Abstract
1. We have investigated the granule size distributions in human and horse eosinophils by time-resolved patch-clamp capacitance measurements. 2. During exocytosis of single granules the electrical capacitance of the plasma membrane increases in discrete steps. The steps in horse cells are about six times larger than those in human cells in accordance with the difference in granule size. 3. In both species a multimodal capacitance step size distribution is observed with a first peak at 6-7 fF corresponding to granules with a diameter of about 450-500 nm and a surface area of about 0.7 microns2, which we call the unit granule. The other peaks in the distributions correspond to multiples of the surface area of these units. 4. These results show that the larger granules are formed by fusion of several unit granules and the final size of mature granules is determined by the number of units allowed to fuse with each other. Whereas in human eosinophils most granules consist of one or two units, most granules of horse eosinophils are formed by fusion of seven to fifteen units. 5. The intracellular fusion events associated with vesicular traffic are believed to occur constitutively. In contrast, our results indicate that a cellular mechanism exists which regulates the size of the mature granules by determining the number of units allowed to fuse with each other. In view of our recent report that granule-granule fusion can be activated by GTP gamma S, this regulation may possibly involve GTP-binding proteins.
Full text
PDF


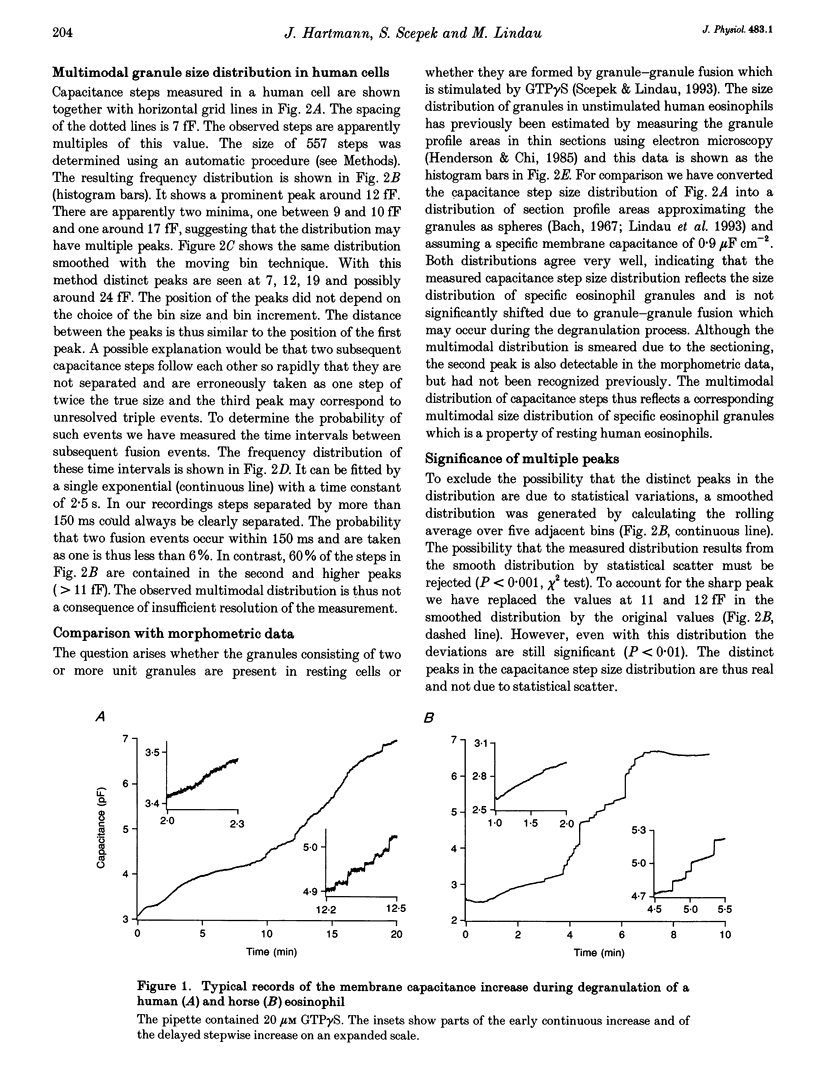
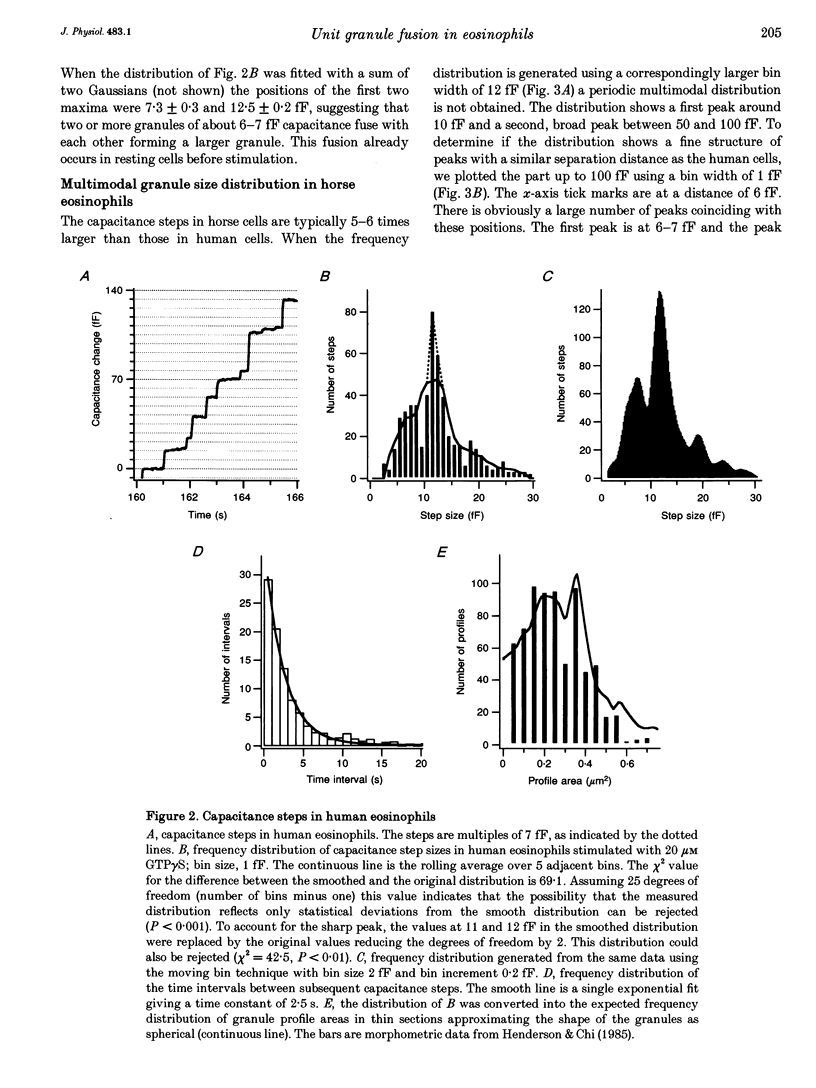
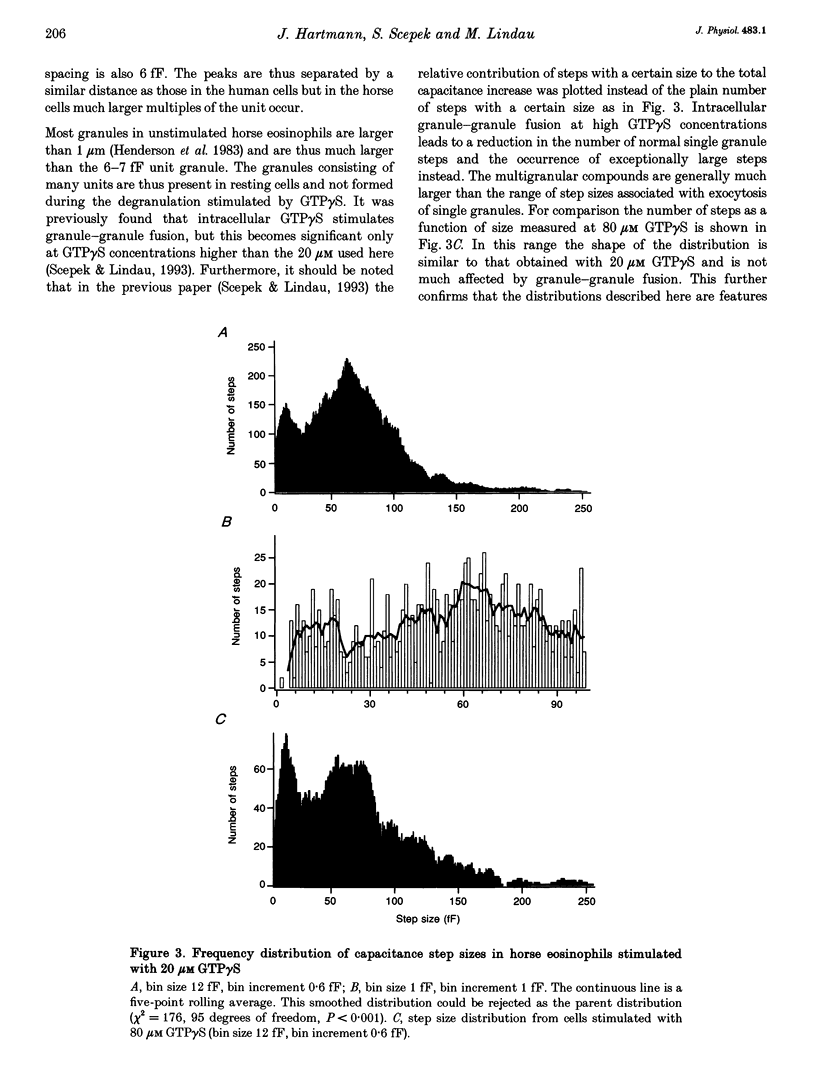
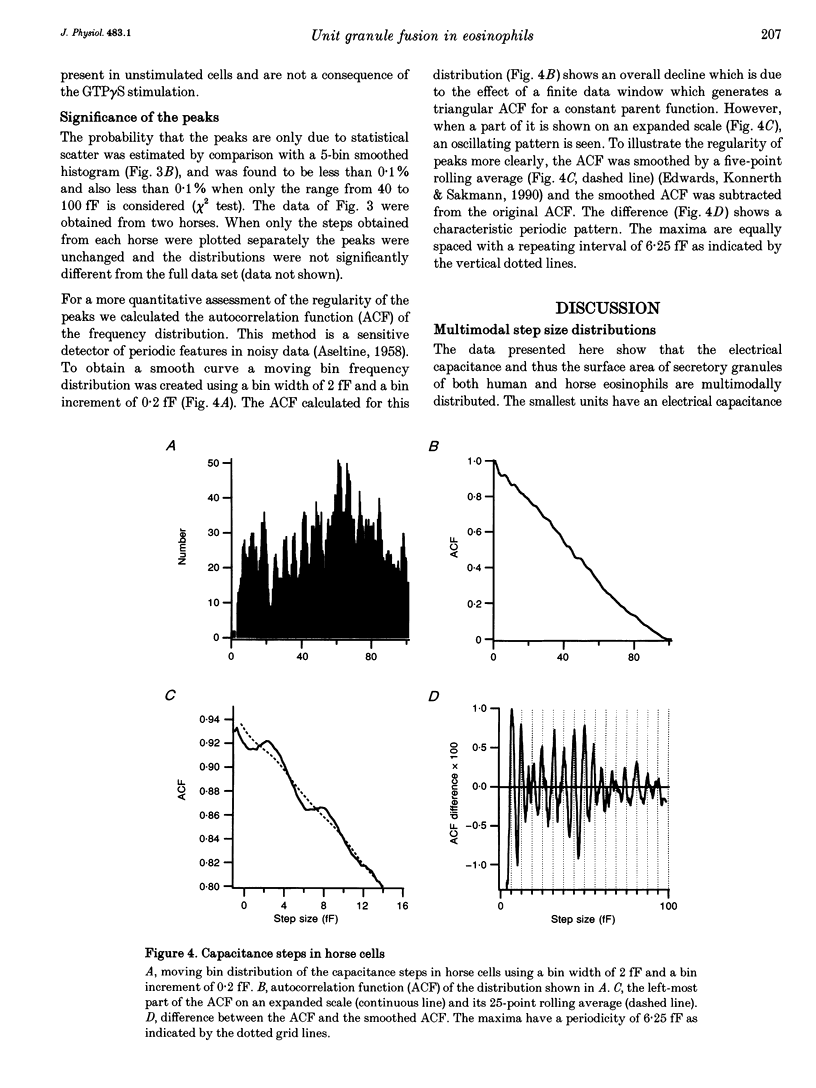


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T., HIRSCH J. G. ISOLATION OF GRANULES FROM EOSINOPHIL LEUCOCYTES AND STUDY OF THEIR ENZYME CONTENT. J Exp Med. 1963 Aug 1;118:277–286. doi: 10.1084/jem.118.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez de Toledo G., Fernandez J. M. Patch-clamp measurements reveal multimodal distribution of granule sizes in rat mast cells. J Cell Biol. 1990 Apr;110(4):1033–1039. doi: 10.1083/jcb.110.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton D. F., Farquhar M. G. Segregation and packaging of granule enzymes in eosinophilic leukocytes. J Cell Biol. 1970 Apr;45(1):54–73. doi: 10.1083/jcb.45.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E. Small GTP-binding proteins in vesicular transport. Trends Biochem Sci. 1990 Dec;15(12):473–477. doi: 10.1016/0968-0004(90)90301-q. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Sanders D. A., McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990 Nov 8;348(6297):125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- Calafat J., Kuijpers T. W., Janssen H., Borregaard N., Verhoeven A. J., Roos D. Evidence for small intracellular vesicles in human blood phagocytes containing cytochrome b558 and the adhesion molecule CD11b/CD18. Blood. 1993 Jun 1;81(11):3122–3129. [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel I., Dvorak A. M., Peters S. P., Schulman E. S., Dvorak H. F., Lichtenstein L. M., Galli S. J. Differences in the volume distributions of human lung mast cell granules and lipid bodies: evidence that the size of these organelles is regulated by distinct mechanisms. J Cell Biol. 1985 May;100(5):1488–1492. doi: 10.1083/jcb.100.5.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel I., Lagunoff D., Bauza M., Chi E. Periodic, multimodal distribution of granule volumes in mast cells. Cell Tissue Res. 1983;228(1):51–59. doi: 10.1007/BF00206264. [DOI] [PubMed] [Google Scholar]
- Hammel I., Lagunoff D., Krüger P. G. Studies on the growth of mast cells in rats. Changes in granule size between 1 and 6 months. Lab Invest. 1988 Oct;59(4):549–554. [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Jörg A., Klebanoff S. J. Horse eosinophil degranulation induced by the ionophore A23187. Ultrastructure and role of phospholipase A2. Am J Pathol. 1983 Jun;111(3):341–349. [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y. Ultrastructural characterization and morphometric analysis of human eosinophil degranulation. J Cell Sci. 1985 Feb;73:33–48. doi: 10.1242/jcs.73.1.33. [DOI] [PubMed] [Google Scholar]
- Leyte A., Barr F. A., Kehlenbach R. H., Huttner W. B. Multiple trimeric G-proteins on the trans-Golgi network exert stimulatory and inhibitory effects on secretory vesicle formation. EMBO J. 1992 Dec;11(13):4795–4804. doi: 10.1002/j.1460-2075.1992.tb05585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D., Jones A., Malinow R. Direct measurement of quantal changes underlying long-term potentiation in CA1 hippocampus. Neuron. 1992 Dec;9(6):1089–1097. doi: 10.1016/0896-6273(92)90068-o. [DOI] [PubMed] [Google Scholar]
- Lindau M., Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988 Feb;411(2):137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- Lindau M., Nüsse O., Bennett J., Cromwell O. The membrane fusion events in degranulating guinea pig eosinophils. J Cell Sci. 1993 Jan;104(Pt 1):203–210. doi: 10.1242/jcs.104.1.203. [DOI] [PubMed] [Google Scholar]
- Lindau M. Time-resolved capacitance measurements: monitoring exocytosis in single cells. Q Rev Biophys. 1991 Feb;24(1):75–101. doi: 10.1017/s0033583500003279. [DOI] [PubMed] [Google Scholar]
- Neher E., Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsse O., Lindau M., Cromwell O., Kay A. B., Gomperts B. D. Intracellular application of guanosine-5'-O-(3-thiotriphosphate) induces exocytotic granule fusion in guinea pig eosinophils. J Exp Med. 1990 Mar 1;171(3):775–786. doi: 10.1084/jem.171.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahamimoff R., Yaari Y. Delayed release of transmitter at the frog neuromuscular junction. J Physiol. 1973 Jan;228(1):241–257. doi: 10.1113/jphysiol.1973.sp010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scepek S., Lindau M. Focal exocytosis by eosinophils--compound exocytosis and cumulative fusion. EMBO J. 1993 May;12(5):1811–1817. doi: 10.1002/j.1460-2075.1993.tb05829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A. Biogenesis of secretory granules. Implications arising from the immature secretory granule in the regulated pathway of secretion. FEBS Lett. 1991 Jul 22;285(2):220–224. doi: 10.1016/0014-5793(91)80805-d. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Flatmark T., Tooze J., Huttner W. B. Characterization of the immature secretory granule, an intermediate in granule biogenesis. J Cell Biol. 1991 Dec;115(6):1491–1503. doi: 10.1083/jcb.115.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]


