Abstract
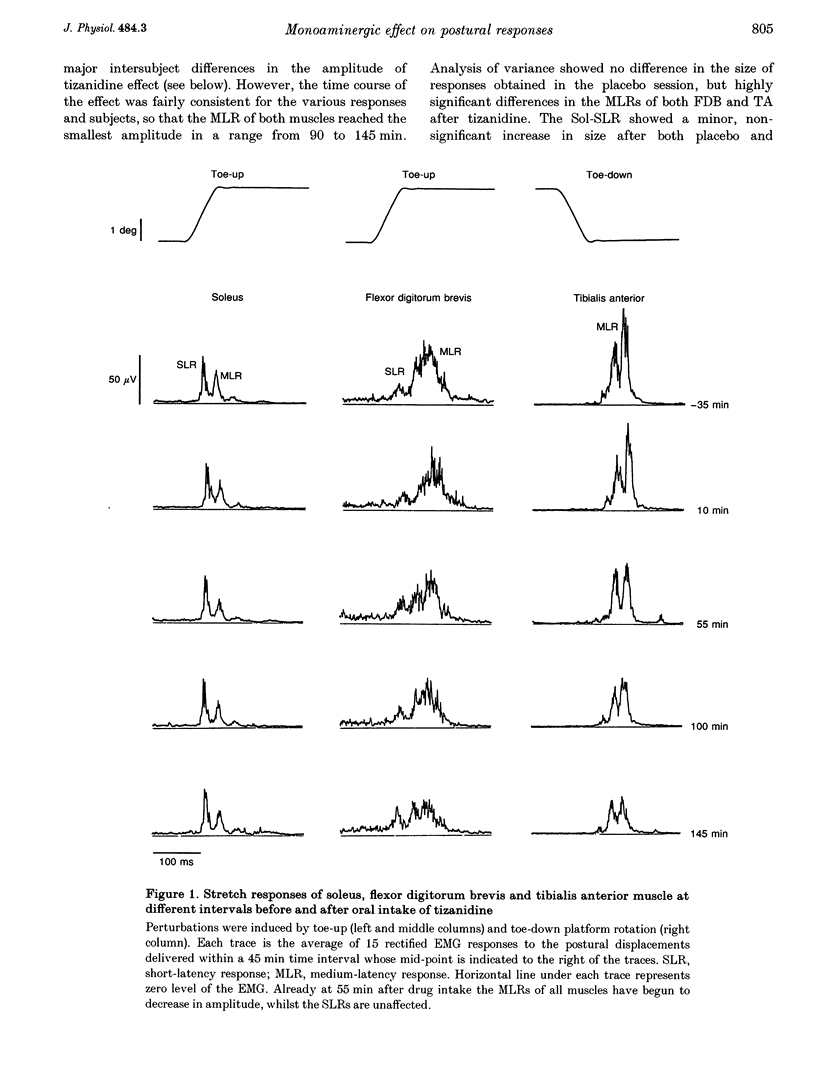
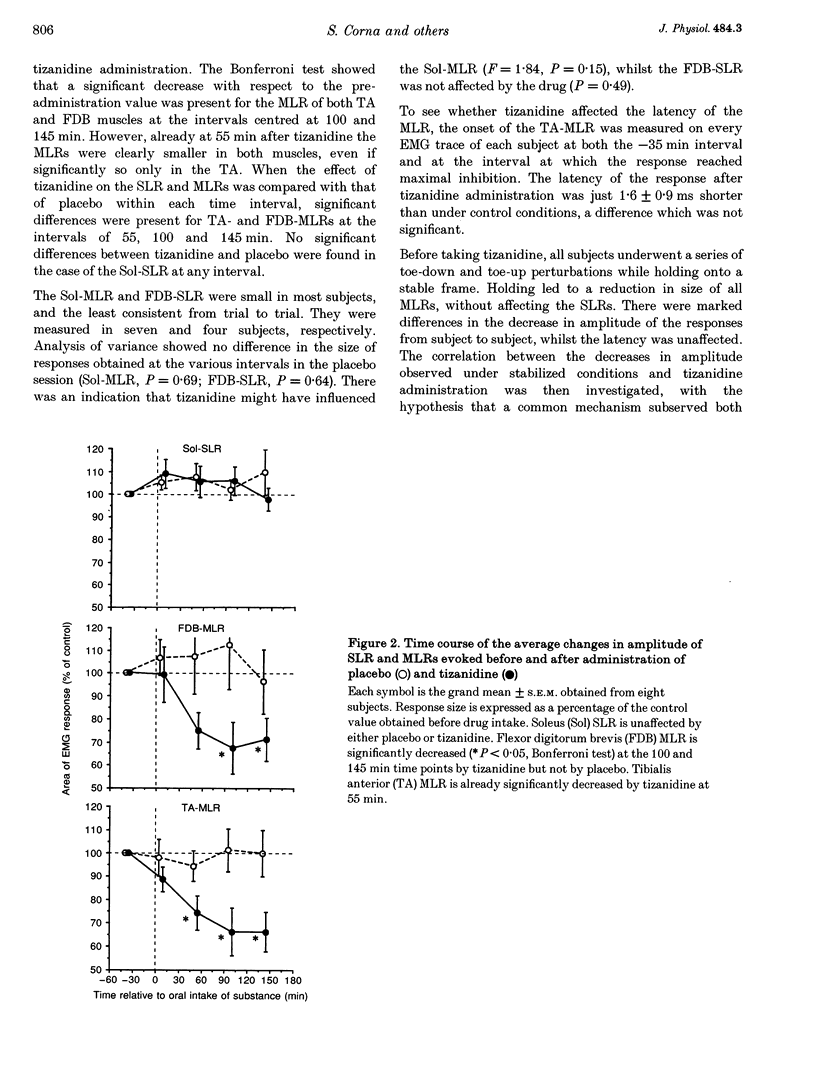
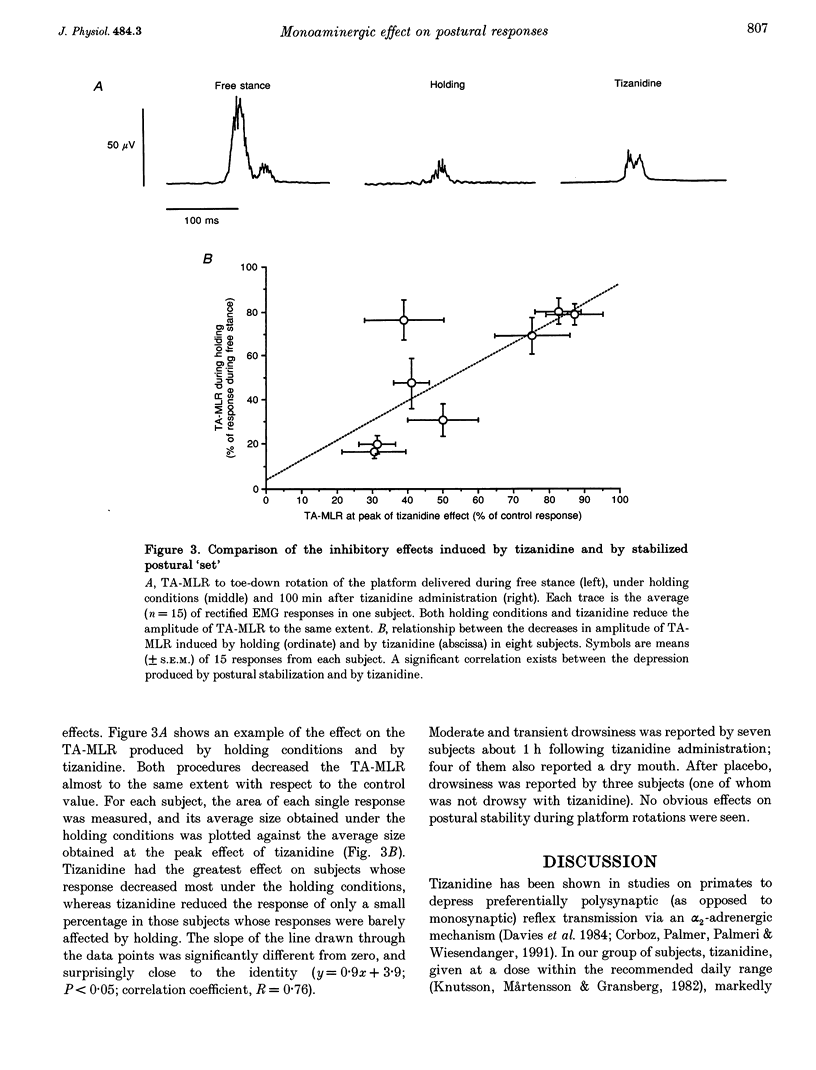
1. In standing humans, toe-up rotation of a platform induces a short-latency (SLR) and a medium-latency response (MLR) in both soleus (Sol) and flexor digitorum brevis (FDB) muscles. Toe-down rotation evokes a MLR in the tibialis anterior (TA). The SLR is the counterpart of the monosynaptic stretch reflex, but the origin of the MLR is still debated. By means of tizanidine (an alpha 2-adrenergic receptor agonist) we tested the hypothesis that the MLR is relayed by group II afferent fibres, since animal data indicate that tizanidine or stimulation of monoaminergic brainstem centres decrease the excitability of spinal interneurones supplied by those fibres. In addition, we compared the effect of the drug on these responses with that induced by stabilization of posture. 2. Eight subjects received tizanidine (150 micrograms kg-1 orally) or placebo, in a single-blind design. Platform rotations were delivered prior to administration and for 3 h afterwards. Both TA- and FDB-MLRs decreased in size, starting from about 1 h after tizanidine administration. Sol-SLR was unaffected. Response latencies were unchanged. Placebo induced no changes in any response. In each subject, the extent of TA-MLR depression induced by holding onto a frame and by tizanidine was superimposable. 3. The selective effect of tizanidine on MLR supports the notion that it is relayed through group II afferent fibres. The similar effects of holding and tizanidine on the response suggests that it is modulated by monoaminergic centres.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bras H., Cavallari P., Jankowska E., McCrea D. Comparison of effects of monoamines on transmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res. 1989;76(1):27–37. doi: 10.1007/BF00253620. [DOI] [PubMed] [Google Scholar]
- Bras H., Jankowska E., Noga B., Skoog B. Comparison of Effects of Various Types of NA and 5-HT Agonists on Transmission from Group II Muscle Afferents in the Cat. Eur J Neurosci. 1990;2(12):1029–1039. doi: 10.1111/j.1460-9568.1990.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Corboz M., Palmer C. I., Palmeri A., Wiesendanger M. Tizanidine-induced depression of polysynaptic cutaneous reflexes in nonanesthetized monkeys is mediated by an alpha 2-adrenergic mechanism. Exp Neurol. 1991 Feb;111(2):210–216. doi: 10.1016/0014-4886(91)90009-2. [DOI] [PubMed] [Google Scholar]
- Davies J., Johnston S. E., Hill D. R., Quinlan J. E. Tizanidine (DS103-282), a centrally acting muscle relaxant, selectively depresses excitation of feline dorsal horn neurones to noxious peripheral stimuli by an action at alpha 2-adrenoceptors. Neurosci Lett. 1984 Jul 27;48(2):197–202. doi: 10.1016/0304-3940(84)90019-3. [DOI] [PubMed] [Google Scholar]
- Dietz V. Human neuronal control of automatic functional movements: interaction between central programs and afferent input. Physiol Rev. 1992 Jan;72(1):33–69. doi: 10.1152/physrev.1992.72.1.33. [DOI] [PubMed] [Google Scholar]
- Eken T., Hultborn H., Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog Brain Res. 1989;80:257–242. doi: 10.1016/s0079-6123(08)62219-0. [DOI] [PubMed] [Google Scholar]
- Emre M., Leslie G. C., Muir C., Part N. J., Pokorny R., Roberts R. C. Correlations between dose, plasma concentrations, and antispastic action of tizanidine (Sirdalud). J Neurol Neurosurg Psychiatry. 1994 Nov;57(11):1355–1359. doi: 10.1136/jnnp.57.11.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson E., Mårtensson A., Gransberg L. Antiparetic and antispastic effects induced by tizanidine in patients with spastic paresis. J Neurol Sci. 1982 Feb;53(2):187–204. doi: 10.1016/0022-510x(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Nardone A., Corrà T., Schieppati M. Different activations of the soleus and gastrocnemii muscles in response to various types of stance perturbation in man. Exp Brain Res. 1990;80(2):323–332. doi: 10.1007/BF00228159. [DOI] [PubMed] [Google Scholar]
- Noga B. R., Bras H., Jankowska E. Transmission from group II muscle afferents is depressed by stimulation of locus coeruleus/subcoeruleus, Kölliker-Fuse and raphe nuclei in the cat. Exp Brain Res. 1992;88(3):502–516. doi: 10.1007/BF00228180. [DOI] [PubMed] [Google Scholar]
- Pompeiano O. Relationship of noradrenergic locus coeruleus neurones to vestibulospinal reflexes. Prog Brain Res. 1989;80:329–319. doi: 10.1016/s0079-6123(08)62228-1. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33(4):281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Schieppati M., Nardone A. Free and supported stance in Parkinson's disease. The effect of posture and 'postural set' on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain. 1991 Jun;114(Pt 3):1227–1244. doi: 10.1093/brain/114.3.1227. [DOI] [PubMed] [Google Scholar]
- Strahlendorf J. C., Strahlendorf H. K., Kingsley R. E., Gintautas J., Barnes C. D. Facilitation of the lumbar monosynaptic reflexes by locus coeruleus stimulation. Neuropharmacology. 1980 Feb;19(2):225–230. doi: 10.1016/0028-3908(80)90143-4. [DOI] [PubMed] [Google Scholar]


