Abstract
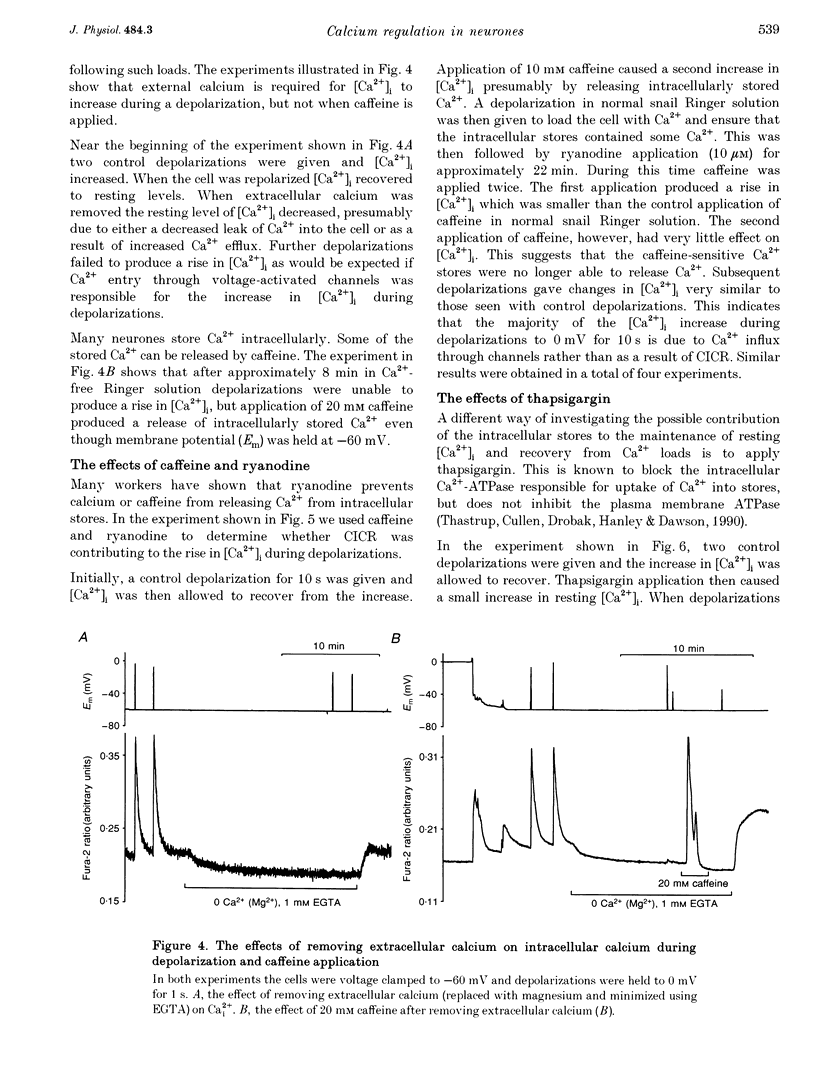
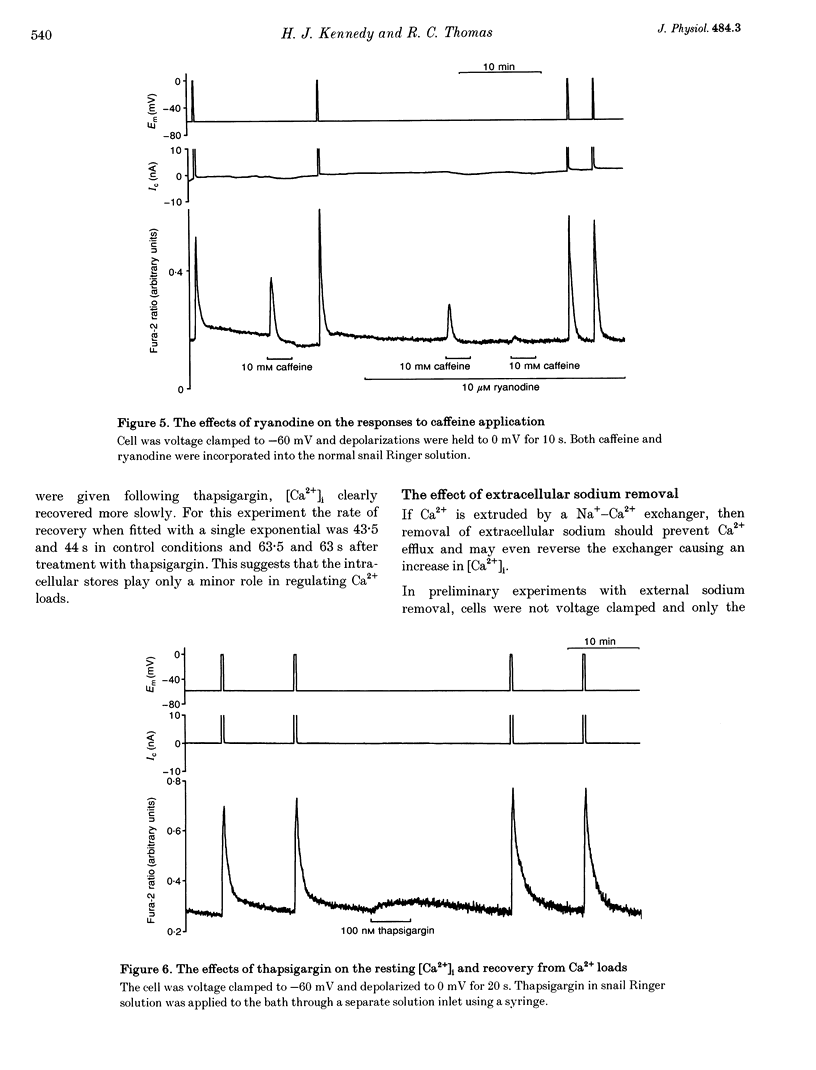
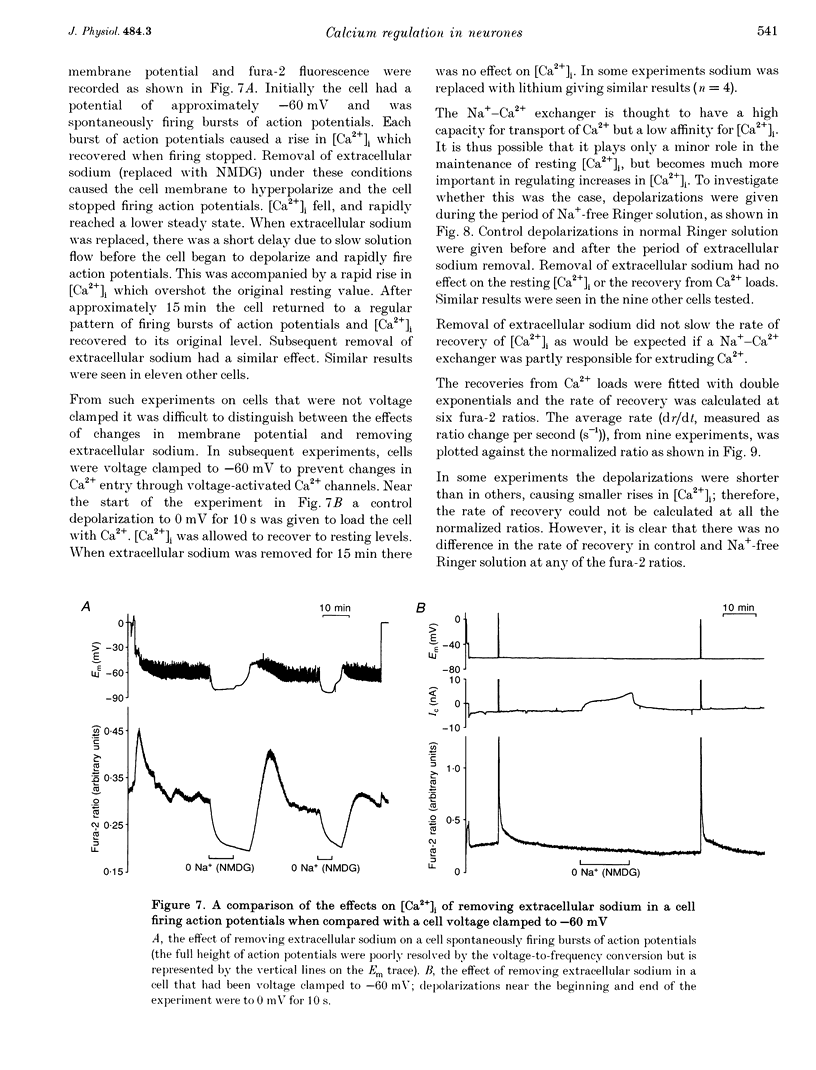
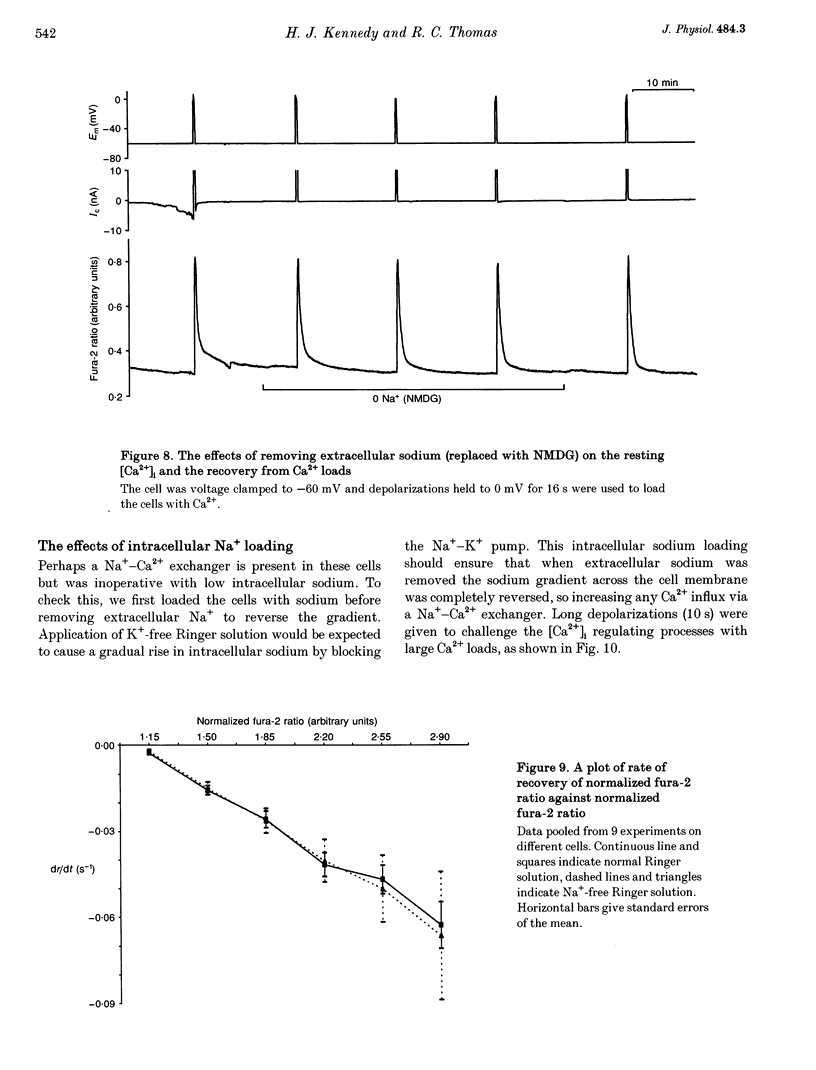
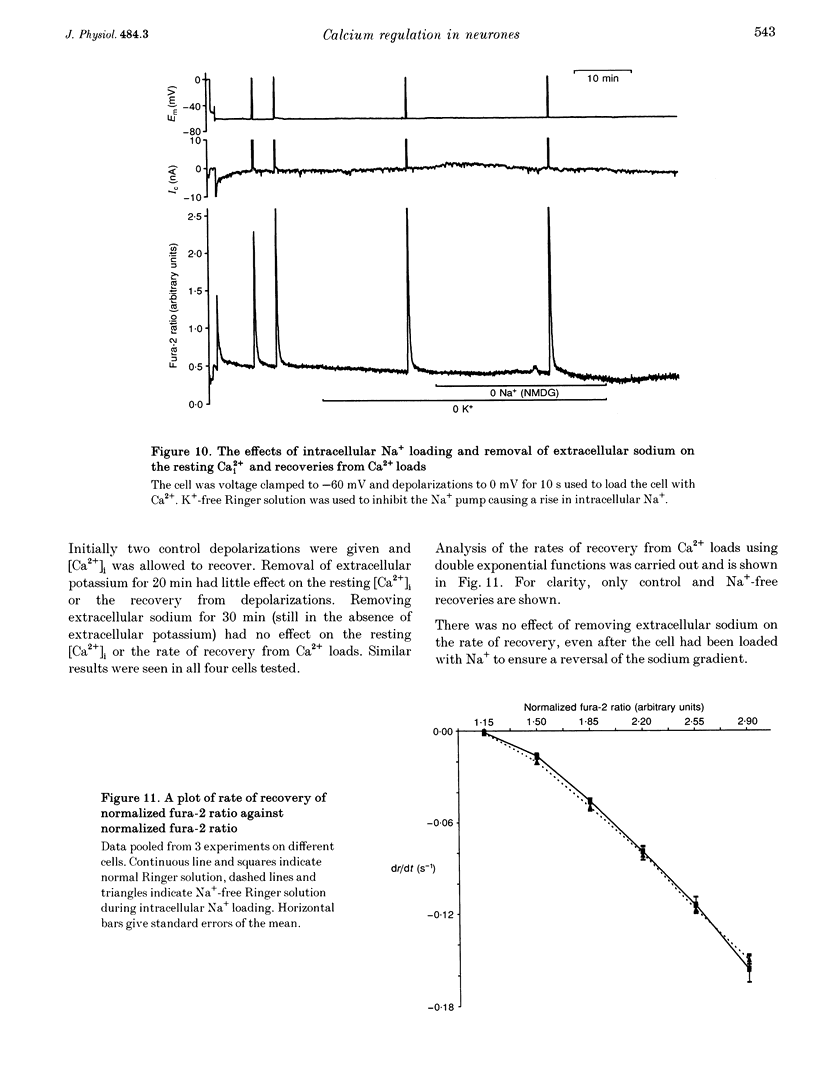
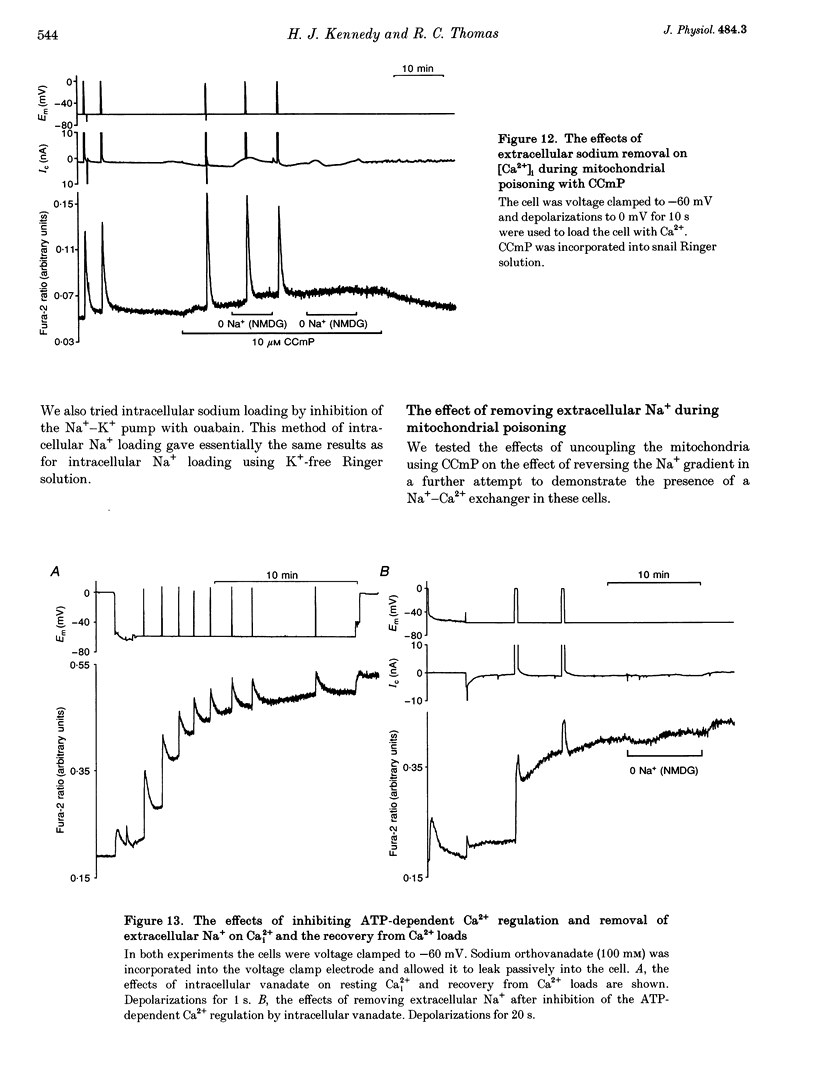
1. We have used both Ca(2+)-sensitive microelectrodes and fura-2 to measure the intracellular free calcium ion concentration ([Ca2+]i or its negative log, pCai) of snail neurones voltage clamped to -50 or -60 mV. Using Ca(2+)-sensitive microelectrodes, [Ca2+]i was found to be approximately 174 nM and pCai, 6.76 +/- 0.09 (mean +/- S.E.M.; n = 11); using fura-2, [Ca2+]i was approximately 40 nM and pCai, 7.44 +/- 0.06 (mean +/- S.E.M., n = 10). 2. Depolarizations (1-20 s) caused an increase in [Ca2+]i which was abolished by removal of extracellular Ca2+, indicating that the rise in [Ca2+]i was due to Ca2+ influx through voltage-activated Ca2+ channels. 3. Caffeine (10-20 mM) caused an increase in [Ca2+]i in the presence or absence of extracellular Ca2+. The effects of caffeine on [Ca2+]i could be prevented by ryanodine. 4. Thapsigargin, an inhibitor of the endoplasmic reticulum Ca(2+)-ATPase, caused a small increase in resting [Ca2+]i and slowed the rate of recovery from Ca2+ loads following 20 s depolarizations. 5. Neither replacement of extracellular sodium with N-methyl-D-glucamine (NMDG), nor loading the cells with intracellular sodium, had any effect on resting [Ca2+]i or the rate of recovery of [Ca2+]i following depolarizations. 6. The mitochondrial uncoupling agent carbonyl cyanide m-chlorophenylhydrazone (CCmP) caused a small gradual rise in resting [Ca2+]i. Removal of extracellular sodium during exposure to CCmP had no further effect on [Ca2+]i. 7. Intracellular orthovanadate caused an increase in resting [Ca2+]i and prevented the full recovery of [Ca2+]i following small Ca2+ loads, but removal of extracellular sodium did not cause a rise in [Ca2+]i. We conclude that there is no Na(+)-Ca2+ exchanger present in the cell body of these neurones and that [Ca2+]i is maintained by an ATP-dependent Ca2+ pump.
Full text
PDF

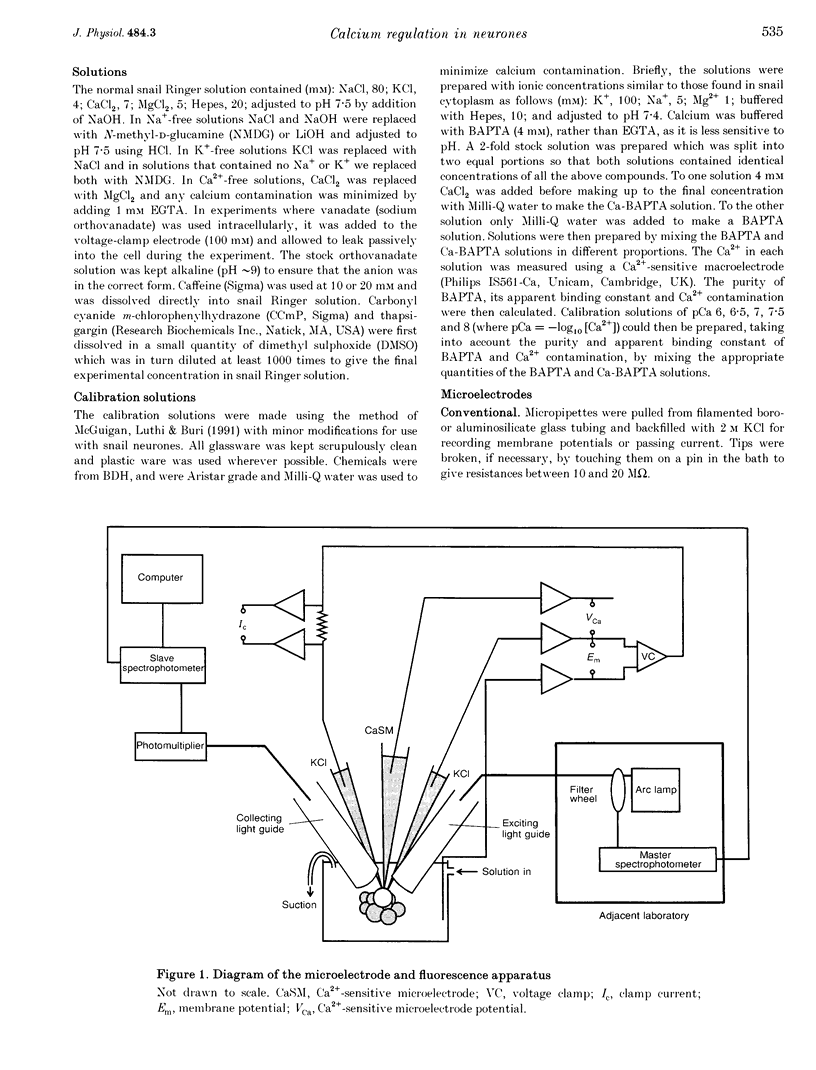

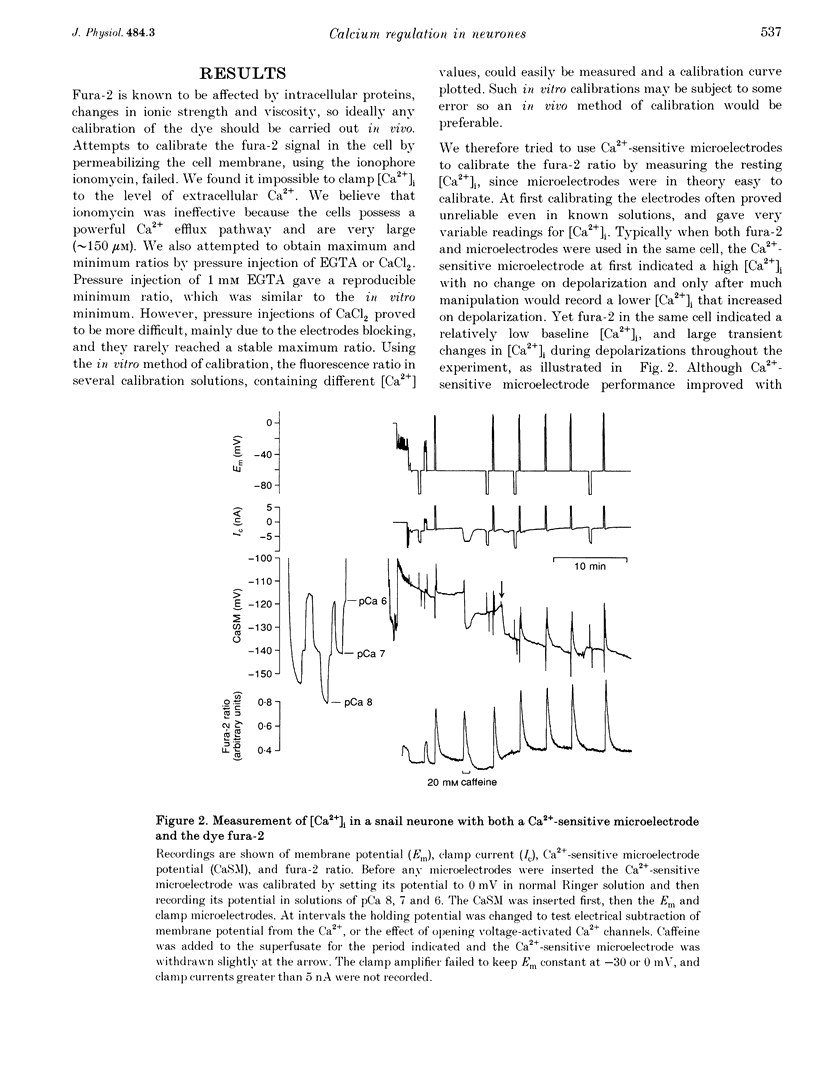
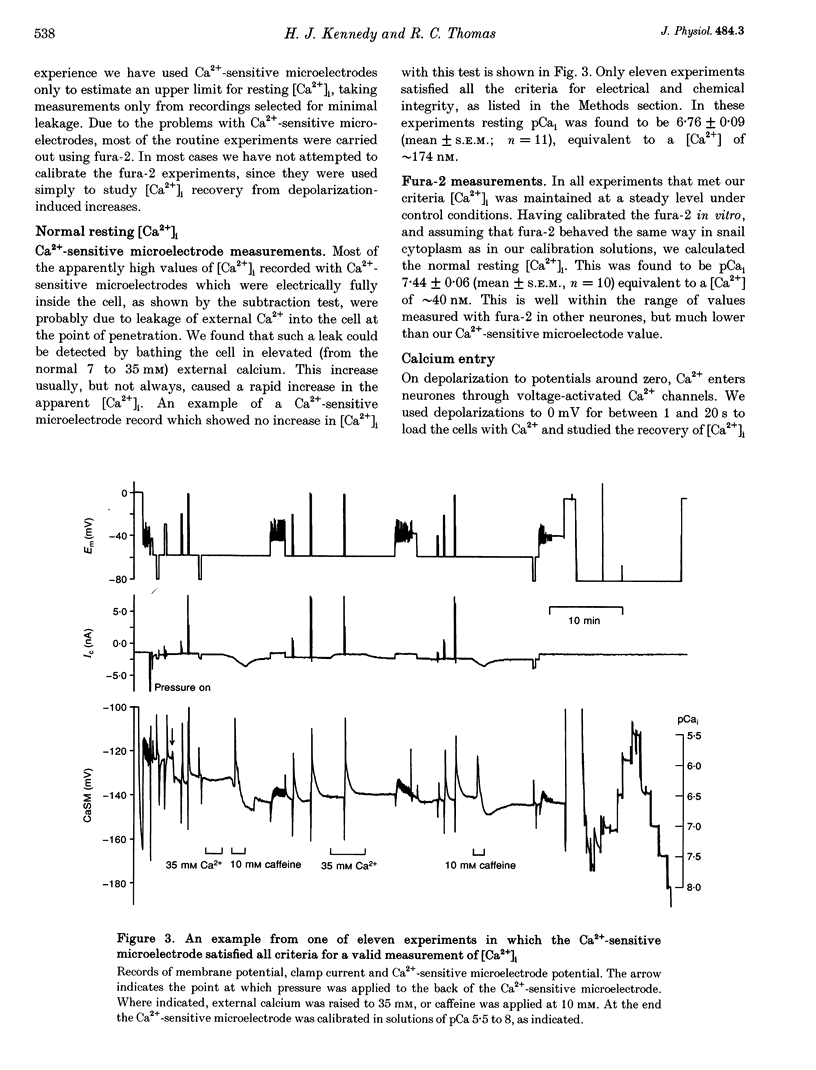




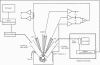
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Leefmans F. J., Rink T. J., Tsien R. Y. Free calcium ions in neurones of Helix aspersa measured with ion-selective micro-electrodes. J Physiol. 1981 Jun;315:531–548. doi: 10.1113/jphysiol.1981.sp013762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Evans M. L., McBain C. J. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurones. J Physiol. 1992 Sep;455:567–583. doi: 10.1113/jphysiol.1992.sp019316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D., Roback J. D., Wainer B. H., Miller R. J., Harrison N. L. Calcium homeostasis in rat septal neurons in tissue culture. Brain Res. 1993 Jan 15;600(2):257–267. doi: 10.1016/0006-8993(93)91381-2. [DOI] [PubMed] [Google Scholar]
- Bond G. H., Hudgins P. M. Inhibition of red cell Ca2+-ATPase by vanadate. Biochim Biophys Acta. 1980 Aug 14;600(3):781–790. doi: 10.1016/0005-2736(80)90480-0. [DOI] [PubMed] [Google Scholar]
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Coutinho O. P., Carvalho A. P., Carvalho C. A. Effect of monovalent cations on Na+/Ca2+ exchange and ATP-dependent Ca2+ transport in synaptic plasma membranes. J Neurochem. 1983 Sep;41(3):670–676. doi: 10.1111/j.1471-4159.1983.tb04793.x. [DOI] [PubMed] [Google Scholar]
- DiPolo R., Rojas H. R., Beaugé L. Vanadate inhibits uncoupled Ca efflux but not Na--Ca exchange in squid axons. Nature. 1979 Sep 20;281(5728):229–230. doi: 10.1038/281228a0. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992 May;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Levy S., Tillotson D. Effects of Na+ and Ca2+ gradients on intracellular free Ca2+ in voltage-clamped Aplysia neurons. Brain Res. 1988 Dec 6;474(2):333–342. doi: 10.1016/0006-8993(88)90447-7. [DOI] [PubMed] [Google Scholar]
- McGuigan J. A., Lüthi D., Buri A. Calcium buffer solutions and how to make them: a do it yourself guide. Can J Physiol Pharmacol. 1991 Nov;69(11):1733–1749. doi: 10.1139/y91-257. [DOI] [PubMed] [Google Scholar]
- Mironov S. L., Usachev YuM, Lux H. D. Spatial and temporal control of intracellular free Ca2+ in chick sensory neurons. Pflugers Arch. 1993 Jul;424(2):183–191. doi: 10.1007/BF00374610. [DOI] [PubMed] [Google Scholar]
- Mironov S. L., Usachev J. M. Caffeine affects Ca uptake and Ca release from intracellular stores: fura-2 measurements in isolated snail neurones. Neurosci Lett. 1991 Feb 25;123(2):200–202. doi: 10.1016/0304-3940(91)90930-r. [DOI] [PubMed] [Google Scholar]
- Müller T. H., Partridge L. D., Swandulla D. Calcium buffering in bursting Helix pacemaker neurons. Pflugers Arch. 1993 Dec;425(5-6):499–505. doi: 10.1007/BF00374877. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Sanchez-Armass S., Weinstein A. M. The regulation of cytosolic calcium in rat brain synaptosomes by sodium-dependent calcium efflux. J Physiol. 1986 Dec;381:17–28. doi: 10.1113/jphysiol.1986.sp016309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Nohmi M., Kuba K. Effects of Na+ gradient on the intracellular Ca2+ oscillation in the sympathetic ganglion cell: Na-Ca exchange in the neurone cell soma? Brain Res. 1984 Dec 17;324(1):171–174. doi: 10.1016/0006-8993(84)90638-3. [DOI] [PubMed] [Google Scholar]
- Poenie M. Alteration of intracellular Fura-2 fluorescence by viscosity: a simple correction. Cell Calcium. 1990 Feb-Mar;11(2-3):85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- Rahamimoff H., Abramovitz E. Calcium transport in a vesicular membrane preparation from rat brain synaptosomes. FEBS Lett. 1978 May 15;89(2):223–226. doi: 10.1016/0014-5793(78)80222-1. [DOI] [PubMed] [Google Scholar]
- Sanchez-Armass S., Blaustein M. P. Role of sodium-calcium exchange in regulation of intracellular calcium in nerve terminals. Am J Physiol. 1987 Jun;252(6 Pt 1):C595–C603. doi: 10.1152/ajpcell.1987.252.6.C595. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Schwiening C. J., Kennedy H. J., Thomas R. C. Calcium-hydrogen exchange by the plasma membrane Ca-ATPase of voltage-clamped snail neurons. Proc Biol Sci. 1993 Sep 22;253(1338):285–289. doi: 10.1098/rspb.1993.0115. [DOI] [PubMed] [Google Scholar]
- Silver I. A., Erecińska M. Intracellular and extracellular changes of [Ca2+] in hypoxia and ischemia in rat brain in vivo. J Gen Physiol. 1990 May;95(5):837–866. doi: 10.1085/jgp.95.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993 May;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth J. L., Thayer S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994 Jan;14(1):348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]