Abstract
Parkinson’s Disease is a progressive neurodegenerative disorder afflicting almost 12 million people. Increased understanding of its complex and heterogenous disease pathology, etiology and symptom manifestations has resulted in the need to design, capture and interrogate substantial clinical datasets. Herein we advocate how advances in the deployment of artificial intelligence models for Federated Data Analysis and Federated Learning can help spearhead coordinated and sustainable approaches to address this grand challenge.
Subject terms: Predictive markers, Predictive markers
Introduction
The drug development process remains an arduous task spanning target identification, drug design, preclinical development, clinical evaluation and, finally, regulatory approval. The current investment required to bring a successful drug to market is estimated to exceed $2B1 in aggregated costs and yielding clinical success rates of <10%2. Within the neurosciences, the success rates are often lower, a consequence of the added complexities in drug transport, brain physiology, and target heterogeneity, prompting many pharmaceutical companies to prioritize investments in other indications3. Despite these challenges the search for new CNS agents continues at a fast pace due to high unmet need, high disease burden and rapidly growing societal needs. Neurodegenerative diseases such as Alzheimer’s and PD represent some of the greatest challenges for the healthcare system, afflicting over 50 million people and inflicting a >$300B economic burden4. Indeed, PD is regarded as the world’s fastest-growing neurodegenerative condition, with a growth that takes the shape of a pandemic5. The latest data show that currently 11.8 million people worldwide are affected by PD6. Reflecting the magnitude of the task, it is little surprise that current efforts involve collaborative approaches between industry, research communities and patient associations, further facilitated in the PD arena by consortia and foundations such as the Critical Path Institute for PD (CPP), Michael J Fox Foundation (MJFF), Accelerating Medicines Partnership Program for PD (AMP-PD), the Parkinson’s Progression Markers initiative (PPMI), the Innovative Medicines Initiative (IMI), and others. Such public-private dialog has already begun to have an impact in the Alzheimer’s Disease (AD) community, where coordinated inputs from patient groups have played a key role in the market access debate for recently approved therapeutics7.
In PD, although symptomatic relief is available through L-Dopa and other treatments, the pursuit of the first disease-modifying therapies continues apace with over sixty active clinical studies involving candidate drugs8, and complimentary approaches investigating lifestyle interventions9. The diversity of pharmacological approaches being pursued (small molecule, biologics, cell & gene therapies, anti-inflammatories) is reflective of the complexities of the disease, with a multifaceted pathophysiology, broad phenotypic heterogeneity and pro-dromal periods which can span several decades before evolving into clinically diagnosable motor and non-motor clinical entities10. There are now suggestions to redefine Parkinson’s biologically based on genetic and biomarker classification systems11–13. For example, a large part of the spectrum of PD and related disorders could be considered “Neuronal alpha-Synuclein Disease” (NSD), in a spectrum, which would also encompass disorders such as dementia with Lewy bodies, which display characteristic Lewy body and Lewy neurite pathology in neurons of the central and peripheral nervous systems11,12. The complexities of designing and evaluating drugs, which might function to attenuate, ameliorate or reverse patient symptoms, are compounded by the lack of validated markers of disease progression. Drug development for PD would benefit from the identification of measures that can more accurately detect changes in the early stages of the disease, to assist in the evaluation of therapeutic agents both in terms of efficacy demonstration and clinical effectiveness.
Traditional assessment instruments such as the Movement Disorders Society revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) are subjective, performed in clinic environments in which patient responses may be affected by a variety of extrinsic factors (fatigue, emotional state etc), and are typically administered episodically, all of which may result in incomplete or inaccurate representation of patients’ actual functioning under real-world conditions. Rating scales are also unable to capture minor sub-clinical changes in patient performance or state, and can be partially reliant on the person’s recall, which can pose additional challenges as cognitive function becomes impaired as the disease advances. They may also include an element of subjectivity derived from the external rater. Digital monitoring technologies offer the promise for more frequent, objective and likely more precise assessments and there is considerable interest in applying both active and passive digital approaches to gather evidence for diagnosis, staging, progression, sign and symptom relevance, and drug efficacy studies14. Working alongside non-profit research foundations, the clinical research community and the pharmaceutical industry, the Critical Path Institute has spearheaded efforts to drive meaningful data capture approaches for digital drug development tools15. A framework for digital biomarker development has been articulated in collaboration with the Digital Medicine Society16, and sustained efforts are underway on how to integrate these data alongside benchmark conventional measures (genetic, serologic, imaging, rating)17. Herein we outline elements that may be useful to capitalize on this momentum, and believe that insights from data sharing or federated learning approaches could help to accelerate the development and approval of the first disease-modifying drugs for PD. Any successful approach will reflect the needs of facilitators (clinical investigators, study delivery teams), end users (patient and advocate community), the approval process (regulators, payers), and developers (pharmaceutical industry, digital technology innovators). A common thread for approaching such a grand ‘moonshot’ challenge is the pivotal role of medical data, including what it contains, when and how it is captured, and how it is stored and made available for analysis. Separately from digital monitoring technologies, striking advances in the federated use of Artificial Intelligence (AI) and Machine Learning (ML) technologies may have the potential to springboard this pursuit by building on foundational frameworks18–20. We also outline the potential for this challenge and offer models for near-term deployment to accelerate standardization and harmonization of measures from which the data are derived.
Application of Federated Learning in drug discovery and development
Drug development programs provide high-quality and well-curated sources of data, but patient numbers in any individual clinical program are small compared to the large volumes of data typically used to train AI/ML models. Combining data from multiple programs could enable more effective use of AI/ML approaches, but this is challenging due to the complexity of acquiring, managing, standardizing and preserving the data. Furthermore, commercially sensitive clinical trial data are considered highly proprietary and any patient-derived data are also subject to strict regulations regarding governance and confidentiality. This makes data sharing challenging and historically federated approaches to data analysis have been limited. The Latin term foederatus roughly translates to ‘federated, combined, and bound by treaty’. This latter phrase holds the key to how drug developers are beginning to work together on complex data projects through codified approaches and pre-agreed principles21. For the purpose of drug development, we use the term Federated Learning (FL) to encompass sharing data, samples or other knowledge in a pre-competitive space to accelerate therapeutic development for the benefit of all parties. Given dramatic developments in AI methodologies, increasing computational power, data storage capacities and data security advances, leveraging FL in drug development should no longer be an insurmountable challenge if barriers to making the data accessible can be removed22. AI has been identified as a game-changing technology by the drug development community and major pharmaceutical companies are investing heavily in its deployment23. Among numerous potential applications, its use in analyzing patient derived data from clinical trials is attracting considerable interest, heightened by the prospect of de-risking through collaborative approaches and high levels of data security.
In the case of federated data sharing networks, collaborating parties can collectively access and analyze geographically distributed datasets through a series of decentralized, interconnected locations (nodes). Each participant maintains control and independence of their respective de-identified database as local policies and procedures will vary based on environment24. This approach has been used for numerous collaborations between clinicians and researchers at academic medical centers and healthcare institutions, overcoming barriers to disparate, inaccessible and siloed datasets. Levels of access and data types under these agreements can be specified to help maintain data sovereignty and security and define the network and its degree of federation (versus conventional open data-sharing repositories). An early example of data sharing was the Alzheimer’s Disease Neuroimaging Initiative (ADNI) formed in 2004 and which paved the way for collaborative data sharing in dementias25. Numerous data repositories have since emerged ranging from the NIH National Centralized Repository for Alzheimer’s Disease (NCRAD)26, to the PPMI, and the Accelerating Medicines Partnership (AMP) program, a precompetitive partnership between government, non-profit and industry27. Another notable example is the collaboration between the Global Alliance for Genomics and Health (GA4GH) and the International Neuroinformatics Coordinating Facility (INCF)28. The partners share multivariate data (imaging, genomic, biomarker, DMPK and phenotypic) in order to help maximize predictive power in clinical studies. Data are shared under the now-established FAIR (Findable, Accessible, Interoperable, and Reusable) principles29. A recent study also examined the potential impact of federated analyses on post-marketing drug safety studies emanating from the EMA, concluding that improvement in precision is attainable30. Adaptations of this approach (query-based data interrogation) have been utilized among partners in the Dementia Platform UK (DPUK)31, the Global Alzheimer’s Association Interactive Network (GAAIN)32, the Alzheimer’s Disease Data Initiative (ADDI)33, the Neurodegenerative Disease Knowledge Portal (NDKP)34, the NIH Cloud Platform Interoperability program (NCPI)35, and the European Medical Information Framework Alzheimer’s Disease program (EMIF-AD)36.
Federated learning (FL) approaches use decentralized raw data held by participants that is not shared or moved but, instead, subjected to AI machine learning models sent to the locus of the data where they are locally trained. These learnings from the analysis are subsequently shared with a global model through updates of parameters as the model evolves. This approach ensures that sensitive (patient) data fidelity and privacy are preserved but pooled data analysis can nonetheless be effected37. Examples of their use in clinical studies range from refinement of clinical trial approaches to drive primary outcomes, identifying optimal patient inclusion criteria, uncovering subtle signals on drug effects in specific populations and comparing longitudinal real-world evidence across different patient cohorts. A well-publicized example of this approach was the MELLODDY (MachinE Learning Ledger Orchestration for Drug DiscoverY) project orchestrated by the Innovative Medicines Initiative in Europe38. Ten pharmaceutical companies participated in the project from 2019-2022 which assessed the potential of model-driven FL to enhance predictive learnings from QSAR data. 20 million small molecule drug candidates across 40,000 biological screens and 2.6 billion proprietary data points were assessed through the SAR data warehouses of the participating institutions. Outcomes were positive with increases of up to 4% on the key RIPtoP (Relative Improvement of Proximity to Perfection) measure. Each consortium partner contributed to specific tasks, and this is regarded as a first-in-kind federated learning project at data warehouse scale in the pharmaceutical industry38. A potential limitation lay in the need for institutions to disclose assay details among partners, thereby precluding the inclusion of highly sensitive and proprietary exemplars.
Another FL-based platform involving the pharmaceutical industry is Effiris. Developed by Lhasa Ltd, this fully functional solution provides secondary pharmacology prediction by interrogation of data from within a consortium of pharmaceutical companies39. Beyond drug discovery there are numerous rapidly developing applications of FL in clinical research relevant to neurodegenerative disease. In one example, FL was used to develop AI models for PD by analyzing speech patterns from different languages40. This is noteworthy as the resulting models were comparable or superior to local approaches based on mono-lingual models and bodes well for a unified (global) approach to speech-based detection of disease. Of direct significance, an evaluation of FL algorithms has been conducted based on multi-omic diagnostic data from people with PD41. Derived from AMP-PD warehouses, which include genomic, transcriptomic and clinic-demographic data, it involved splitting of the PPMI and PDBP cohorts for the development of the training set. The performance of the federated algorithm derived from aggregation strategies was within 2% of the top-performing central ML algorithm, suggesting high potential for collaborative deployment41.
FL strategies are also being deployed among clinical networks. For example, the Mayo Clinic has established a new venture, the Clinical Data Analytics Platform, allowing external parties to interrogate its data for algorithm refinement42. Similarly, Kings College in London have established an FL network among four teaching hospitals and three Universities to stimulate improvements among clinical pathways43. Of note, the patient data represents approximately 33% of London’s 9 million population. Examples of FL in clinical research include the Federated Tumor Segmentation Tool network, a consortium of 30 institutes committed to refinement in the detection of tumor boundaries44, the Kaapana Project involving radiologic imaging data among 36 University hospitals in Germany45, and AI for value based healthcare (AI4VBH) where 12 UK hospital trusts are deploying FL to improve patient pathways46. In all cases a primary consideration is ensuring data privacy laws are adhered to (HIPAA, GDPR) and that the approaches are future-proofed as far as possible for potential changes in these laws that might impact use. This is especially important in neurodegenerative diseases, where prodromal periods can last decades and longitudinal monitoring may span over multiple decades.
The examples cited above bode well for the deployment of FL approaches in PD and other neurodegenerative indications with their utility in diagnostics clearly demonstrated. A grand challenge however will be to deploy such approaches for understanding and modeling longitudinal progression in PD. This will require sustained and coordinated long-term effort among multiple stakeholders, and would benefit from harmonized approaches at the outset. Herein we outline how this might be pursued.
A harmonized FL model for Parkinson’s Disease?
The pursuit of therapeutics for PD is evolving and dynamic with over 130 active clinical trials roughly split between symptomatic therapies and disease-modifying therapies8. Therapeutics span all drug modalities and include repurposed and reformulated agents and a limited number of nondrug-based interventions. Although encouraging, there is a dramatic reduction in interventions transitioning from phase 2 to 3 and this may reflect the heterogeneity and complexities of the disease and its tapestry of symptoms. Sources of variability include:
Genomic and epigenetic variants affecting PD and comorbidities
Prodromal periods that can exceed 10 years
Differing rates of disease progression
Episodic assessment methods, which cannot fully represent changes occurring in the underlying disease
Heterogeneity in motor and non-motor manifestations
Differential impact of demographics, lifestyle variables, co-morbidities and polypharmacy on symptom manifestation and drug efficacy
Given the magnitude of the challenge faced there have been sustained and coordinated efforts on clinical care and trial design resulting in substantial and growing data repositories around the globe47. Compounding the complexity of clinical studies is the realization that current methods of assessment for detection, staging and monitoring are imprecise, complicating and potentially limiting the inter-operability of datasets48. Recent advances in Digital Health Technologies (DHT’s) seem poised to help address this problem as they offer the potential for standardized, quantified means for collection, storage, analysis and sharing of archival quality data to track disease over multi-decade timelines (Table 1)49.
Table 1.
Selected clinical and observational studies in PD utilizing digital technologies84
| Technology | Exploratory measurements | NCT | Sponsor | #Months/Subjects |
|---|---|---|---|---|
| Smartphone | Symptom self-management | 05120609 | Beats medical | 12/40 |
| Wearable devices | Multiple activities | 05529121 | VA medical center | 48/85 |
| Wearable devices | Motor function correlations | 03681015 | University of Rochester | 35/132 |
| IoMT & smart objects | Activities of daily living | 05830253 | ICS Maugeri SpA | 36/30 |
| Multiple devices | Cognitive and motor | 04139551 | University of Oxford | 86/300 |
| Smartwatch | Motor and activities of daily living | 04985539 | Radboud Medical Center | 40/144 |
| Smartphone | Speech analysis | 05421832 | Northwestern U | 24/120 |
| Digital walking aid | Freezing of gait | 03978507 | Sourasky Medical Center | 51/62 |
| Digital on-body sensor | Functional studies | 05874739 | Newcastle / NHS Trust | 26/751 |
| Eye tracking | Digital phenotyping | 05638477 | Misericordiae Hospital | 20/122 |
| Smartphone/watch | Motor, cognition, speech | 03100149 | Hoffman-La Roche | 104/316 |
| Smartwatch | Cognitive and motor | 03364894 | Radboud Medical Center | 75/520 |
| Contactless sensor | Gait and sleep metrics | 05363046 | BlueRock Therapeutics | 24/50 |
| Contactless sensor | Gait and sleep metrics | 06344026 | Aspen Neuroscience | 60/9 |
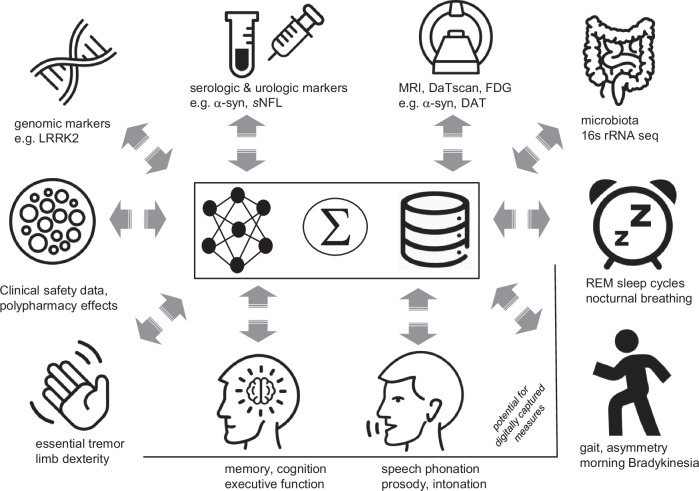
Beyond patient care, such data is also critical to inform decisions by regulatory bodies for enabling medical product decisions, and providers and payers for prescribing and reimbursement decisions. Accordingly, we sit at a pivotal juncture where decisions need to be made on what data are most relevant to serve different stakeholder needs, how we can best capture such, and what format is the most conducive to shared learnings. The latter is of key significance if we are to harness the transformative promise of AI and FL to advance treatment options in PD. In terms of progress toward this goal, a number of PD cohorts have established multi-modal datasets derived from traditional and digital measures including the Oxford Parkinson’s Disease Centre (OPDC), MJFF, PPMI50–52, the Personalized Parkinson’s Project (PPP)53, and the CPP global consortium17. As an example, the latter, overseen by the Critical Path Institute (CPath), hosts an aggregated database made available to qualified research investigators, which contains observational cohort and clinical trial data from 31 datasets covering over 15,000 participants. An overview of the complexity and diversity of clinically relevant patient-derived data is depicted in Fig. 1, representing a blend of conventional and digitally derived assessment methods.
Fig. 1.
Diversity of clinical data from people with PD in the CPath database.
In each category, there are growing volumes of data available from a wide variety of clinical, epidemiological and longitudinal studies, including clinician-reported outcomes, patient-reported outcomes, digital, imaging and physiological data that is ideally suited to use of FL methods to identify definitive signals. Moreover, as these data evolve over time, including symptom modulation by patients’ prescribed therapeutics (including for comorbidities e.g., GLP-1 inhibitors for Diabetes Mellitus), they could also represent de facto, real world phase 4 clinical data. A major opportunity clearly exists for FL approaches to iteratively identify and integrate orthogonal learnings from these various datasets. Such an approach may have the potential to identify and address biases inherent in existing datasets (e.g. from demographic, comorbidity) and present an opportunity to promote inclusivity in data capture for future interventional and observational studies.
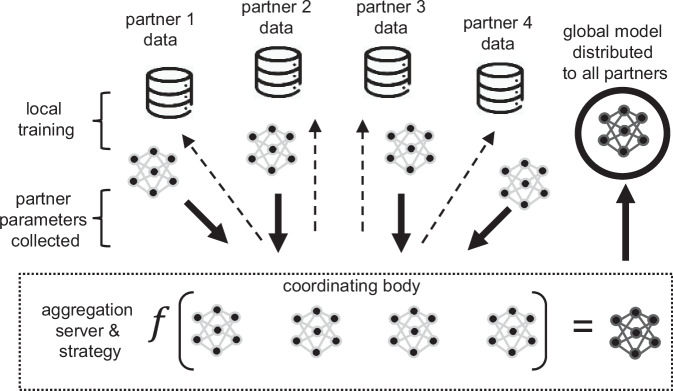
In addition to designing optimal FL networks for data analysis, the question of network composition itself is also pertinent. The existing FL networks for drug development have involved clinical research centers, nonprofit organizations, and pharmaceutical companies. In the present case, the engagement of technical developers (hardware, firmware, software) may also be critical, as they play a key role in the devices used to capture and calibrate patient data. This could encompass large and smaller technology companies, whose business models will differ widely in terms of deliverable timelines, monetization formats, and incentivization strategies54. This is likely to raise new questions about intellectual property, and ownership of any AI/ML models trained through the FL process. There is also the omnipresent concern regarding data privacy and security when sharing among this industry group and the need to strictly adhere to informed consent protocols pertaining to when the data was captured and its intended use. Such concerns are readily addressable under the tenets of FL approaches (as opposed to Federated Data Analyses) as source data are retained by the owner, and rigid firewalls protect both its fidelity and sovereignty55. Likewise, these data could also play an enabling role in informing post-marketing assessment and safety monitoring. A representative overview of a hypothetical FL model composed of four collaborating partners is outlined in Fig. 256. Source data are preserved at each client site but algorithms are trained on the datasets and are then aggregated and subsequently distributed in the form of a global model. A third-party, nonprofit coordinating body could play a pivotal role in enabling and promoting such practices in PD (and potentially other disease areas) as exemplified by the CPath, the Foundation for the National Institutes of Health AMP®-PD program, and MJFF. Several of these third parties also oversee consolidated datasets that could help in refining the algorithms, for the benefit of data generated by the independent partners and other interested parties (Fig. 2).
Fig. 2.
Hypothetical Federated learning network composed of four partners with a fifth, nonprofit coordinating body overseeing aggregation and strategy.
Defining a three-year roadmap for FL-inspired approaches to PD
In order to capitalize on the momentum surrounding FL methods, fundamental decisions are needed on the scope and nature of data to be assessed. We outline three key parameters that should be considered as part of a roadmap to success in the design and adoption of a FL model in PD.
What should be measured and when?
Given the wide range of data sources (Fig. 1) there is a need to agree on common sub-features of highest relevance for drug development. For example, measures such as bradykinesia57, gait58, tremor59, falls60, sleep61, and speech pattern abberations62 are all Concepts of Interest (COI’s) that might be considered depending on various Contexts of Use (CoU’s). Digital versions of these measures come with obvious advantages, including being objective in nature, the possibility of being measured more frequently than is possible for periodic in-clinic visits, ecological validity (as assessments are performed in patients’ real-world, day-to-day environments), and the promise of being more sensitive to dynamic changes compared to in-clinic. Two challenges, however, present themselves: 1) the need to demonstrate that any changes measured have direct relevance to the patient’s disease status and quality of life and 2) it has yet to be determined from longitudinal studies whether variability between patients in rates of progression will nullify the advantage that precision of measurement might bring when using digital measures as study outcomes.
In terms of imaging data, the power of DaTscan and potentially multimodal imaging modalities, including PET, may provide insights63, as underscored in recent AD research64. Finally, fluid markers such as α-synuclein derived from CSF, serum and potentially other non-invasive matrices might play a key role6, and additional emerging fluid biomarkers such as NfL and GFAP can be considered. Collectively these represent potentially powerful tools but how they can be best leveraged for the drug development process remains an open question, and one which is crucial for determining their intended use in terms of CoU. A clear opportunity exists, however, for FL approaches to integrate insights from multiple studies that collect different subsets of information from diverse types of behavioral and physiological measures.
How should we measure it?
Though many of the analytics in Fig. 1 are deployed under highly standardized conditions, there is considerable variability in the capture of cognitive, motor, and non-motor changes. The use of wearables or smartphone devices to capture patient data has been exemplified by numerous studies, including the landmark OXQUIP65, and WATCH-PD studies66,67, and there is compelling evidence that device-derived data can complement traditional, physician conducted, assessments68. Importantly, digital measures and biomarkers can give insight into the patient’s functioning outside of in-clinic visits, i.e. at home, which may well be very different from how the patient performs while being examined in a clinical environment, as illustrated by a recent 12-month comparative study69. Given the rapidly evolving digital technologies that can capture real-time data longitudinally, there is also the consideration of the merits of passive over active measuring methods for long term monitoring61,70. For example, passive contactless home sensors employing Artificial Intelligence can analyze wireless signals that bounce off patients’ bodies to measure gait speed, mobility, respiration, and sleep metrics, while patients go about their daily activities without the need for wearable devices. These sensors have been shown to provide signals (digital measures / biomarkers) for detecting PD71, as well as monitoring disease progression and response to treatment72, and are now being deployed for at-home monitoring in PD clinical trials73,74. Such approaches offer the potential for reducing patient bias and burden, and potentially increasing likelihood of capturing clean, standardized data over time. With all approaches it will be imperative to also consider best practice in data collection methodology and metadata documentation in order to permit accurate interpretation in future years. Capturing raw data, for example, would enable future processing with newer, refined algorithms as they become available.
Leveraging existing data sources
A key enabler in pursuing the vision outlined in Fig. 2 will be to springboard from the insights provided by existing data sources for the development and refinement of operating models and algorithm design. Among numerous opportunities, data from the Oxford Parkinson’s Disease Cohort50, PPMI51, MJFF52, AMP®-PD75, Mobilise-D76, PPP53, OXQUIP65, the Global Parkinson’s Genetics Program77, and European Platform for Neurodegenerative Diseases78 are potential sources. Machine learning techniques are already well advanced across these initiatives, but there is acknowledgment that multi-modal longitudinal data still remains relatively under-utilized, especially for early PD79.
Arriving at consensus on these topics will require active coordination from an independent entity, with partner foundations, researchers and sponsor companies and the Movement Disorders clinical community. It will also be essential to engage with the regulatory bodies at an early stage to ensure key principles and plans are aligned with regulatory expectations. Key pivotal issues to achieve consensus on include:
Inclusion criteria and consent for participation in FL approaches
Addressing potential limitations and biases within datasets
System architecture
Standardization of minimal datasets, protocols and algorithms
Data heterogeneity
Privacy/security
Information leakage
Traceability and accountability
Ownership of IP in models trained on federated data
Roles and accountabilities of consortia members
As a next step we anticipate facilitating a series of stakeholder workshops at upcoming events in the annual cycle of PD-focused research gatherings, including congresses (AD/PD, MDS etc.) and CPP meetings to bring like-minded experts together for formal and informal exchange. Ideally such fora will be driven by defined outcomes and goals, and codified, for example, in a three-year roadmap resulting in design of a viable FL framework. In addition to capitalizing on momentum building through AI technologies, other developments in data sharing and access may contribute to this grand challenge. For example, NIH guidelines for grantees now require an acceptable data monitoring and sharing plan (DMSP)80. Given that NIH invests over $30B annually in biomedical grant awards, such efforts could contribute substantively to our shared knowledge base in PD and related neurodegenerative and cognitive diseases through engagement in an FL network. Indeed, the potential to exploit commonalities with research data captured across neurodegenerative diseases could be prioritized. Federated learning models applied to digital, imaging and clinical data in Alzheimer’s Disease offer obvious potential, and the global prevalence ( > 50 million dementia cases and expected to double every twenty years) underscores the magnitude of the opportunity. In the case of PD there is also the opportunity to more fully understand the idiosyncrasies of related conditions with shared pathological mechanisms and symptom presentation, such as PSP, which is often misdiagnosed as PD in early stages. Through a more comprehensive approach to data analysis this might also allow enhanced applicability of any therapeutic developments to benefit broader patient populations. Also prescient is the realization that in order to achieve near-term progress in clinical development we may need to develop novel endpoints which allow faster readout, as has been demonstrated in the oncology field81. Given the nature of PD and typically narrow window of eligibility for many disease-modifying therapy (DMT) clinical trials, such an approach may further enrich our corpus of clinical data for subsequent deployment of FL methods, and also help further addressing potential bias in clinical studies.
Conclusion and recommendations
The age-old anecdote that ‘it takes a village to raise a child’ embodies well the challenge and community collaboration needed in order to successfully introduce therapies for PD. The confluence of new technologies, clinical insight and patient engagement seems poised to benefit from the wave of momentum surrounding AI methods in drug development. The hope is that through a combination of best practices in data sharing and FL strategies the clinical development of disease-modifying therapies for PD can accelerate. We approach this opportunity at a pivotal time where the biological definition of PD is refining our approach to disease staging, new sensor technologies become powerful allies for the diagnosis and assessment of patients, and computational firepower offers unparalleled opportunities for data analysis and collective learning. The potential for precompetitive collaboration models is substantial and may offer new dimensions beyond PD and neuroscience82. This said, to be successful we must learn from prior experiences regarding data sharing, and accept this challenge with focus, commitment and resolve83. In terms of concrete next steps, this author group intends to embark upon identification of harmonized standards (common minima) of what to measure and how to measure for the purposes of supporting and enabling FL principles even further. We would like to encourage all involved in PD research and development to carefully consider data sharing and FL approaches based on their evident potential to advance science, disease understanding, and help develop breakthrough treatments for PD. It is indeed our duty and responsibility to ensure this opportunity is capitalized upon, for the benefit of the millions of patients and their families afflicted by this disease.
Author contributions
A.K., J.A., C.A., B.B., C.C., J.C., J.Co., D.D., M.D., J.E., L.G., E.G., D.H., F.H., E.I., T.K., D.K., C.K., M.L., J.M., K.M., K.Mc., A.M., M.M., G.P., M.J.P., J.R., L.R., S.S., A.S., T.S., D.S., C.S.-F., J.W., and G.J. contributed substantively to the development and editing of the manuscript, read and approved the final version, and attest accountability for its content.
Competing interests
AK, LG and GJ are all employees of Novartis Pharmaceuticals and may hold common stock in the company. MD and JR are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options. JA has received research support from the Michael J. Fox Foundation for Parkinson’s Research, NIH/NINDS, the Huntington Study Group, and PhotoPharmics; received compensation as a consultant/steering committee/advisory board member from the Huntington Study Group, the Parkinson Study Group, Sana Biotechnology, Biohaven, and the Michael J. Fox Foundation for Parkinson’s Research. CA is supported by the National Institute for Health Research Oxford Biomedical Research Centre and received research grant support from UCB Pharma, MSD, EPSRC and has been a consultant for UCB and J & J. BRB: No declaration. CC has been a consultant for AbbVie, Mission Therapeutics and Roche and received research funding from Parkinson’s UK, Edmond J Safra Foundation, National Institute of Health and Care Research and Cure Parkinson’s. JCe has been a consultant for VanquaBio, MiCure, Elsie Bio, has stock/options from Vanqua Bio, Micure, Immunobrain Checkpoint and has received grant funding from ASAP, the Marcus Foundation. JCo is an employee of AbbVie Pharmaceuticals. DTD: No declaration. JE is an employee of Biogen. EG is an employee of UCB Pharmaceuticals. DH has been a consultant for the Critical Path Institute, a share holder and employee of Panoramic Digital Health and received funding from the EPSRC, and France Relance. FH is employed part-time at Clario. ESI is an employee of Koneksa Health and may own company stock. TK is an employee of Takeda Pharmaceuticals. DK No declaration. CK No declaration. ML is a consultant to F. Hoffmann– La Roche Ltd via Inovigate. JM has consulted for and received funding from the Michael J Fox Foundation for Parkinson’s Research. KM No declaration. KMc No declaration. AM has been a consult for Koneksa and received grant funding from MJFF, JPND, US Department of Defense, and the EU IMI. MM No declaration. GP is an employee of F. Hoffmann La-Roche. MJP is an employee of Sanofi, and may hold shares and/or stock options in the company. LR has received honorarium from the MJ Fox Foundation and grant funding from EU, NIHR, MRC, EPSRC, Cure Parkinson’s Trust, PDUK, Dunhill Medical Trust. SS No declaration. AS has been a consultant to the following companies in the past year: Acadia, Boerhinger-Ingelheim, GE Healthcare, Wave Life Sciences, Inhibikase, Prevail, Mitzubishi and Alertity Therapeutics. He has served on DSMBs for the Huntington Study Group and The Healey ALS Consortium (Massachusetts General Hospital).He has received grant funding from the Michael J. Fox Foundation, NIA and NINDS. TS has served as a consultant for AskBio, Amneal, Blue Rock Therapeutics, Critical Path for Parkinson’s Consortium (CPP), Denali, General Electric, Kyowa, Neuroderm/ MTPA, Prevail/ Lilly, Roche, Sanofi, Sinopia, Takeda and Vanqua Bio. She has served on the ad board for AskBio, Amneal, Biohaven, Denali, GAIN, General Electric, Kyowa, MJFF, Neuron23, Parkinson Study Group, Prevail/ Lilly, and Roche. She has also served as a member of the scientific advisory board of Koneksa, Neuroderm/ MTPA, Sanofi and UCB. Dr. Simuni has received research funding from Amneal, Biogen, Neuroderm, Prevail, Roche, UCB and is an investigator for NINDS, MJFF, Parkinson’s Foundation. DS No declaration. CS-F No declaration. JAW is an employee of Koneksa Health and may own stock and/or stock options. No funding is associated with the production of this manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schlander, M., Hernandez-Villafuerte, K., Cheng, C. Y., Mestre-Ferrandiz, J. & Baumann, M. How Much Does It Cost to Research and Develop a New Drug? A Systematic Review and Assessment. Pharmacoeconomics39, 1243–1269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun, D., Gao, W., Hu, H. & Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B.12, 3049–3062 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gribkoff, V. K. & Kaczmarek, L. K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology120, 11–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skaria, A. P. The Economic and Societal Burden of Alzheimer Disease: Managed Care Considerations. Am. J. Manag Care.28, S188–S196 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Dorsey, E. R. & Bloem, B. R. The Parkinson Pandemic-A Call to Action. JAMA Neurol.75, 9–10 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shlomo, Y. et al. The epidemiology of Parkinson’s disease. Lancet403, 283–292 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings, J., Apostolova, L. & Rabinovici, G. D. Lecanemab: Appropriate Use Recommendations. J. Prev. Alzheimers Dis.10, 362–377 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarthing, K. et al. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update. J. Parkinsons Dis.13, 427–439 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen Daalen, J. M., Schootemeijer, S., Richard, E., Darweesh, S. K. L. & Bloem, B. R. Lifestyle Interventions for the Prevention of Parkinson Disease: A Recipe for Action. Neurology99, 42–51 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet397, 2284–2303 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Höglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol.23, 191–204 (2024). [DOI] [PubMed] [Google Scholar]
- 12.Simuni, T., Chahine, L. M. & Poston, K. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol.23, 178–190 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Cardoso, F. et al. A Statement of the MDS on Biological Definition, Staging, and Classification of Parkinson’s Disease. Mov. Disord.39, 259–266 (2024 Feb). [DOI] [PubMed] [Google Scholar]
- 14.Khanna, A. & Jones, G. Toward Personalized Medicine Approaches for Parkinson Disease Using Digital Technologies. JMIR Form. Res.7, e47486 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mammen, J. R., Speck, R. M. & Stebbins, G. M. Mapping Relevance of Digital Measures to Meaningful Symptoms and Impacts in Early Parkinson’s Disease. J. Parkinsons Dis.13, 589–607 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldsack, J. C., Coravos, A. & Bakker, J. P. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). npj Digit. Med.3, 55 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephenson, D., Alexander, R. & Aggarwal, V. Precompetitive Consensus Building to Facilitate the Use of Digital Health Technologies to Support Parkinson Disease Drug Development through Regulatory Science. Digit Biomark.4, 28–49 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephenson, D. et al. Transforming Drug Development for Neurological Disorders: Proceedings from a Multidisease Area Workshop. Neurotherapeutics20, 1682–1691 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, Q., Joshi, A., Standing, J. F. & van der Graaf, P. H. Artificial Intelligence/Machine Learning: The New Frontier of Clinical Pharmacology and Precision Medicine. Clin. Pharm. Ther.115, 637–642 (2024). [DOI] [PubMed] [Google Scholar]
- 20.Podichetty, J. T. et al. Accelerating healthcare innovation: the role of Artificial intelligence and digital health technologies in critical path institute’s public-private partnerships. Clin. Transl. Sci.17, e13851 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niazi, S. K. The coming of age of AI/ML in drug discovery, development, clinical testing and manufacturing: The FDA perspectives. Drug Des. Devel. Ther.17, 2691–2725 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Witte, D. et al. A Federated Data Analysis Approach for the Evaluation of Surrogate Endpoints Statistics in medicine, submitted.
- 23.Paul, D. et al. Artificial intelligence in drug discovery and development. Drug Discov. Today26, 80–93 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallock, H. et al. Federated Networks for Distributed Analysis of Health Data. Front Public Health9, 712569 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen, R. C., Aisen, P. S. & Beckett, L. A. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology74, 201–209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edler, M. C., et al. (2023). Implementing new technologies to enhance specimen quality and researcher value at the National Centralized Repository for Alzheimer’s Disease and Related Dementias. Alzheimer’s Dementia. 19. 10.1002/alz.078840.
- 27.Iwaki, H., Leonard, H. L. & Makarious, M. B. Uniformed Services University of the Health Sciences Associates; AMP PD Whole Genome Sequencing Working Group; AMP PD consortium. Accelerating Medicines Partnership: Parkinson’s Disease. Genetic Resource. Mov. Disord.36, 1795–1804 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.GA4GH and the International Neuroinformatics Coordinating Facility (INCF) launch community focused on neuroscience data interoperability, https://www.ga4gh.org/.
- 29.Wilkinson, M. D., Dumontier, M. & Aalbersberg, I. J. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data.3, 160018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gedeborg, R. et al. Federated analyses of multiple data sources in drug safety studies. Pharmacoepidemiol Drug Saf.32, 279–286 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Bauermeister, S., Orton, C. & Thompson, S. The Dementias Platform UK (DPUK) Data Portal. Eur. J. Epidemiol.35, 601–611 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toga, A. W., Neu, S. C., Bhatt, P., Crawford, K. L. & Ashish, N. The Global Alzheimer’s Association Interactive Network. Alzheimers Dement.12, 49–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toga, A. W. et al. The pursuit of approaches to federate data to accelerate Alzheimer’s disease and related dementia research: GAAIN, DPUK, and ADDI. Front Neuroinform.17, 1175689 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.https://ndkp.hugeamp.org/.
- 35.https://www.ncpi-acc.org/.
- 36.Lovestone, S., EMIF Consortium. The European medical information framework: A novel ecosystem for sharing healthcare data across Europe. Learn Health Syst.4, e10214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahman A. et al. Federated learning-based AI approaches in smart healthcare: concepts, taxonomies, challenges and open issues. Cluster Comput. 2022 Aug 17:1-41. 10.1007/s10586-022-03658-4. [DOI] [PMC free article] [PubMed]
- 38.Heyndrickx, W., Mervin, L. & Morawietz, T. MELLODDY: Cross-pharma Federated Learning at Unprecedented Scale Unlocks Benefits in QSAR without Compromising Proprietary Information. J. Chem. Inf. Model.10.1021/acs.jcim.3c00799 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.https://www.lhasalimited.org/.
- 40.Sarlas, S., Kalafatelis, A., Alexandridis, G., Kourtis, M. A., Trakadas. P. 2023. Exploring Federated Learning for Speech-based Parkinson’s Disease Detection. In The 18th International Conference on Availability, Reliability and Security (ARES 2023), August 29–September 01, 2023, Benevento, Italy. ACM, New York, NY, USA 6 Pages. 10.1145/3600160.3605088.
- 41.Danek, B. et al. Federated Learning for multi-omics: a performance evaluation in Parkinson’s disease. Patterns5, 100945 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.https://newsnetwork.mayoclinic.org/discussion/mayo-clinic-launches-its-first-platform-initiative/.
- 43.Rehman, M. H. U. et al. Federated learning for medical imaging radiology. Br. J. Radiol.96, 20220890 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pati S., et al. The federated tumor segmentation (FeTS) tool: an open-source solution to further solid tumor research. Phys Med Biol. 2022 Oct 12;67: 10.1088/1361-6560/ac9449. [DOI] [PMC free article] [PubMed]
- 45.Scherer, J., Nolden, M. & Kleesiek, J. Joint Imaging Platform for Federated Clinical Data Analytics. JCO Clin. Cancer Inform.4, 1027–1038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AI4VBH - AI Centre for Value Based Healthcare. Available online at: https://www.aicentre.co.uk/projects.
- 47.Tanguy, A., Jönsson, L. & Ishihara, L. Inventory of real world data sources in Parkinson’s disease. BMC Neurol.17, 213 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendricks, R. M. & Khasawneh, M. T. An Investigation into the Use and Meaning of Parkinson’s Disease Clinical Scale Scores. Parkinsons Dis.2021, 1765220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chandrabhatla, A. S., Pomeraniec, I. J. & Ksendzovsky, A. Co-evolution of machine learning and digital technologies to improve monitoring of Parkinson’s disease motor symptoms. npj Digit. Med.5, 32 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Griffanti, L. et al. Cohort profile: the Oxford Parkinson’s Disease Centre Discovery Cohort MRI substudy (OPDC-MRI). BMJ Open.10, e034110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.S. Kanagaraj, M. S. Hema and M. N. Guptha, “Performance analysis of Classification methods for Parkinson’s Disease with PPMI Dataset,” 2021 International Conference on Advancements in Electrical, Electronics, Communication, Computing and Automation (ICAECA), Coimbatore, India, 2021, pp. 1-5, 10.1109/ICAECA52838.2021.9675588.
- 52.Vollstedt, E. J., Schaake, S. & Lohmann, K. MJFF Global Genetic Parkinson’s Disease Study Group. Embracing Monogenic Parkinson’s Disease: The MJFF Global Genetic PD Cohort. Mov. Disord.38, 286–303 (2023). [DOI] [PubMed] [Google Scholar]
- 53.Bloem, B. R. et al. The Personalized Parkinson Project: examining disease progression through broad biomarkers in early Parkinson’s disease. BMC Neurol.19, 160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.As an exemplar, the Critical Path Institute have established a consortium of technology providers who are collaborating to develop best practices for the electronic implementation of clinical outcome assessments (https://c-path.org/program/electronic-clinical-outcome-assessment-consortium/).
- 55.Wen, J. et al. A survey on federated learning: challenges and applications. Int J. Mach. Learn Cybern.14, 513–535 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.https://www.datatecnica.com/blog/federated-learning-in-multimodal-heatlhcare-data-preprint.
- 57.Isaacson, S. H., Pahwa, R., Pappert, E. J. & Torres-Russotto, D. Evaluation of morning bradykinesia in Parkinson’s disease in a United States cohort using continuous objective monitoring. Clin. Park Relat. Disord.6, 100145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Biase, L. et al. Gait Analysis in Parkinson’s Disease: An Overview of the Most Accurate Markers for Diagnosis and Symptoms Monitoring. Sens. (Basel).20, 3529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarakad, A. & Jankovic, J. Essential Tremor and Parkinson’s Disease: Exploring the Relationship. Tremor Other Hyperkinet Mov. (N. Y).8, 589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva de Lima, A. L. et al. Home-based monitoring of falls using wearable sensors in Parkinson’s disease. Mov. Disord.35, 109–115 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mirelman, A. et al. Tossing and Turning in Bed: Nocturnal Movements in Parkinson’s Disease. Mov. Disord.35, 959–968 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Wiesman, A. I., Donhauser, P. W. & Degroot, C. Aberrant neurophysiological signaling associated with speech impairments in Parkinson’s disease. npj Parkinsons Dis.9, 61 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Depierreux, F., Parmentier, E. & Mackels, L. Parkinson’s disease multimodal imaging: F-DOPA PET, neuromelanin-sensitive and quantitative iron-sensitive MRI. npj Parkinsons Dis.7, 57 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ning, K., Cannon, P. B., Yu, J., Shenoi, S., Wang, L., Alzheimer’s Disease Neuroimaging Initiative & Sarkar, J. 3D convolutional neural networks uncover modality-specific brain-imaging predictors for Alzheimer’s disease sub-scores. Brain Inform.11, 5 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sotirakis, C., Su, Z. & Brzezicki, M. A. Identification of motor progression in Parkinson’s disease using wearable sensors and machine learning. npj Parkinsons Dis.9, 142 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams, J. L., Kangarloo, T. & Tracey, B. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. npj Parkinsons Dis.9, 64 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adams, J. L., Kangarloo, T. & Gong, Y. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study over 12 months. npj Parkinsons Dis.10, 112 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antonini, A. et al. Toward objective monitoring of Parkinson’s disease motor symptoms using a wearable device: wearability and performance evaluation of PDMonitor®. Front Neurol.14, 1080752 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burq, M. et al. Virtual exam for Parkinson’s disease enables frequent and reliable remote measurements of motor function. NPJ Digit Med.5, 65 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teipel, S. et al. Use of nonintrusive sensor-based information and communication technology for real-world evidence for clinical trials in dementia. Alzheimers Dement.14, 1216–1231 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang, Y., Yuan, Y. & Zhang, G. Artificial intelligence-enabled detection and assessment of Parkinson’s disease using nocturnal breathing signals. Nat. Med28, 2207–2215 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu, Y., Zhang, G. & Tarolli, C. G. Monitoring Gait at Home with Radio Waves in Parkinson’s Disease: a Marker of Severity, Progression, and Medication Response. Sci. Transl. Med.14, eadc9669 (2022). [DOI] [PubMed] [Google Scholar]
- 73.BlueRock Therapeutics to incorporate wearable and invisible contactless digital health technologies from Rune Labs and Emerald Innovations in Parkinson’s disease clinical trial. https://www.prnewswire.com/news-releases/bluerock-therapeutics-to-incorporate-wearable-and-invisible-contactless-digital-health-technologies-from-rune-labs-and-emerald-innovations-in-parkinsons-disease-clinical-trial-301770876.html.
- 74.Neuroscience to Partner with Rune Labs and Emerald Innovations to Incorporate both Active and Passive Digital Health Monitoring in Trial Ready Cohort Screening Study. https://www.prnewswire.com/news-releases/aspen-neuroscience-to-partner-with-rune-labs-and-emerald-innovations-to-incorporate-both-active-and-passive-digital-health-monitoring-in-trial-ready-cohort-screening-study-301908824.html.
- 75.https://amp-pd.org.
- 76.https://mobilise-d.eu/.
- 77.https://gp2.org.
- 78.https://discover.epnd.org.
- 79.Gerraty, R. T. et al. Machine learning within the Parkinson’s progression markers initiative: Review of the current state of affairs. Front Aging Neurosci.15, 1076657 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.https://grants.nih.gov/grants/guide/notice-files/NOT-OD-21-013.html.
- 81.Hoo, R., Chua, K. L. M., Panda, P. K., Skanderup, A. J. & Tan, D. S. W. Precision Endpoints for Contemporary Precision Oncology Trials. Cancer Discov.14, 573–578 (2024). [DOI] [PubMed] [Google Scholar]
- 82.Altshuler, J. S. et al. Opening up to precompetitive collaboration. Sci. Transl. Med.2, 52cm26 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Wagner, J. A. et al. The Biomarkers Consortium: practice and pitfalls of open-source precompetitive collaboration. Clin. Pharm. Ther.87, 539–42 (2010). [DOI] [PubMed] [Google Scholar]
- 84.All available at clinicaltrial.gov. For DiME database see: https://dimesociety.org/library-of-digital-endpoints/.