Abstract
1. Synaptic actions in the thoracic spinal cord of individual expiratory bulbospinal neurones were studied in anaesthetized cats by the use of two techniques: (i) the monosynaptic connections to motoneurones were assessed by cross-correlations between the discharges of the neurones and efferent discharges in the internal intercostal nerves of several segments bilaterally; and (ii) distributions of terminal and focal synaptic potentials were measured by extracellular spike-triggered averaging in the thoracic ventral horn. 2. Monosynaptic connections were identified by both the durations and timings of observed cross-correlation peaks, taking into account accurate conduction velocity measurements derived from collision tests and from spike-triggered averaging. Discrimination was made against peaks resulting from presynaptic synchronization. 3. Monosynaptic connections to motoneurones were identified for twenty-three out of twenty-seven neurones. The connections to nerves on the side ipsilateral to the cell somata were, on average, about 36% of the strength of those on the contralateral side. The overall strength of the connections was about twice as strong as previous estimates for similar connections from inspiratory bulbospinal neurones to phrenic motoneurones. The monosynaptic pathway was calculated to be able to provide most of the depolarization for the motoneurones concerned and therefore was likely to be the main determinant of their firing patterns under the conditions of these experiments. 4. However, taking into account previous measurements it is considered possible that these connections may only involve a minority of motoneurones, perhaps only 10% of the expiratory population. Thus, in general, the control of the whole pool of expiratory motoneurones, despite the strong monosynaptic connections measured here, is suggested to be mainly dependent on spinal interneurones, as has been concluded previously for inspiratory motoneurones. 5. Spike-triggered averaging revealed that nearly all neurones gave signs of collaterals in each of the segments investigated (T7, T8 or T9), as shown by the presence of terminal potentials or focal synaptic potentials, but the projection within a given thoracic segment was non-uniform, in that large-amplitude potentials were more common in the rostral than the caudal part of the segment. This non-uniformity could be a factor involved in the apparently non-heterogeneous connections to the motoneurones.
Full text
PDF

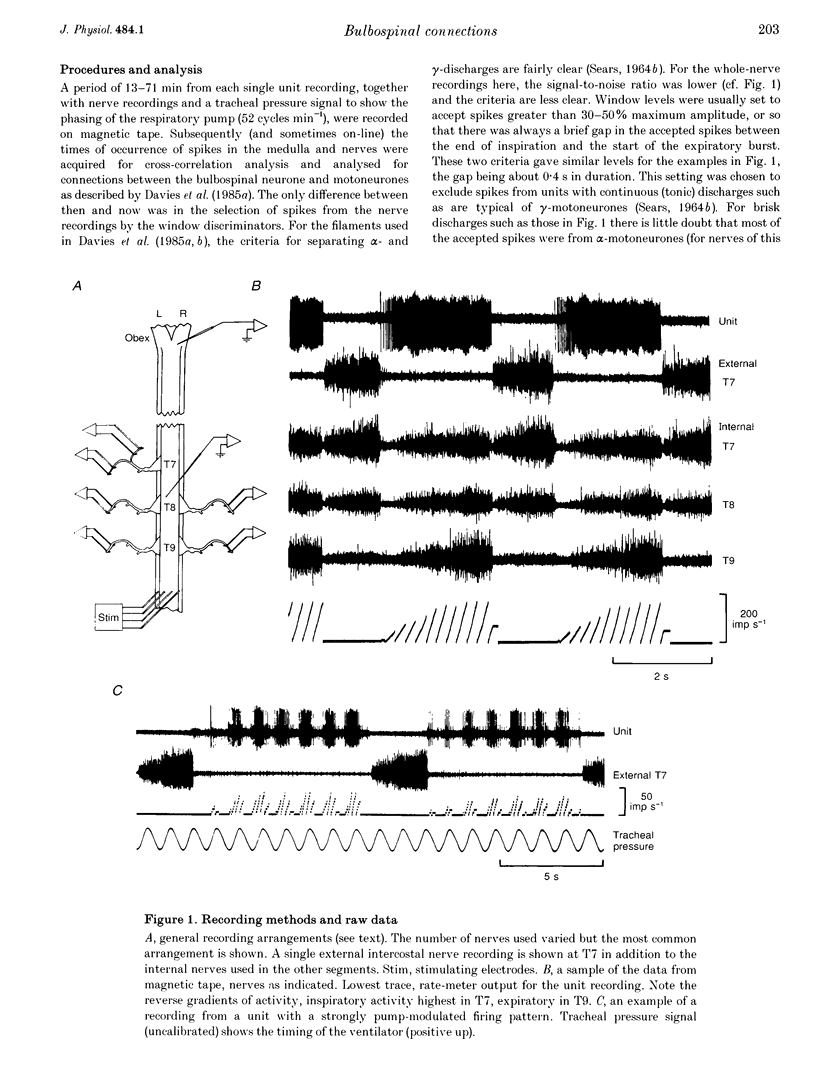

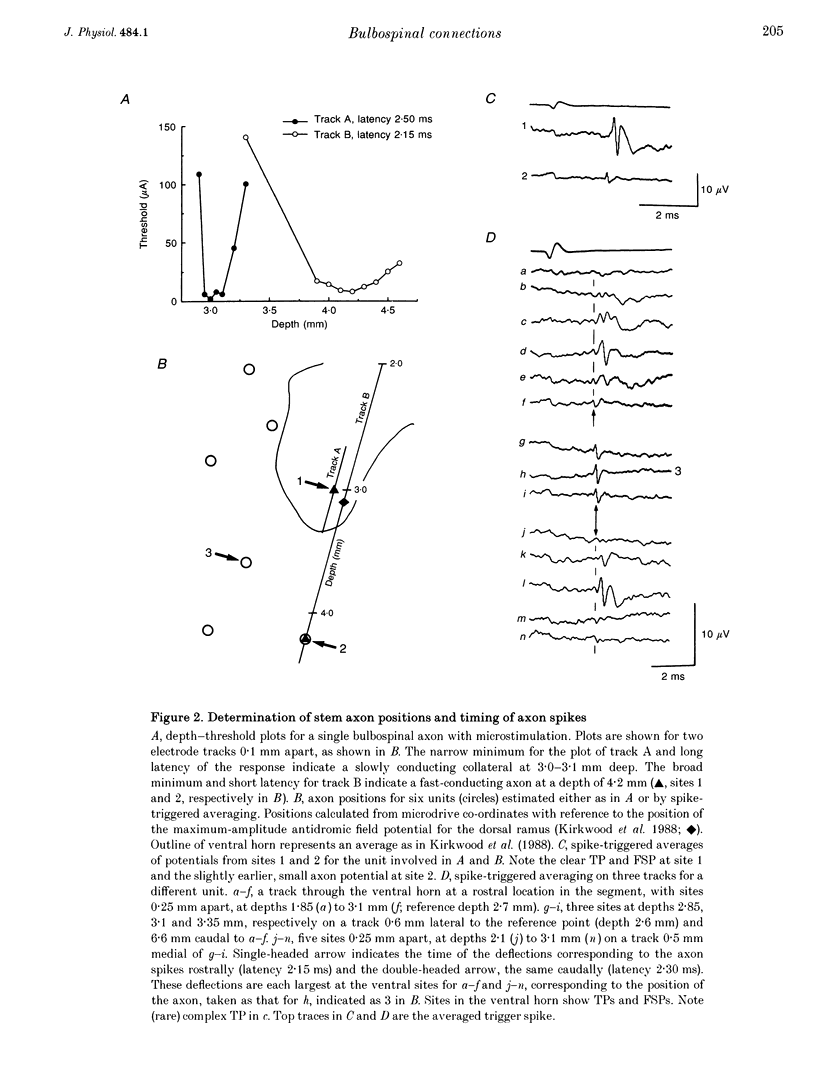
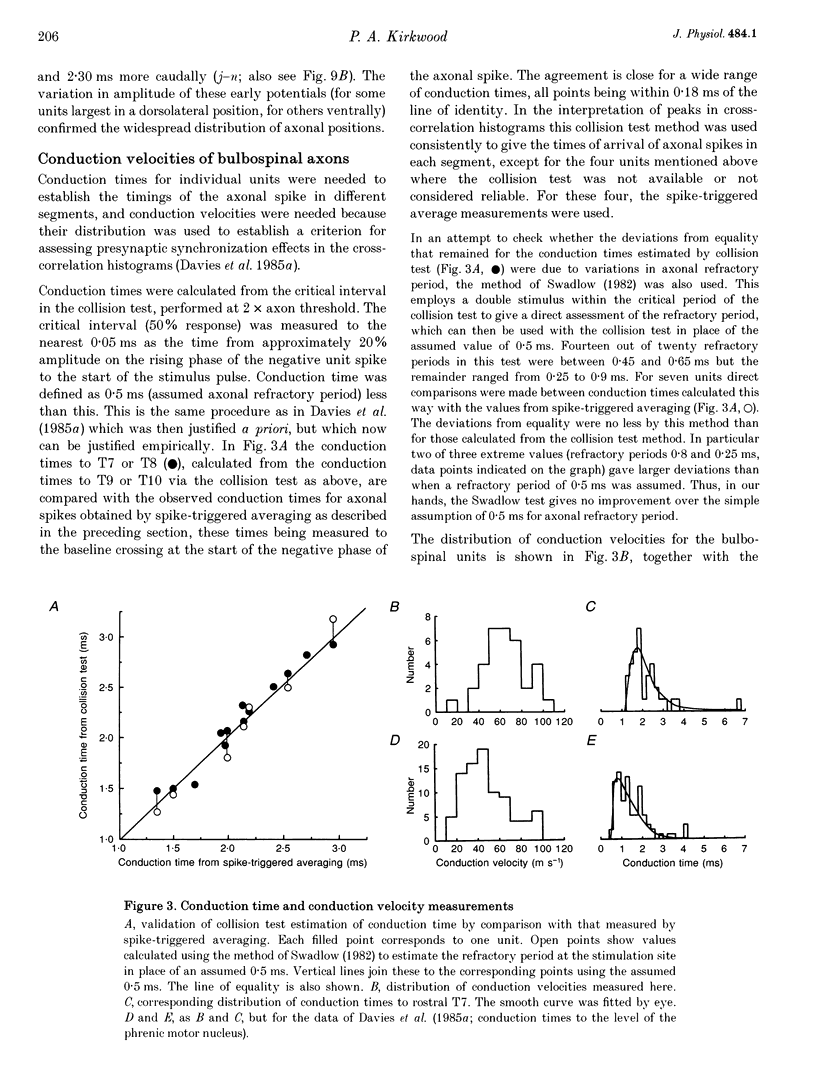

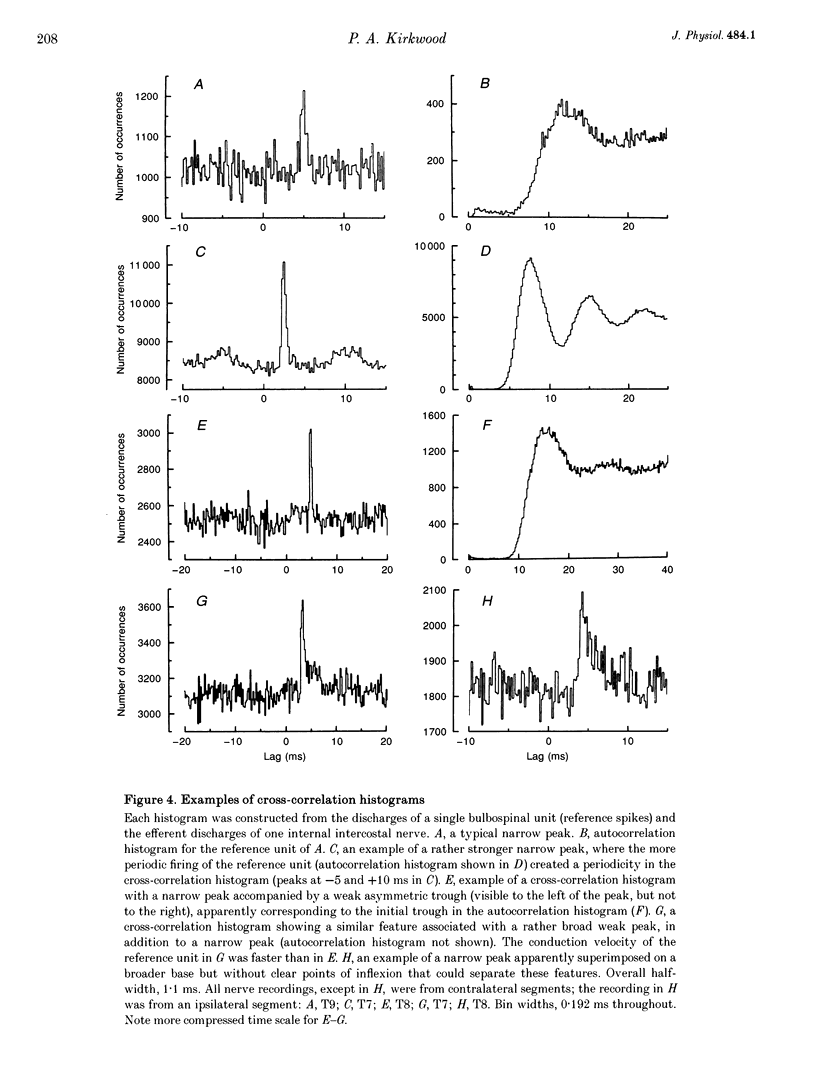













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellingham M. C., Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990 Nov 12;533(1):141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Feldman J. L., Sommer D. Caudal medullary expiratory neurone and internal intercostal nerve discharges in the cat: effects of lung inflation. J Physiol. 1985 Nov;368:147–178. doi: 10.1113/jphysiol.1985.sp015851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope T. C., Fetz E. E., Matsumura M. Cross-correlation assessment of synaptic strength of single Ia fibre connections with triceps surae motoneurones in cats. J Physiol. 1987 Sep;390:161–188. doi: 10.1113/jphysiol.1987.sp016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The detection of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:33–62. doi: 10.1113/jphysiol.1985.sp015845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. G., Kirkwood P. A., Sears T. A. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985 Nov;368:63–87. doi: 10.1113/jphysiol.1985.sp015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kubin L. Projection of pulmonary rapidly adapting receptors to the medulla of the cat: an antidromic mapping study. J Physiol. 1986 Apr;373:63–86. doi: 10.1113/jphysiol.1986.sp016035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick T. E., Berger A. J. Axonal projections of single bulbospinal inspiratory neurons revealed by spike-triggered averaging and antidromic activation. J Neurophysiol. 1985 Jun;53(6):1590–1603. doi: 10.1152/jn.1985.53.6.1590. [DOI] [PubMed] [Google Scholar]
- Dick T. E., Viana F., Berger A. J. Electrophysiological determination of the axonal projections of single dorsal respiratory group neurons to the cervical spinal cord of cat. Brain Res. 1988 Jun 28;454(1-2):31–39. doi: 10.1016/0006-8993(88)90800-1. [DOI] [PubMed] [Google Scholar]
- Grélot L., Milano S., Portillo F., Miller A. D. Respiratory interneurons of the lower cervical (C4-C5) cord: membrane potential changes during fictive coughing, vomiting, and swallowing in the decerebrate cat. Pflugers Arch. 1993 Nov;425(3-4):313–320. doi: 10.1007/BF00374181. [DOI] [PubMed] [Google Scholar]
- Jakus J., Tomori Z., Stránsky A. Activity of bulbar respiratory neurones during cough and other respiratory tract reflexes in cats. Physiol Bohemoslov. 1985;34(2):127–136. [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. Synaptic actions of single interneurones mediating reciprocal Ia inhibition of motoneurones. J Physiol. 1972 May;222(3):623–642. doi: 10.1113/jphysiol.1972.sp009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Munson J. B., Sears T. A., Westgaard R. H. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988 Jan;395:161–192. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Schmid K., Otto M., Sears T. A. Focal blockade of single unit synaptic transmission by iontophoresis of antagonists. Neuroreport. 1991 Apr;2(4):185–188. doi: 10.1097/00001756-199104000-00006. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Schmid K., Sears T. A. Functional identities of thoracic respiratory interneurones in the cat. J Physiol. 1993 Feb;461:667–687. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Tuck D. L., Westgaard R. H. Variations in the time course of the synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:105–135. doi: 10.1113/jphysiol.1982.sp014223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Restoration of function in external intercostal motoneurones of the cat following partial central deafferentation. J Physiol. 1984 May;350:225–251. doi: 10.1113/jphysiol.1984.sp015198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L. E., Borison H. L. Respiratory mechanics of vomiting in decerebrate cats. Am J Physiol. 1974 Mar;226(3):738–743. doi: 10.1152/ajplegacy.1974.226.3.738. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol. 1987 Jun;57(6):1837–1853. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Tan L. K., Suzuki I. Control of abdominal and expiratory intercostal muscle activity during vomiting: role of ventral respiratory group expiratory neurons. J Neurophysiol. 1987 Jun;57(6):1854–1866. doi: 10.1152/jn.1987.57.6.1854. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Yates B. J. Evaluation of role of upper cervical inspiratory neurons in respiration, emesis and cough. Brain Res. 1993 Mar 19;606(1):143–147. doi: 10.1016/0006-8993(93)91582-d. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price W. M., Batsel H. L. Respiratory neurons participating in sneeze and in response to resistance to expiration. Exp Neurol. 1970 Dec;29(3):554–570. doi: 10.1016/0014-4886(70)90080-4. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. SOME PROPERTIES AND REFLEX CONNEXIONS OF RESPIRATORY MOTONEURONES OF THE CAT'S THORACIC SPINAL CORD. J Physiol. 1964 Dec;175:386–403. doi: 10.1113/jphysiol.1964.sp007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Kirkwood P. A., Munson J. B., Shen E., Sears T. A. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol. 1993 Feb;461:647–665. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y., Yamaguchi T., Futami T. Multiple axon collaterals of single corticospinal axons in the cat spinal cord. J Neurophysiol. 1986 Mar;55(3):425–448. doi: 10.1152/jn.1986.55.3.425. [DOI] [PubMed] [Google Scholar]
- Swadlow H. A. Antidromic activation: measuring the refractory period at the site of axonal stimulation. Exp Neurol. 1982 Feb;75(2):514–519. doi: 10.1016/0014-4886(82)90179-0. [DOI] [PubMed] [Google Scholar]
- Taylor A., Stephens J. A., Somjen G., Appenteng K., O'Donovan M. J. Extracellular spike triggered averaging for plotting synaptic projections. Brain Res. 1978 Jan 27;140(2):344–348. doi: 10.1016/0006-8993(78)90466-3. [DOI] [PubMed] [Google Scholar]


