Antioxidant Activity of Moringa oleifera Leaf Extract by the Appropriate Extraction Method. Industrial Crops & Products . 2013;44:566–571. [Google Scholar]
- 28.Hashim F J., Vichitphan S., Boonsiri P., Vichitphan K. Neuroprotective Assessment of Moringa oleifera Leaves Extract Against Oxidative-Stress-Induced Cytotoxicity in SHSY5Y Neuroblastoma Cells. Plants . 2021;10:p. 889. doi: 10.3390/plants10050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva R. F. M., Pogačnik L. Polyphenols From Food and Natural Products: Neuroprotection and Safety. Antioxidants . 2020;9:p. 61. doi: 10.3390/antiox9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alqahtani W. S., Albasher G. Moringa oleifera Lam. Extract Rescues Lead‐Induced Oxidative Stress, Inflammation, and Apoptosis in the Rat Cerebral Cortex. Journal of Food Biochemistry . 2021;45 doi: 10.1111/jfbc.13579. [DOI] [PubMed] [Google Scholar]
- 31.Porter A. G., Jänicke R. U. Emerging Roles of Caspase-3 in Apoptosis. Cell Death & Differentiation . 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 32.Ayaz M., Sadiq A., Junaid M., et al. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Frontiers in Aging Neuroscience . 2019;11 doi: 10.3389/fnagi.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cichon N., Saluk-Bijak J., Gorniak L., Przyslo L., Bijak M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants . 2020;9:p. 1035. doi: 10.3390/antiox9111035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J., Yang W., Suo D., et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Frontiers in Pharmacology . 2018;9 doi: 10.3389/fphar.2018.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tragulpakseerojn J., Yamaguchi N., Pamonsinlapatham P., et al. Anti-Proliferative Effect of Moringa oleifera Lam (Moringaceae) Leaf Extract on Human Colon Cancer HCT116 Cell Line. Tropical Journal of Pharmaceutical Research . 2017;16:p. 371. [Google Scholar]
- 36.Bian X., Wang Y., Yang R., et al. Anti‐Fatigue Properties of the Ethanol Extract of Moringa oleifera Leaves in Mice. Journal of the Science of Food and Agriculture . 2023;103:5500–5510. doi: 10.1002/jsfa.12628. [DOI] [PubMed] [Google Scholar]
- 37.Liu M., Ding H., Wang H., et al. Moringa oleifera Leaf Extracts Protect BMSC Osteogenic Induction Following Peroxidative Damage by Activating the PI3K/Akt/Foxo1 Pathway. Journal of Orthopaedic Surgery and Research . 2021;16:p. 150. doi: 10.1186/s13018-021-02284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information can be found online in the Supporting Information section.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


