Abstract
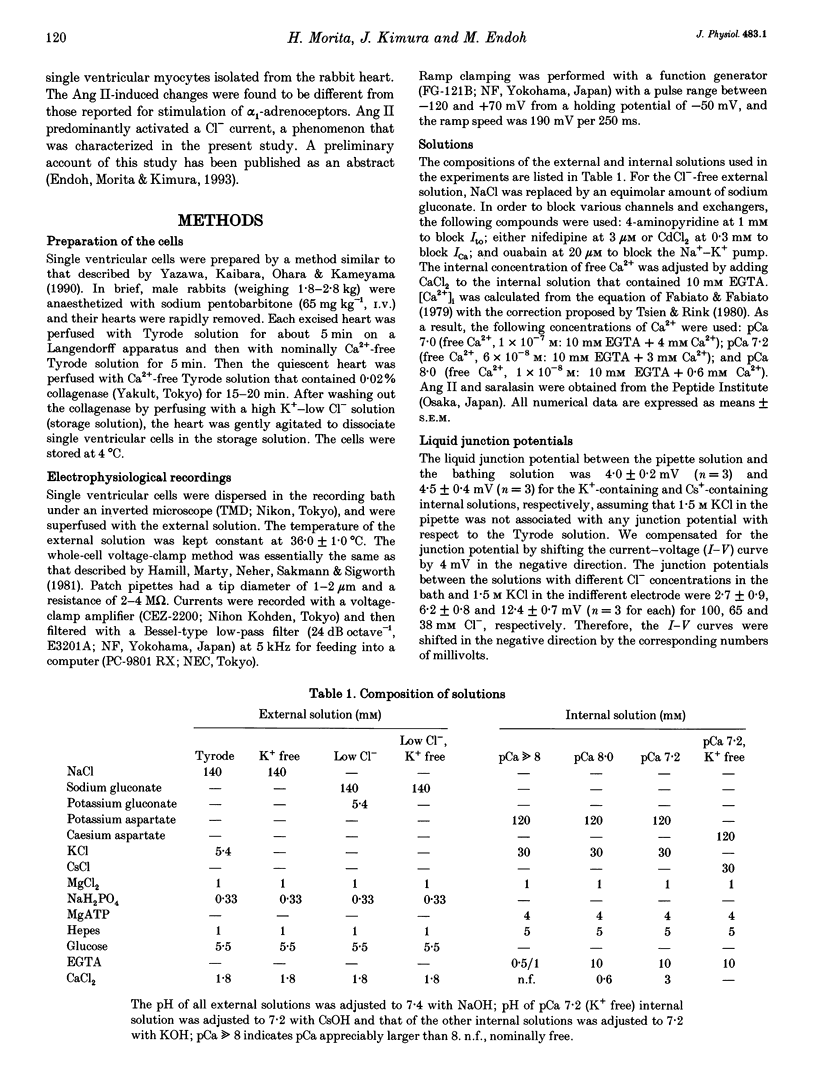
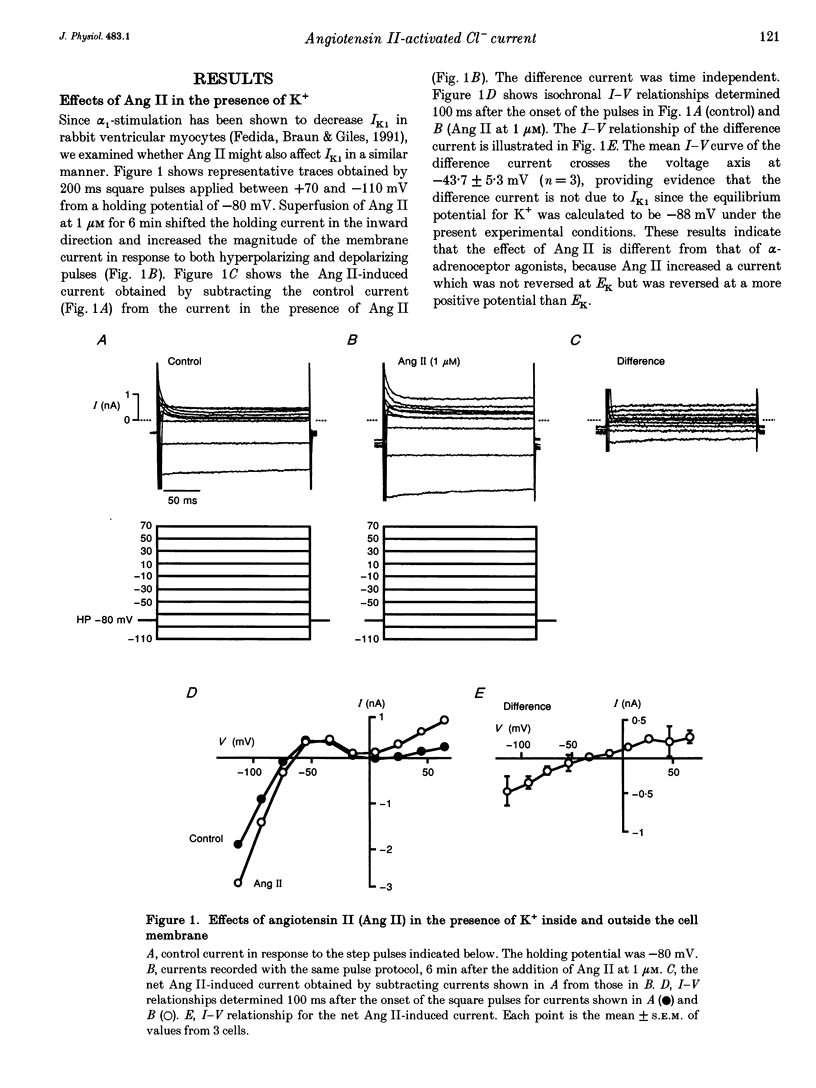
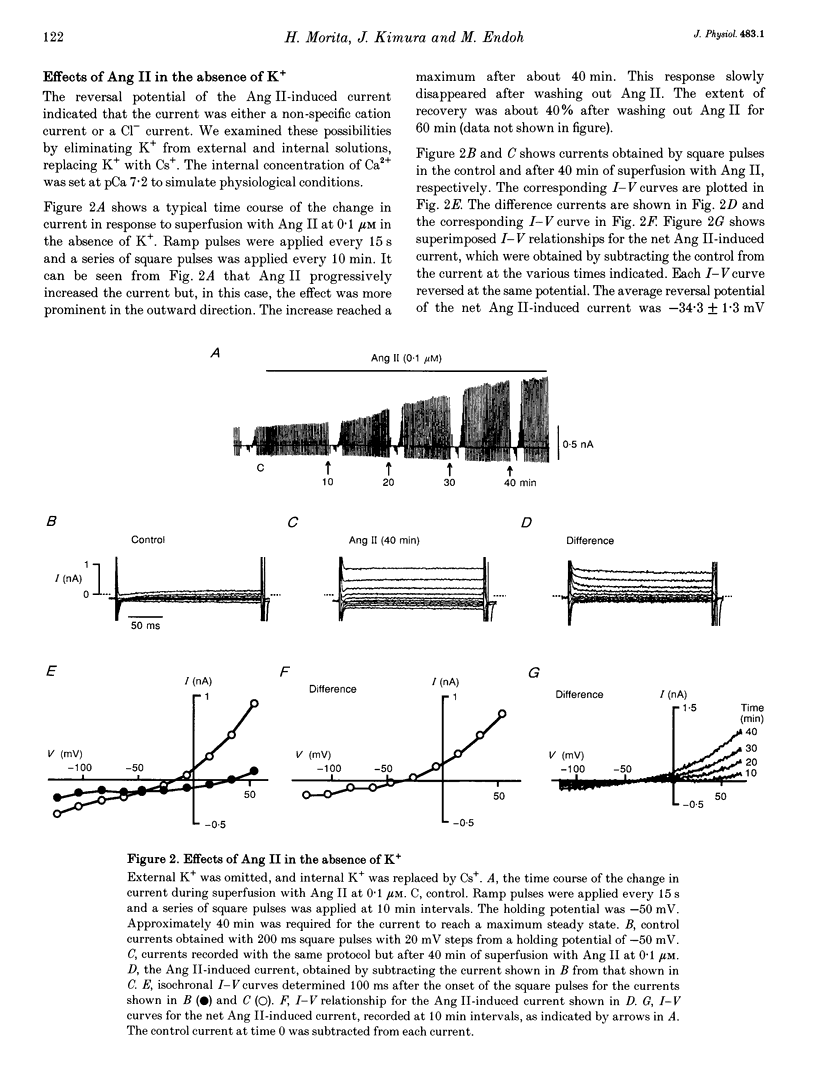
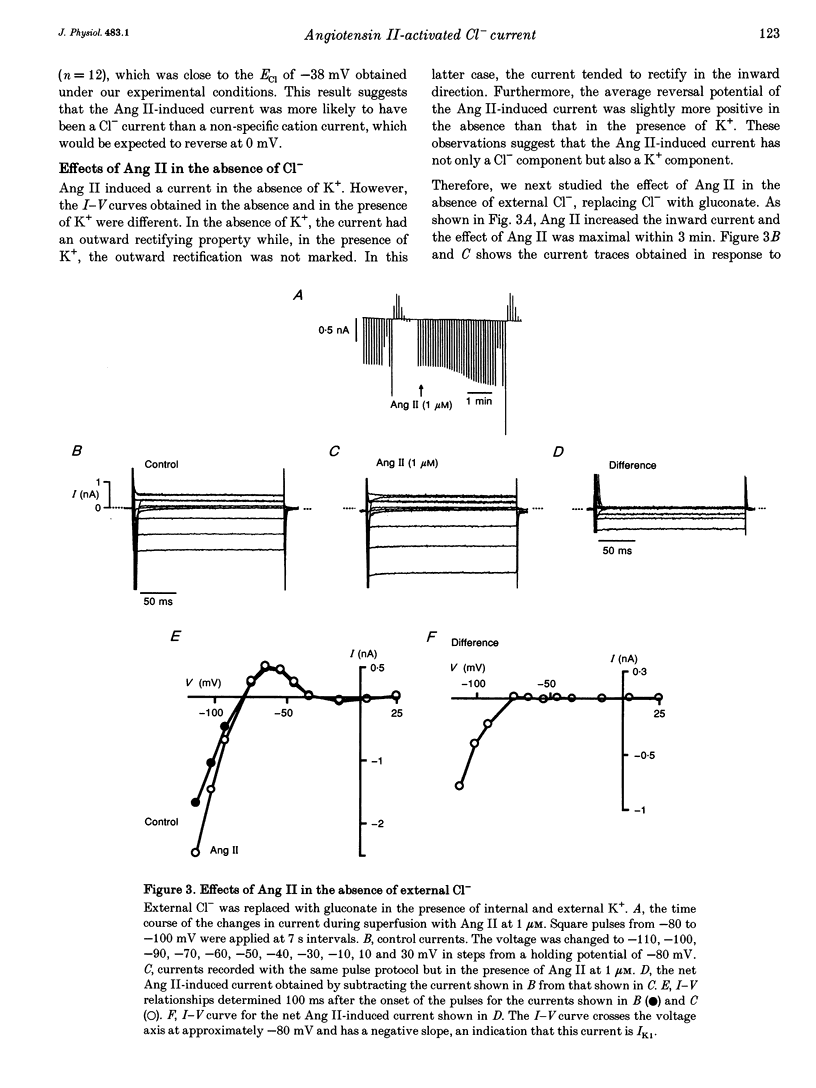
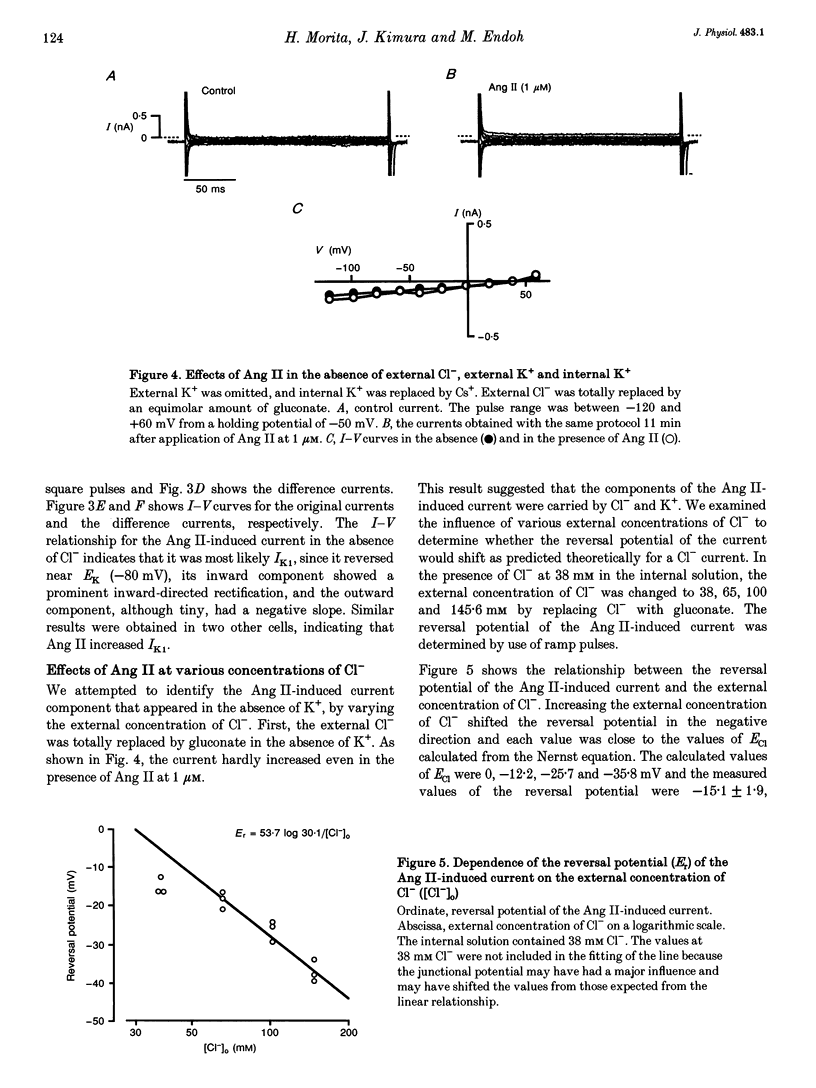
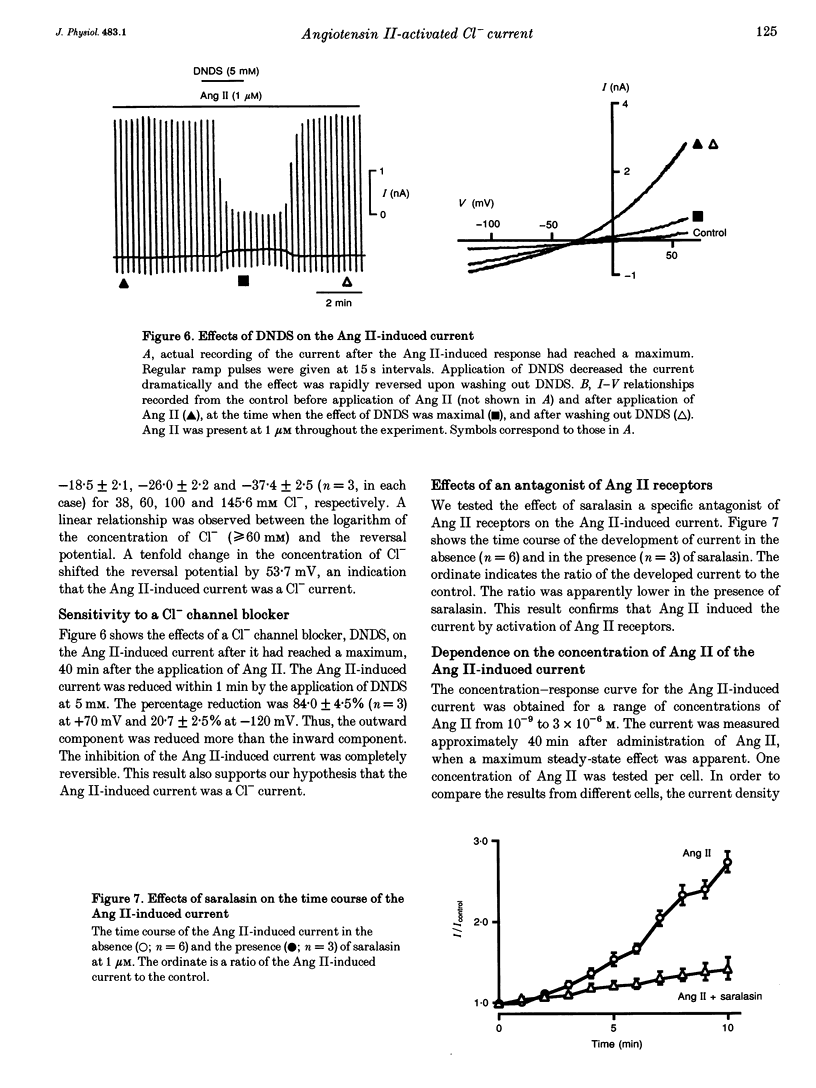
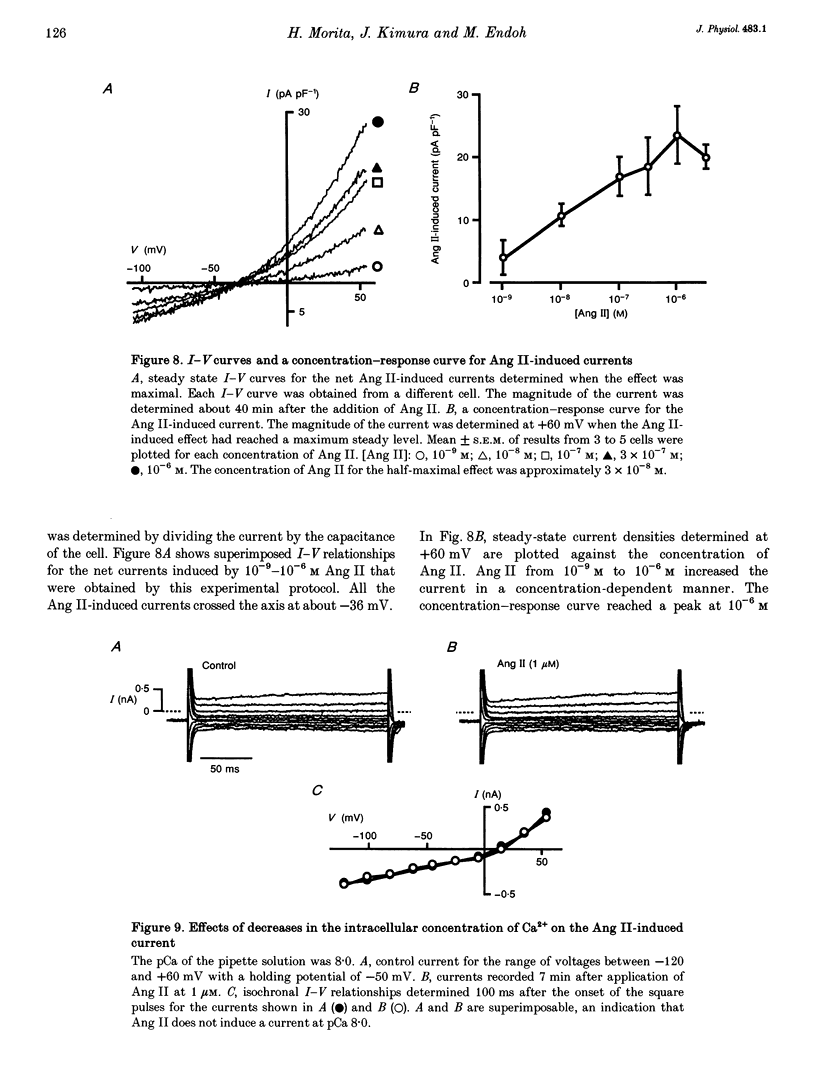
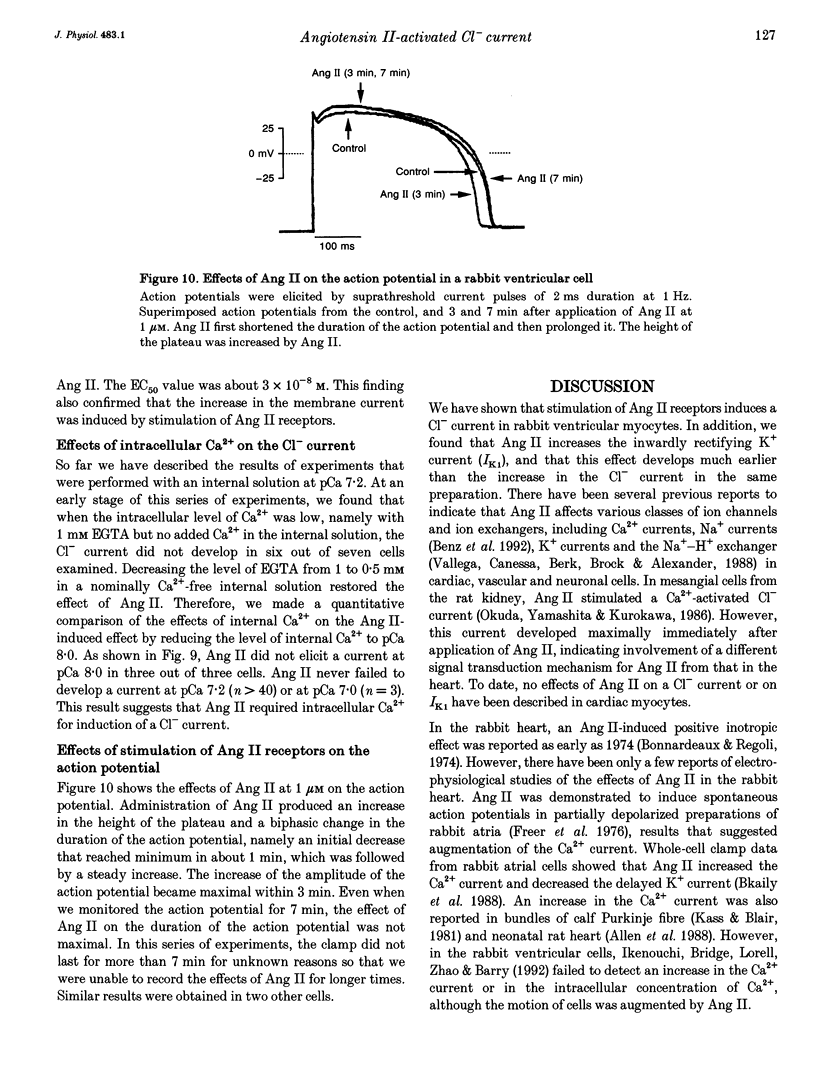
1. The effects of angiotensin II (Ang II) on membrane currents were investigated in single ventricular myocytes from the rabbit heart by the whole-cell voltage-clamp method. 2. In the presence of an inhibitor of Ca2+ currents (nifedipine at 3 microM or CdCl2 at 0.3 mM) and a beta-adrenoceptor blocker (bupranolol at 1 microM), 1 microM Ang II significantly increased the membrane conductance. 3. After elimination of K+ from external and internal solutions and its replacement by Cs+, Ang II at 0.1 microM increased an outwardly rectifying current that reached a maximum after about 40 min. The effect was concentration dependent (10(-9)-10(-6) M) and was inhibited by saralasin, an antagonist of Ang II receptors. 4. The reversal potential of the Ang II-induced current in the absence of K+ was compatible with the Cl- equilibrium potential at various external concentrations of Cl-. 5. A Cl- channel blocker, 4,4'-dinitrostilbene-2,2'-disulphonic acid (DNDS, at 5 mM), reversibly decreased the Ang II-induced current. 6. The Ang II-induced current developed when the internal solution contained Ca2+ (pCa 7.2 or 7.0) but not when it contained 10 mM EGTA without Ca2+. 7. Besides developing a Cl- current, Ang II at 1 microM increased the inwardly rectifying K+ current (IK1) and this effect reached maximum within 3 min. 8. The effect of Ang II on the action potential was biphasic: the duration of the action potential was initially reduced and then it was increased. 9. These results suggest that Ang II induces a Cl- current that appears likely to modulate the action potential in rabbit ventricular myocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen I. S., Cohen N. M., Dhallan R. S., Gaa S. T., Lederer W. J., Rogers T. B. Angiotensin II increases spontaneous contractile frequency and stimulates calcium current in cultured neonatal rat heart myocytes: insights into the underlying biochemical mechanisms. Circ Res. 1988 Mar;62(3):524–534. doi: 10.1161/01.res.62.3.524. [DOI] [PubMed] [Google Scholar]
- Baker K. M., Booz G. W., Dostal D. E. Cardiac actions of angiotensin II: Role of an intracardiac renin-angiotensin system. Annu Rev Physiol. 1992;54:227–241. doi: 10.1146/annurev.ph.54.030192.001303. [DOI] [PubMed] [Google Scholar]
- Baker K. M., Singer H. A. Identification and characterization of guinea pig angiotensin II ventricular and atrial receptors: coupling to inositol phosphate production. Circ Res. 1988 May;62(5):896–904. doi: 10.1161/01.res.62.5.896. [DOI] [PubMed] [Google Scholar]
- Benz I., Herzig J. W., Kohlhardt M. Opposite effects of angiotensin II and the protein kinase C activator OAG on cardiac Na+ channels. J Membr Biol. 1992 Nov;130(2):183–190. doi: 10.1007/BF00231895. [DOI] [PubMed] [Google Scholar]
- Bkaily G., Peyrow M., Sculptoreanu A., Jacques D., Chahine M., Regoli D., Sperelakis N. Angiotensin II increases Isi and blocks IK in single aortic cell of rabbit. Pflugers Arch. 1988 Sep;412(4):448–450. doi: 10.1007/BF01907567. [DOI] [PubMed] [Google Scholar]
- Bonnardeaux J. L., Regoli D. Action of angiotensin and analogues on the heart. Can J Physiol Pharmacol. 1974 Feb;52(1):50–60. doi: 10.1139/y74-007. [DOI] [PubMed] [Google Scholar]
- Chen S. A., Chang M. S., Chiang B. N., Cheng K. K., Lin C. I. Electromechanical effects of angiotensin in human atrial tissues. J Mol Cell Cardiol. 1991 Apr;23(4):483–493. doi: 10.1016/0022-2828(91)90172-i. [DOI] [PubMed] [Google Scholar]
- Endoh M., Hiramoto T., Ishihata A., Takanashi M., Inui J. Myocardial alpha 1-adrenoceptors mediate positive inotropic effect and changes in phosphatidylinositol metabolism. Species differences in receptor distribution and the intracellular coupling process in mammalian ventricular myocardium. Circ Res. 1991 May;68(5):1179–1190. doi: 10.1161/01.res.68.5.1179. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fedida D., Braun A. P., Giles W. R. Alpha 1-adrenoceptors in myocardium: functional aspects and transmembrane signaling mechanisms. Physiol Rev. 1993 Apr;73(2):469–487. doi: 10.1152/physrev.1993.73.2.469. [DOI] [PubMed] [Google Scholar]
- Fedida D., Braun A. P., Giles W. R. Alpha 1-adrenoceptors reduce background K+ current in rabbit ventricular myocytes. J Physiol. 1991 Sep;441:673–684. doi: 10.1113/jphysiol.1991.sp018772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer R. J., Pappano A. J., Peach M. J., Bing K. T., McLean M. J., Vogel S., Sperelakis N. Mechanism for the postive inotropic effect of angiotensin II on isolated cardiac muscle. Circ Res. 1976 Aug;39(2):178–183. doi: 10.1161/01.res.39.2.178. [DOI] [PubMed] [Google Scholar]
- Hagiwara N., Masuda H., Shoda M., Irisawa H. Stretch-activated anion currents of rabbit cardiac myocytes. J Physiol. 1992 Oct;456:285–302. doi: 10.1113/jphysiol.1992.sp019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harvey R. D., Clark C. D., Hume J. R. Chloride current in mammalian cardiac myocytes. Novel mechanism for autonomic regulation of action potential duration and resting membrane potential. J Gen Physiol. 1990 Jun;95(6):1077–1102. doi: 10.1085/jgp.95.6.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M., Hwang T. C., Gadsby D. C. Pipette GTP is essential for receptor-mediated regulation of Cl- current in dialysed myocytes from guinea-pig ventricle. J Physiol. 1992 Sep;455:235–246. doi: 10.1113/jphysiol.1992.sp019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihata A., Endoh M. Pharmacological characteristics of the positive inotropic effect of angiotensin II in the rabbit ventricular myocardium. Br J Pharmacol. 1993 Apr;108(4):999–1005. doi: 10.1111/j.1476-5381.1993.tb13497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C. I. Franz Volhard Lecture. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992 Dec;10(7):S13–S26. [PubMed] [Google Scholar]
- Kass R. S., Blair M. L. Effects of angiotensin II on membrane current in cardiac Purkinje fibers. J Mol Cell Cardiol. 1981 Sep;13(9):797–809. doi: 10.1016/0022-2828(81)90237-6. [DOI] [PubMed] [Google Scholar]
- Kem D. C., Johnson E. I., Capponi A. M., Chardonnens D., Lang U., Blondel B., Koshida H., Vallotton M. B. Effect of angiotensin II on cytosolic free calcium in neonatal rat cardiomyocytes. Am J Physiol. 1991 Jul;261(1 Pt 1):C77–C85. doi: 10.1152/ajpcell.1991.261.1.C77. [DOI] [PubMed] [Google Scholar]
- Lindpaintner K., Ganten D. The cardiac renin-angiotensin system. An appraisal of present experimental and clinical evidence. Circ Res. 1991 Apr;68(4):905–921. doi: 10.1161/01.res.68.4.905. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Lacerda A. E., Brown A. M. Angiotensin II modulates cardiac Na+ channels in neonatal rat. Circ Res. 1989 Dec;65(6):1804–1809. doi: 10.1161/01.res.65.6.1804. [DOI] [PubMed] [Google Scholar]
- Moravec C. S., Schluchter M. D., Paranandi L., Czerska B., Stewart R. W., Rosenkranz E., Bond M. Inotropic effects of angiotensin II on human cardiac muscle in vitro. Circulation. 1990 Dec;82(6):1973–1984. doi: 10.1161/01.cir.82.6.1973. [DOI] [PubMed] [Google Scholar]
- Nilius B., Tytgat J., Albitz R. Modulation of cardiac Na channels by angiotensin II. Biochim Biophys Acta. 1989 Dec 14;1014(3):259–262. doi: 10.1016/0167-4889(89)90221-8. [DOI] [PubMed] [Google Scholar]
- Okuda T., Yamashita N., Kurokawa K. Angiotensin II and vasopressin stimulate calcium-activated chloride conductance in rat mesangial cells. J Clin Invest. 1986 Dec;78(6):1443–1448. doi: 10.1172/JCI112734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Tseng G. N. Cell swelling increases membrane conductance of canine cardiac cells: evidence for a volume-sensitive Cl channel. Am J Physiol. 1992 Apr;262(4 Pt 1):C1056–C1068. doi: 10.1152/ajpcell.1992.262.4.C1056. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vallega G. A., Canessa M. L., Berk B. C., Brock T. A., Alexander R. W. Vascular smooth muscle Na+-H+ exchanger kinetics and its activation by angiotensin II. Am J Physiol. 1988 Jun;254(6 Pt 1):C751–C758. doi: 10.1152/ajpcell.1988.254.6.C751. [DOI] [PubMed] [Google Scholar]
- Walsh K. B. Activation of a heart chloride current during stimulation of protein kinase C. Mol Pharmacol. 1991 Sep;40(3):342–346. [PubMed] [Google Scholar]
- Xu Y., Sandirasegarane L., Gopalakrishnan V. Protein kinase C inhibitors enhance endothelin-1 and attenuate vasopressin and angiotensin II evoked [Ca2+]i elevation in the rat cardiomyocyte. Br J Pharmacol. 1993 Jan;108(1):6–8. doi: 10.1111/j.1476-5381.1993.tb13430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa K., Kaibara M., Ohara M., Kameyama M. An improved method for isolating cardiac myocytes useful for patch-clamp studies. Jpn J Physiol. 1990;40(1):157–163. doi: 10.2170/jjphysiol.40.157. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Gibbons W. R. Calcium-activated chloride current in rabbit ventricular myocytes. Circ Res. 1991 Feb;68(2):424–437. doi: 10.1161/01.res.68.2.424. [DOI] [PubMed] [Google Scholar]


