Abstract
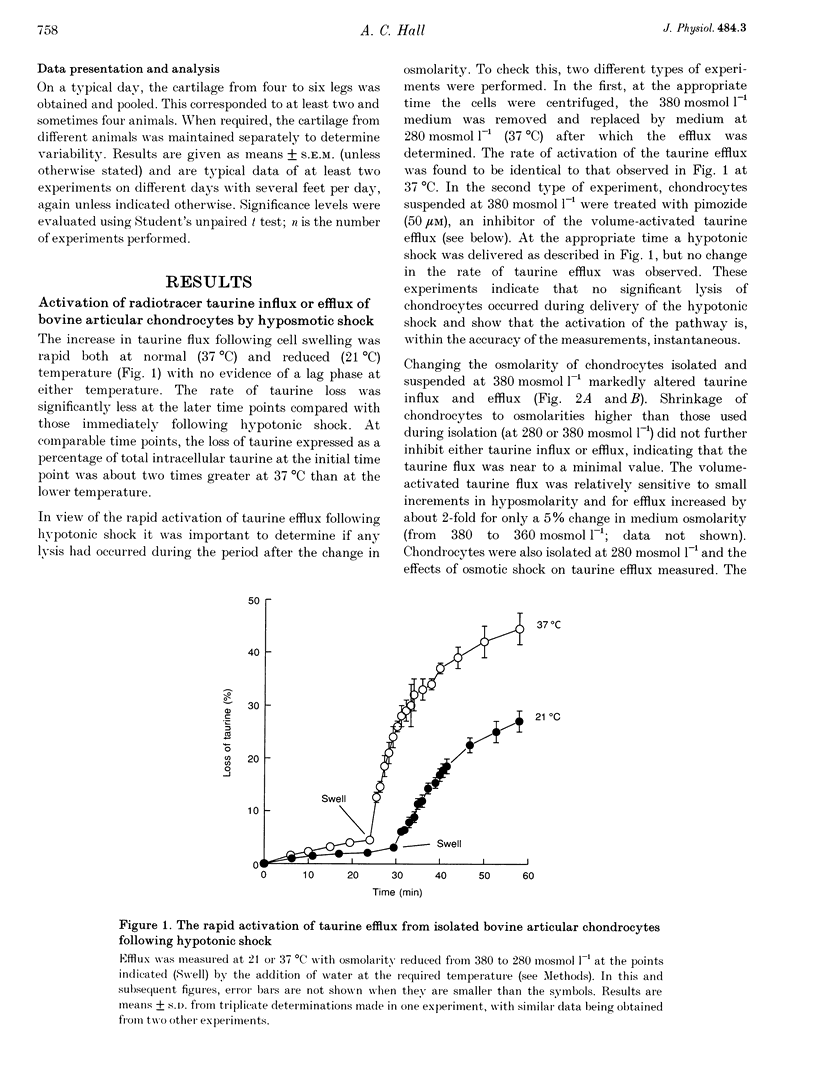
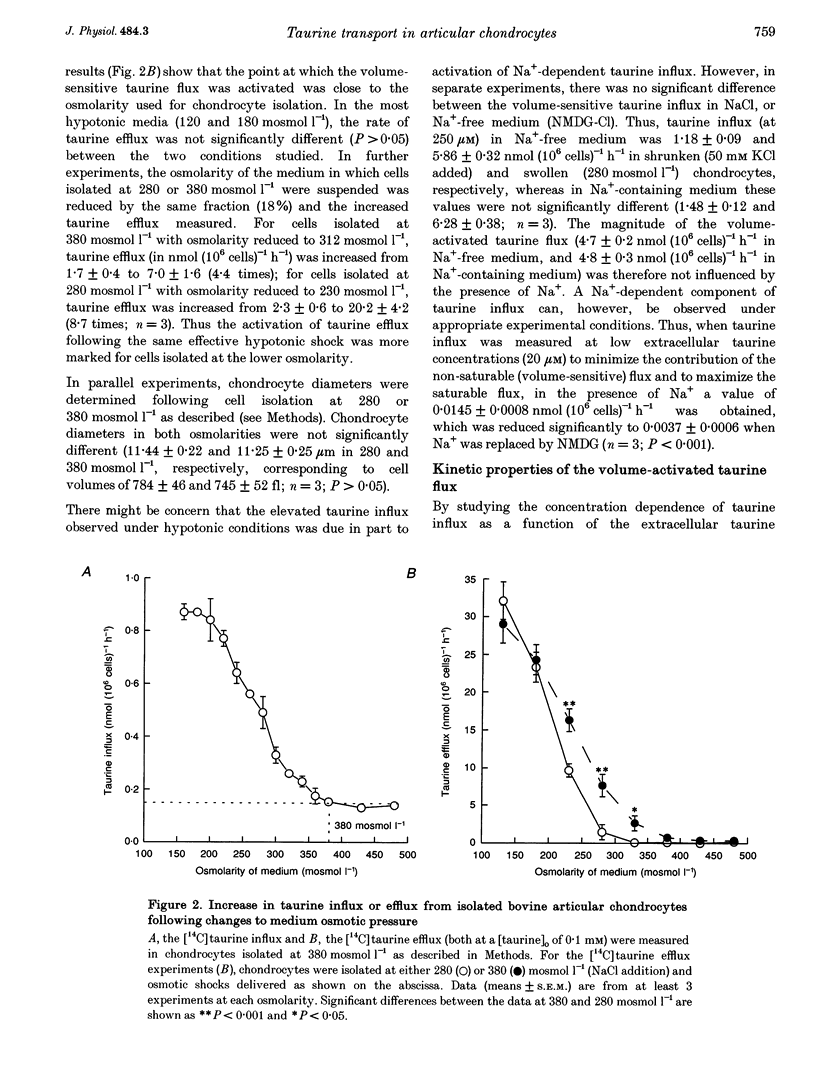
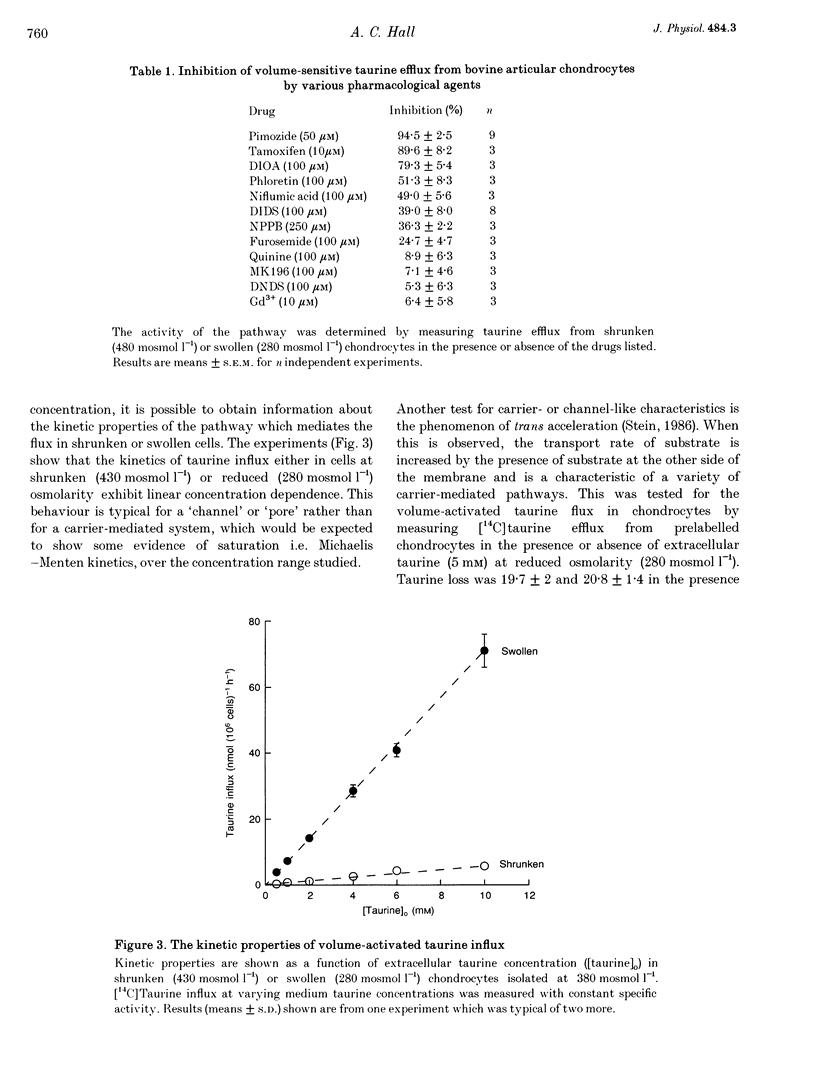
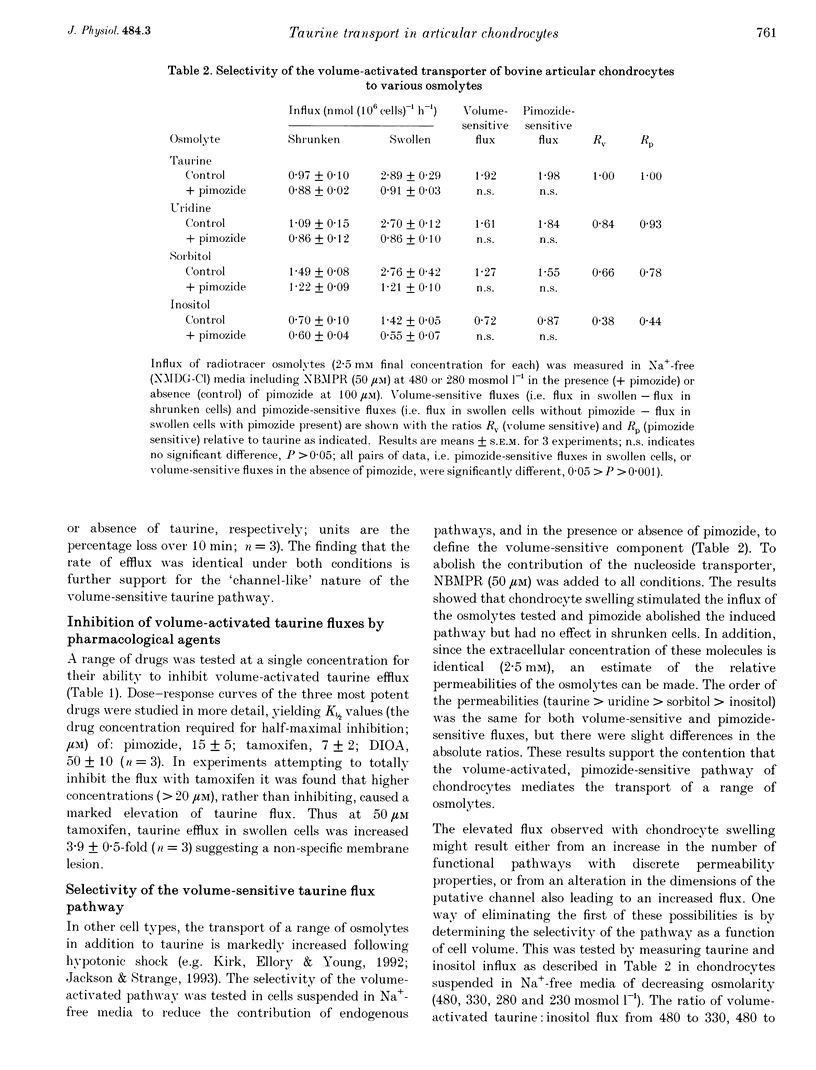
1. The swelling of bovine articular chondrocytes isolated from, or in situ within, cartilage by hypotonic shock rapidly activated the efflux or influx of radiolabelled taurine, an amino acid involved in volume regulation in a range of other cell types. 2. When chondrocytes were isolated by the use of collagenase into media of 280 or 380 mosmol l-1, the activation of taurine efflux was at about the osmolarity of the isolating medium, but it was more marked for a given hypotonic shock in the cells isolated at the lower osmolarity. The volume of chondrocytes following isolation in these two osmolarities was the same, suggesting that the cells possess volume regulatory capacity. 3. In isolated chondrocytes, the induced pathway had some of the characteristics of a volume-activated channel, i.e. no transport saturation with increasing substrate concentration, and lack of trans acceleration. The pattern of inhibition of the volume-activated pathway by pharmacological blockers (e.g. pimozide, [(dihydro-indenyl)oxy]alkanoic acid (DIOA) and tamoxifen) differed from that described for a similar pathway in other cell types. 4. The transport of other potential osmolytes (uridine, sorbitol and inositol) was stimulated by cell swelling, independent of sodium and inhibited by pimozide with a selectivity ratio of taurine, 1.00; uridine, 0.84; sorbitol, 0.66; and inositol, 0.38. The selectivity of taurine: inositol was not altered at different cell volumes. 5. The intracellular taurine concentration of chondrocytes within cartilage was low (about 2 mmol (l cell water)-1) showing a negligible role for taurine as an osmolyte during recovery from cell swelling. The swelling-induced loss of taurine from chondrocytes in situ was largely inhibited by pimozide and other drugs, showing that despite the rigid nature of cartilage, the chondrocytes were osmotically sensitive within the extracellular matrix.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballatori N., Boyer J. L. Taurine transport in skate hepatocytes. II. Volume activation, energy, and sulfhydryl dependence. Am J Physiol. 1992 Mar;262(3 Pt 1):G451–G460. doi: 10.1152/ajpgi.1992.262.3.G451. [DOI] [PubMed] [Google Scholar]
- Banderali U., Roy G. Activation of K+ and Cl- channels in MDCK cells during volume regulation in hypotonic media. J Membr Biol. 1992 Mar;126(3):219–234. doi: 10.1007/BF00232319. [DOI] [PubMed] [Google Scholar]
- Bender A. S., Neary J. T., Blicharska J., Norenberg L. O., Norenberg M. D. Role of calmodulin and protein kinase C in astrocytic cell volume regulation. J Neurochem. 1992 May;58(5):1874–1882. doi: 10.1111/j.1471-4159.1992.tb10064.x. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992 Apr;262(4 Pt 1):C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Christensen O., Hoffmann E. K. Cell swelling activates K+ and Cl- channels as well as nonselective, stretch-activated cation channels in Ehrlich ascites tumor cells. J Membr Biol. 1992 Jul;129(1):13–36. doi: 10.1007/BF00232052. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Wolowyk M. W., Young J. D. Volume-sensitive taurine transport in fish erythrocytes. J Membr Biol. 1987;96(1):45–56. doi: 10.1007/BF01869333. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Nazaret C., Hannaert P. A., Cragoe E. J., Jr Demonstration of a [K+,Cl-]-cotransport system in human red cells by its sensitivity to [(dihydroindenyl)oxy]alkanoic acids: regulation of cell swelling and distinction from the bumetanide-sensitive [Na+,K+,Cl-]-cotransport system. Mol Pharmacol. 1988 Jun;33(6):696–701. [PubMed] [Google Scholar]
- Garcia-Romeu F., Cossins A. R., Motais R. Cell volume regulation by trout erythrocytes: characteristics of the transport systems activated by hypotonic swelling. J Physiol. 1991;440:547–567. doi: 10.1113/jphysiol.1991.sp018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L., Brill S. R. Volume-activated taurine efflux from skate erythrocytes: possible band 3 involvement. Am J Physiol. 1991 May;260(5 Pt 2):R1014–R1020. doi: 10.1152/ajpregu.1991.260.5.R1014. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- Hall A. C., Urban J. P., Gehl K. A. The effects of hydrostatic pressure on matrix synthesis in articular cartilage. J Orthop Res. 1991 Jan;9(1):1–10. doi: 10.1002/jor.1100090102. [DOI] [PubMed] [Google Scholar]
- Hardison W. G., Weiner R. Taurine transport by rat hepatocytes in primary culture. Biochim Biophys Acta. 1980 May 8;598(1):145–152. doi: 10.1016/0005-2736(80)90272-2. [DOI] [PubMed] [Google Scholar]
- Hodge W. A., Fijan R. S., Carlson K. L., Burgess R. G., Harris W. H., Mann R. W. Contact pressures in the human hip joint measured in vivo. Proc Natl Acad Sci U S A. 1986 May;83(9):2879–2883. doi: 10.1073/pnas.83.9.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Lambert I. H. Amino acid transport and cell volume regulation in Ehrlich ascites tumour cells. J Physiol. 1983 May;338:613–625. doi: 10.1113/jphysiol.1983.sp014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Jackson P. S., Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993 Dec;265(6 Pt 1):C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Jennings M. L., al-Rohil N. Kinetics of activation and inactivation of swelling-stimulated K+/Cl- transport. The volume-sensitive parameter is the rate constant for inactivation. J Gen Physiol. 1990 Jun;95(6):1021–1040. doi: 10.1085/jgp.95.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuś García J., Sánchez Olea R., Pasantes-Morales H. Taurine release associated to volume regulation in rabbit lymphocytes. J Cell Biochem. 1991 Feb;45(2):207–212. doi: 10.1002/jcb.240450212. [DOI] [PubMed] [Google Scholar]
- Kirk K., Ellory J. C., Young J. D. Transport of organic substrates via a volume-activated channel. J Biol Chem. 1992 Nov 25;267(33):23475–23478. [PubMed] [Google Scholar]
- Lambert I. H., Hoffmann E. K. Regulation of taurine transport in Ehrlich ascites tumor cells. J Membr Biol. 1993 Jan;131(1):67–79. doi: 10.1007/BF02258535. [DOI] [PubMed] [Google Scholar]
- Law R. O. Amino acids as volume-regulatory osmolytes in mammalian cells. Comp Biochem Physiol A Comp Physiol. 1991;99(3):263–277. doi: 10.1016/0300-9629(91)90001-s. [DOI] [PubMed] [Google Scholar]
- Motais R., Guizouarn H., Garcia-Romeu F. Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochim Biophys Acta. 1991 Oct 10;1075(2):169–180. doi: 10.1016/0304-4165(91)90248-f. [DOI] [PubMed] [Google Scholar]
- Pasantes Morales H., Schousboe A. Volume regulation in astrocytes: a role for taurine as an osmoeffector. J Neurosci Res. 1988 Aug;20(4):503–509. doi: 10.1002/jnr.490200415. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H., Murray R. A., Sánchez-Olea R., Morán J. Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. Am J Physiol. 1994 Jan;266(1 Pt 1):C172–C178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Politis A. D. Ca2(+)-activated cell volume recovery mechanisms. Annu Rev Physiol. 1990;52:27–42. doi: 10.1146/annurev.ph.52.030190.000331. [DOI] [PubMed] [Google Scholar]
- Rasmusson R. L., Davis D. G., Lieberman M. Amino acid loss during volume regulatory decrease in cultured chick heart cells. Am J Physiol. 1993 Jan;264(1 Pt 1):C136–C145. doi: 10.1152/ajpcell.1993.264.1.C136. [DOI] [PubMed] [Google Scholar]
- Roy G., Malo C. Activation of amino acid diffusion by a volume increase in cultured kidney (MDCK) cells. J Membr Biol. 1992 Oct;130(1):83–90. doi: 10.1007/BF00233740. [DOI] [PubMed] [Google Scholar]
- Sánchez Olea R., Pasantes-Morales H., Lázaro A., Cereijido M. Osmolarity-sensitive release of free amino acids from cultured kidney cells (MDCK). J Membr Biol. 1991 Apr;121(1):1–9. doi: 10.1007/BF01870646. [DOI] [PubMed] [Google Scholar]
- Urban J. P., Hall A. C., Gehl K. A. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993 Feb;154(2):262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Valverde M. A., Mintenig G. M., Sepúlveda F. V. Differential effects of tamoxifen and I- on three distinguishable chloride currents activated in T84 intestinal cells. Pflugers Arch. 1993 Dec;425(5-6):552–554. doi: 10.1007/BF00374885. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]


