Abstract
Dystonia, typically characterized by slow repetitive involuntary movements, stiff abnormal postures, and hypertonia, is common among individuals with cerebral palsy (CP). Dystonia can interfere with activities and have considerable impact on motor function, pain/comfort, and ease of caregiving. Although pharmacological and neurosurgical approaches are used clinically in individuals with CP and dystonia that is causing interference, evidence to support these options is limited. This clinical practice guideline update comprises 10 evidence‐based recommendations on the use of pharmacological and neurosurgical interventions for individuals with CP and dystonia causing interference, developed by an international expert panel following the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach. The recommendations are intended to help inform clinicians in their use of these management options for individuals with CP and dystonia, and to guide a shared decision‐making process in selecting a management approach that is aligned with the individual's and the family's values and preferences.
This clinical practice guideline provides evidence‐based clinical recommendations for the use of pharmacological and neurosurgical management options for individuals with dystonia and cerebral palsy (CP), alongside practical considerations and suggestions for future research priorities. It was developed by a panel of clinicians with diverse expertise following the GRADE process, with constructive input from individuals with CP and dystonia and their families.

This clinical practice guideline is commented by Lumsden on pages 1116–1117 of this issue.
Plain language summary: https://onlinelibrary.wiley.com/doi/10.1111/dmcn.15979
Abbreviations
- AACPDM
American Academy for Cerebral Palsy and Developmental Medicine
- DBS
deep brain stimulation
- GABA
γ‐aminobutyric acid
- GRADE
Grading of Recommendations, Assessment, Development and Evaluations
- ITB
intrathecal baclofen
- PICO
population, intervention, comparator, outcome
What this paper adds
Dystonia management in cerebral palsy should be individualized and informed by shared decision‐making with the individual/family.
Individualized trials of oral/enteral/transdermal medications should inform the most suitable option.
Botulinum neurotoxin A injections are suggested for focal/segmental dystonia causing interference.
Intrathecal baclofen and deep brain stimulation are suggested when dystonia is severe and not responsive to oral/enteral/transdermal medications.
Additional well‐designed trials are needed to strengthen evidence informing recommendations.
BACKGROUND
 Dystonia is defined as ‘a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation’.
1
When muscle contractions are sustained, individuals with cerebral palsy (CP) and dystonia present with stiff postures and hypertonia. In dystonia, the hypertonia (sometimes referred to as dystonic hypertonia) is characterized by resistance to passive stretch of a muscle across a joint, which is present at low speeds, fluctuates with repeated stretches, and where tactile stimulus (e.g. light touching) or purposeful movement of another body part triggers increased tone or increased involuntary postures.
2
,
3
When muscle contractions are intermittent, dystonia typically presents with slow repetitive involuntary movements.
4
Dystonia is defined as ‘a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation’.
1
When muscle contractions are sustained, individuals with cerebral palsy (CP) and dystonia present with stiff postures and hypertonia. In dystonia, the hypertonia (sometimes referred to as dystonic hypertonia) is characterized by resistance to passive stretch of a muscle across a joint, which is present at low speeds, fluctuates with repeated stretches, and where tactile stimulus (e.g. light touching) or purposeful movement of another body part triggers increased tone or increased involuntary postures.
2
,
3
When muscle contractions are intermittent, dystonia typically presents with slow repetitive involuntary movements.
4
Dystonia is frequently present in individuals with CP. CP refers to ‘a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to non‐progressive disturbances that occurred in the developing fetal or infant brain’. 5 Dystonia exists among individuals with the dystonic subtype of dyskinetic CP, defined by the Surveillance of Cerebral Palsy in Europe network as being dominated by abnormal postures and hypertonia. 6 Dystonia is also frequently present in individuals with predominantly spastic CP, up to 75% of whom have mixed hypertonia patterns. 7 Dystonia can affect function (including speech), participation, ease of caregiving, well‐being, and is associated with pain and musculoskeletal deformity. 8 , 9
Aims
In partnership with the American Academy for Cerebral Palsy and Developmental Medicine (AACPDM), recommendations for pharmacological and neurosurgical management of individuals with CP and dystonia were published in 2018. 10 This paper advances these recommendations by implementing the revised methodology developed by the AACPDM Care Pathways Committee 11 and following the Guideline Development Checklist – Care Pathway, a modified version of the Guidelines International Network – McMaster Guideline Development Checklist. 12 It represents a clinical practice guideline comprising recommendations informed by a systematic review of evidence and assessment of benefits and harms, developed using Grading of Recommendations, Assessment, Development and Evaluations (GRADE) methodology. 13
Recognizing the complexity of managing dystonia in individuals with CP, this paper aims to synthesize and appraise evidence for outcomes important to individuals with CP and dystonia, formulate recommendations related to pharmacological and neurosurgical interventions based on best available evidence, and identify research priorities. These efforts are intended to provide clinicians with the tools needed to engage individuals with CP and dystonia and their families in an effective shared decision‐making process. This guideline is for clinicians in settings where management is provided to individuals with CP and dystonia, often led by advanced care providers in multidisciplinary teams. It is important to highlight that it is not intended to inform policy or reimbursement decisions.
Target population
This guideline is for individuals of all ages with CP and dystonia who are experiencing stiff dystonic postures, hypertonia, and slow involuntary movements that are causing interference, whether it is part of the predominant dystonic subtype of dyskinetic CP or an additional feature of predominant spastic CP (as defined by the criteria of the Surveillance of Cerebral Palsy in Europe). 6 Operational definitions of the populations described in the recommendations are provided in Table 1, alongside additional neurological definitions for reference.
TABLE 1.
Definitions.
| Operational definitions relating to populations described in recommendations | |
| Dystonia | A movement disorder characterized by ‘sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous. Dystonia is often initiated or worsened by voluntary action and associated with overflow muscle activation.’ 1 |
| Generalized dystonia | Dystonia affecting all or most of the body (i.e. trunk and at least two other body parts). 1 |
| Focal or segmental dystonia | Dystonia predominantly affecting a single limb or truncal part (focal) or a few adjacent body regions (segmental). 14 |
| Dystonia causing interference | Dystonia interfering with the individual's activities, causing some disruption of sleep, pain or discomfort, difficulties sitting, or challenges associated with ease of caregiving. |
| Severe dystonia | Sustained dystonia that is severely restricting the individual's activities, sleep, comfort, or ease of caregiving. |
| Dystonia not responsive to oral/enteral/transdermal dystonia medications | Individuals whose symptoms have not adequately responded to two or more optimized oral, enteral or transdermal dystonia medications (i.e. little to no meaningful progress towards individualized goals) or who develop intolerable side effects. |
| Additional neurological definitions | |
| Spastic CP | A type of CP with increased tone associated with spasticity (‘a velocity‐dependent resistance of a muscle to stretch – resistance to externally imposed movement increases with increasing speed of stretch’). 2 |
| Dyskinetic CP | A type of CP with ‘involuntary, uncontrolled, recurring, and occasionally stereotyped movements. The primitive reflex patterns predominate, and the muscle tone is varying’. 6 |
| Chorea | A hyperkinetic involuntary movement consisting of ‘an ongoing (continuous) random‐appearing sequence of one or more discrete involuntary movements or movement fragments’. 15 They frequently appear to be more rapid than dystonia. |
| Athetosis | A ‘slow, continuous, involuntary writhing movement that prevents maintenance of a stable posture’. 15 |
| Myoclonus | A ‘sequence of repeated, often non‐rhythmic, brief shock‐like jerks due to sudden involuntary contraction or relaxation of one or more muscles’. 15 |
| Status dystonicus | ‘Status dystonicus is characterized by the development of increasingly frequent or continuous severe episodes of generalized dystonic spasms (contractions) and requires urgent (hospital) management’. 16 Status dystonicus is also referred to as dystonic storm or dystonic crisis. |
It is important to highlight that challenges exist in how dystonia is defined in the terminology of CP subtypes. Mixed presentations of CP are common, creating challenges in distinguishing the predominant CP subtype. 7 For example, the Surveillance of Cerebral Palsy in Europe divides dyskinetic CP into two predominant subtypes: dystonic CP, dominated by abnormal postures and hypertonia; and choreoathetotic CP, dominated by hyperkinesia (a combination of chorea and athetotic involuntary movements) and hypotonia. 6 However, dystonic CP often has truncal hypotonia, and dystonia can be present in the choreoathetotic CP subtype to a lesser degree, creating a ‘mixed’ presentation. In addition, although the typical speed of involuntary movements associated with dystonia is slow, when dystonic postures change quickly, it can be difficult to distinguish dystonia from chorea and myoclonic jerks. 15
Dystonia has been classified both as a cause of hypertonia (increased resistance to passive stretch of a muscle across a joint) 2 and as a hyperkinetic movement disorder (unwanted excess movement). 15 Pharmacological and neurosurgical interventions can differ in their effect on hypertonia and hyperkinesia. An example is the dopamine depletor tetrabenazine, 17 where the underlying mechanism of action focuses on suppressing rapid hyperkinetic movements but may worsen ‘stiff’ postures or slow involuntary movements. This Care Pathway focuses on interventions directed towards individuals with dystonia and CP experiencing stiff dystonic postures/hypertonia and slow involuntary movements that are causing interference. Interventions directed towards management of rapid hyperkinetic movements (e.g. tetrabenazine) and recommendations related to emergency management of status dystonicus, increasingly frequent or continuous severe episodes of generalized dystonic spasms (contractions) requiring urgent (hospital) management, 16 , 18 are beyond the scope of this paper.
How to use this guideline
Optimal understanding will be enhanced by consulting the systematic review developed to support this paper. 19 This guideline is intended as a starting point for shared decision‐making, not as a standard of care. Dystonia associated with CP is rarely managed in isolation, and optimal management is often achieved by a multidisciplinary team. Pharmacological or neurosurgical management should only be considered when dystonia is causing interference. It is important to first optimize general health and introduce rehabilitation strategies to promote function, comfort, and participation.
A single recommendation was developed for each PICO (‘population, intervention, comparator, outcome’) question. When a comparator is not stated, a situation where the intervention is not received is implied. The strength of each recommendation presented in this guideline is expressed as strong or conditional. The words ‘we recommend’ are used for strong recommendations and ‘we suggest’ for conditional recommendations. Table 2 provides GRADE's interpretation of strong and conditional recommendations.
TABLE 2.
Interpretation of strong and conditional recommendations. 20
| Strong recommendation | Conditional recommendation | |
|---|---|---|
| Wording in the guideline | We recommend … | We suggest … |
| For clinicians | Most individuals should receive the recommended course of action. Adherence to this recommendation according to the guideline could be used as a quality criterion or performance indicator. Formal decision aids are not likely to be needed to help individuals make decisions consistent with their values and preferences. | Recognize that different choices will be appropriate for different individuals, and that you help each individual and family arrive at a management decision consistent with their values and preferences. Decision aids may be useful in helping individuals making decisions consistent with their values and preferences. Clinicians should expect to spend more time with individuals and families when working towards a decision. |
| For individuals with cerebral palsy and dystonia and their families | Most individuals in this situation would want the recommended course of action and only a small proportion would not. | Most individuals in this situation would want the suggested course of action, but many would not. |
When evidence is limited, there is a close balance between desirable and undesirable effects, or there is likely to be important uncertainty or variation in individual values and preferences, a recommendation of conditional strength is typically more suitable. A conditional recommendation implies that there is likely to be important variation in decision‐making in pursuing an intervention. In these cases, engaging in a shared decision‐making process is essential. Clinical implementation considerations accompanying recommendations of conditional strength reflect the need for flexibility in management and specify factors to discuss with families when applying these recommendations and reaching a decision on whether to pursue a particular management option. This approach reflects the nature of conditional recommendations, which imply that while most individuals in the given situation would want the suggested course of action, some would not.
METHOD
This guideline was developed using GRADE and following AGREE II and AACPDM Care Pathway standards. Key points are discussed below, with detailed methods provided in Appendix S1.
Evidence synthesis and appraisal
Best available evidence was synthesized and appraised for 10 PICO questions. This process is described in detail in section 4 of Appendix S1 (Evidence Synthesis & Appraisal). An overview of the study selection process is provided in Appendix S1. For most interventions, direct evidence (i.e. specific to individuals with CP and dystonia) was sought from the systematic review specifically developed to inform this guideline. 19 When no published studies directly addressing a PICO question were identified (i.e. oral/enteral [e.g. gastrostomy tube] baclofen, benzodiazepines, gabapentin, medical cannabis), evidence focused on an indirectly applicable population was sought from existing systematic reviews. When multiple reviews were identified, those of highest quality (as appraised by the AMSTAR 2 tool) were selected. When limited data were available from these reviews, ancillary searches were conducted to identify best available supporting evidence. Findings were synthesized and appraised following GRADE and summarized in evidence profiles (Appendix S2) and summary of findings tables (Appendix S3).
Evidence to recommendations
The guideline panel, including two developmental paediatricians (DF, JR), three paediatric neurologists (KH, J‐PL, JWM), two paediatric physical therapists (AH, EM), one paediatric occupational therapist (HG), and one consumer representative with lived experience (BA), convened during virtual meetings. A GRADE methodologist (YF‐Y) facilitated discussions. Evidence was summarized and GRADE Evidence‐to‐Decision frameworks were used to guide the panel through a transparent process by reaching judgements on key criteria relevant to decision‐making: evidence of desirable and undesirable effects (as presented in ‘summary of findings’ tables), evidence certainty, values and preferences, economic considerations, and issues relating to equity, acceptability, and feasibility. On the basis of these judgements, the panel reached consensus on the direction, strength, and wording of each recommendation. This process is described in detail in section 5 of Appendix S1 (Evidence to Recommendations).
Stakeholder involvement
A consumer group of individuals with CP and dystonia and their caregivers were engaged at every stage of the guideline development process. The group included two individuals with CP and dystonia and five caregivers. An initial focus group elicited consumer experiences with dystonia management options, values and preferences, and outcomes of importance. Consumer perspectives contributed to informing the panel's judgements. Consumer group members provided essential feedback on recommendations. Draft recommendations also underwent review by a group of 30 clinicians with broad geographical representation and clinical expertise (feedback in Appendix S4). The guideline was reviewed and appraised by the AACPDM Care Pathways Committee, then posted publicly for comment by clinical stakeholders (feedback and responses in Appendix S5). A final review was completed by the AACPDM Care Pathways Committee and Board of Directors.
RECOMMENDATIONS
Recommendations are provided below. An interpretation aid presenting the 10 evidence‐based recommendations alongside considerations for implementation is provided in Figure 1. Each recommendation is accompanied by clinical implementation considerations, followed by a brief summary of Evidence‐to‐Decision domains that informed the panel's recommendation. For more details, please consult the Evidence‐to‐Decision frameworks provided in Appendix S3. This clinical practice guideline consists of recommendations of conditional strength only. This moves the clinician/individual with CP into shared decision‐making with a discussion of the perceived balance of potential benefits and side effects and the values/preferences of the individual with CP before deciding for or against a trial of the intervention for the individual. Hence all interventions in this Care Pathway can be considered in this context.
FIGURE 1.
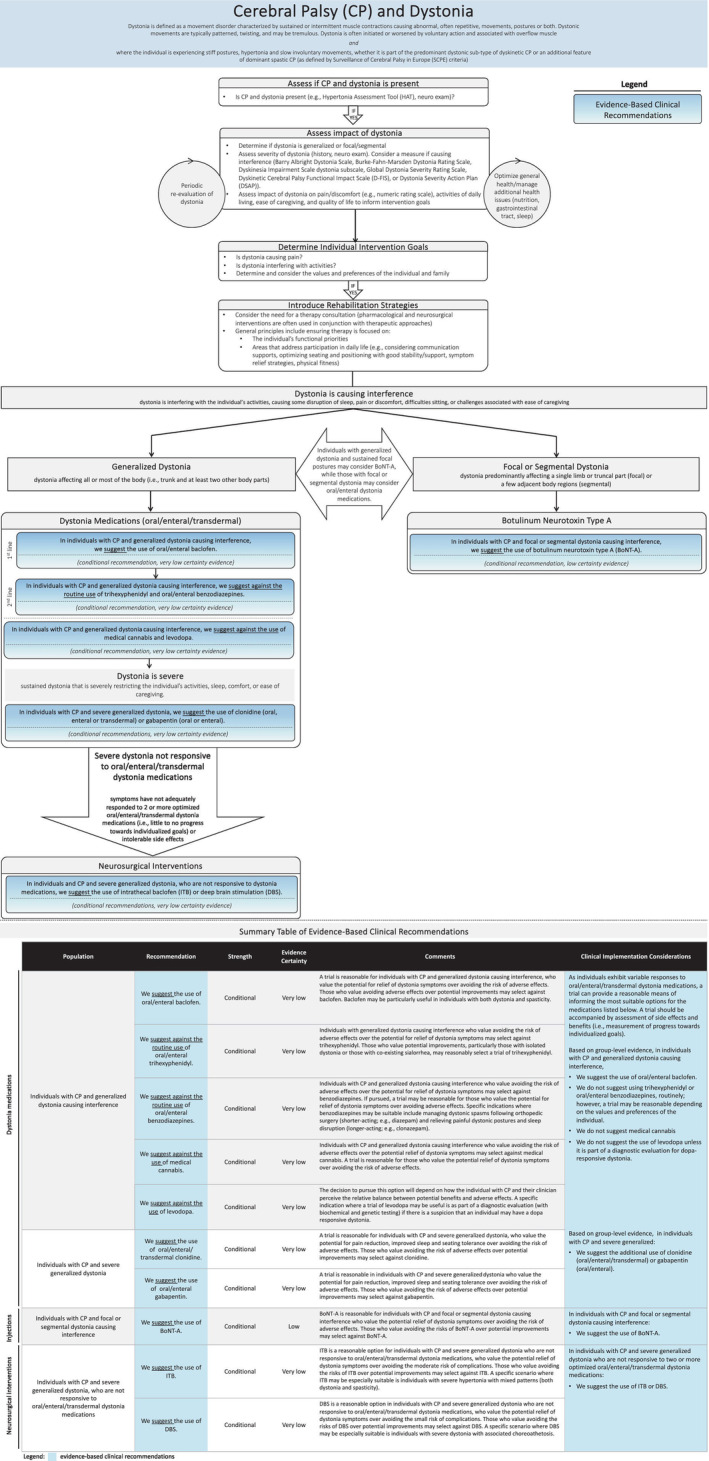
Interpretation aid for the care pathway.
Dystonia medications
Oral/enteral baclofen
Should individuals with CP and generalized dystonia causing interference receive oral/enteral baclofen versus not receiving oral/enteral baclofen?
Recommendation 1
In individuals with CP and generalized dystonia causing interference, we suggest the use of oral/enteral baclofen (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
Oral/enteral baclofen can be viewed as a first‐line consideration. A trial is reasonable for individuals with CP and generalized dystonia causing interference, who value the potential for relief of dystonia symptoms over avoiding the risk of adverse effects (e.g. central nervous system [CNS] drowsiness, dizziness, confusion 21 ). Those who value avoiding adverse effects over potential improvements may select against baclofen. Baclofen may be particularly useful in individuals with both dystonia and spasticity. Clinicians should be careful to administer with slow dose escalation to mitigate side effects such as drowsiness. If discontinued, a slow wean avoids the risk of withdrawal.
Baclofen, an agonist of γ‐aminobutyric acid (GABA)B receptors, has been commonly used but not directly evaluated for individuals with CP and dystonia. 22 , 23 The panel's recommendation was based on indirect evidence predominantly from individuals with spastic CP. 24 , 25 , 26 , 27 The panel judged anticipated desirable effects to be trivial. A small improvement in achievement of individualized goals with baclofen was supported by a single randomized controlled trial, although the evidence is very uncertain. 28 Undesirable effects were judged to be small, and the balance between benefits and harms to favour neither receiving oral/enteral baclofen or not. The panel agreed that, given the very low‐certainty evidence across oral/enteral/transdermal pharmacological options and small adverse event profile for baclofen, a trial is warranted contingent on the individual's values and preferences. A conditional recommendation for oral/enteral baclofen was therefore formulated.
Trihexyphenidyl
Should individuals with CP and generalized dystonia causing interference receive oral/enteral trihexyphenidyl versus not receiving oral/enteral trihexyphenidyl?
Recommendation 2
In individuals with CP and generalized dystonia causing interference, we suggest against the routine use of oral/enteral trihexyphenidyl (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
Trihexyphenidyl can be viewed as a second‐line consideration. Individuals with generalized dystonia causing interference who value avoiding the risk of adverse effects (such as nausea and CNS side effects [e.g. dizziness, difficulty with concentration], and blurred vision 21 ) over the potential for relief of dystonia symptoms may select against trihexyphenidyl. Those who value potential improvements, particularly those with isolated dystonia or those with coexisting sialorrhea, 29 may reasonably select a trial of trihexyphenidyl. If pursued, a trial should be undertaken, individual response monitored, and the medication slowly discontinued in the absence of benefit.
Trihexyphenidyl is an anticholinergic agent that acts as an antagonist to muscarinic receptors, including those in the CNS, with highest affinity for M1 muscarinic receptors. The panel judged anticipated desirable and undesirable effects to be trivial and small respectively, although the evidence is very uncertain. 19 Given no clear improvements in desirable effects reported by best available evidence, the balance of benefits and harms was judged to probably favour not receiving trihexyphenidyl. In addition to possible important uncertainty/variability in values and preferences, these judgements led to a conditional recommendation against routine use.
Benzodiazepines
Should individuals with CP and generalized dystonia causing interference receive oral/enteral benzodiazepines versus not receiving oral/enteral benzodiazepines?
Recommendation 3
In individuals with CP and generalized dystonia causing interference, we suggest against the routine use of oral/enteral benzodiazepines (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
Benzodiazepines can be viewed as a second‐line consideration. Individuals with CP and generalized dystonia causing interference who value avoiding the risk of adverse effects (e.g. impairment of memory, drowsiness/fatigue, paradoxical reaction with disinhibition, 21 tolerance, and possibility of dependence) over the potential for relief of dystonia symptoms may select against benzodiazepines. If pursued, a trial may be reasonable for those who value the potential for relief of dystonia symptoms over avoiding adverse effects. Specific indications where benzodiazepines may be suitable include managing dystonic spasms following orthopaedic surgery (shorter‐acting; e.g. diazepam) and relieving painful dystonic postures and sleep disruption (longer‐acting; e.g. clonazepam). There is a risk of withdrawal with abrupt discontinuation.
Benzodiazepines potentiate the inhibitory postsynaptic action of GABA by modulating the GABAA receptor. Evidence available to inform the recommendation was limited to studies in populations other than CP and dystonia, including spastic 24 , 27 , 30 and dyskinetic 31 CP and status dystonicus. 18 , 32 The panel judged anticipated desirable and undesirable effects to be small and moderate respectively, although the evidence is very uncertain. The balance of benefits and harms favoured neither receiving benzodiazepines or not. The moderate adverse event profile, possible important uncertainty/variability in individual values and preferences, and variable acceptability led to a conditional recommendation against routine use.
Clonidine
Should individuals with CP and severe generalized dystonia receive oral/enteral/transdermal clonidine versus not receiving oral/enteral/transdermal clonidine?
Recommendation 4
In individuals with CP and severe generalized dystonia, we suggest the use of oral/enteral/transdermal clonidine (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
A trial is reasonable for individuals with CP and severe generalized dystonia, who value the potential for pain reduction, improved sleep, and seating tolerance over avoiding the risk of adverse effects (such as bradycardia, hypotension, drowsiness 21 ). Those who value avoiding the risk of adverse effects over potential improvements may select against clonidine. Clonidine should be carefully weaned, as a withdrawal syndrome can occur with rebound hypertension. Sleep–wake cycle monitoring can inform dosing. Clonidine can be delivered by oral, enteral, or transdermal administration. 33
Clonidine is an agonist of α2‐adrenergic receptors. Direct evidence specific to CP and dystonia 19 supplemented with evidence focused on status dystonicus 33 led the panel to judge the anticipated desirable and undesirable effects to both be small, although the evidence is very uncertain. The balance of benefits and harms was judged to probably favour the intervention, driven by improvements in dystonia, goal achievement, sleep, and ease of caregiving. While it is important to be aware of possible important uncertainty/variability in values and preferences, a conditional recommendation for clonidine in the presence of severe generalized dystonia was formulated.
Gabapentin
Should individuals with CP and severe generalized dystonia receive oral/enteral gabapentin versus not receiving oral/enteral gabapentin?
Recommendation 5
In individuals with CP and severe generalized dystonia, we suggest the use of oral/enteral gabapentin (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
A trial is reasonable in individuals with CP and severe generalized dystonia who value the potential for pain reduction, improved sleep, and seating tolerance over avoiding the risk of adverse effects (such as CNS side effects [e.g. dizziness, drowsiness, risk of respiratory depression] and neuropsychiatric effects). Those who value avoiding the risk of adverse effects over potential improvements may select against gabapentin. A specific indication where gabapentin may be particularly suitable is in individuals with severe generalized dystonia with painful generalized or focal/segmental dystonia, although evidence to support use in this context is needed. In rare cases, gabapentin may induce a severe hypersensitivity reaction (e.g. drug reaction with eosinophilia and systemic symptoms). 21 Gabapentin should be introduced slowly, with dosing adjusted according to response. The medication should be weaned slowly in the absence of response.
Gabapentin is a GABA analogue originally used for partial seizures, but increasingly adopted for neuropathic pain syndromes. Evidence was limited to studies of indirect populations, specifically individuals with ‘severe dystonia’ but not necessarily CP. 34 , 35 Additional data relating to undesirable effects were sought from populations in which the use of gabapentin is more common (i.e. pain, epilepsy). 36 , 37 The panel judged anticipated desirable effects to be moderate, including improvements in dystonia, pain, and ease of caregiving, and undesirable effects to be small, although the evidence is very uncertain. The balance of benefits and harms was judged to probably favour the intervention. While the panel acknowledged possible important uncertainty/variability in values and preferences, a conditional recommendation for gabapentin in the presence of severe generalized dystonia was formulated.
Medical cannabis
Should individuals with CP and generalized dystonia causing interference receive medical cannabis versus not receiving medical cannabis?
Recommendation 6
In individuals with CP and generalized dystonia causing interference, we suggest against the use of medical cannabis (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
Individuals with CP and generalized dystonia causing interference who value avoiding the risk of adverse effects (such as somnolence, nausea/vomiting, and behavioural changes 38 , 39 , 40 , 41 ) over the potential relief of dystonia symptoms may select against medical cannabis. A trial is reasonable for those who value the potential relief of dystonia symptoms over avoiding the risk of adverse effects. There is limited evidence evaluating appropriate dosing and safe therapeutic proportions of Δ9‐tetrahydrocannabinol and cannabidiol. A preference exists, particularly in children, for a higher cannabidiol:Δ9‐tetrahydrocannabinol ratio to minimize psychoactive effects. Some pharmacological cannabinoids are available; however, products produced outside regulations can vary in content, Δ9‐tetrahydrocannabinol:cannabidiol ratios, and reproducibility. Legislation allowing use can vary by region and individual awareness of regulations if travelling to different regions is encouraged.
The main therapeutic chemicals in medical cannabis are Δ9‐tetrahydrocannabinol and cannabidiol, which differ in mechanism and effect. While not included in the first iteration of this guideline, interest in medical cannabis for CP and dystonia has since emerged given improvements in spasticity/spasms in multiple sclerosis and pain in other populations. Evidence was of very low certainty and limited to indirect studies focused predominantly on spasticity. 39 Additional information related to undesirable effects was sought from studies of epilepsy and primary dystonia. 40 , 41 The panel judged anticipated desirable and undesirable effects to be trivial and small respectively, although the evidence is very uncertain. Given the lack of clear desirable effects and possible risk of adverse effects, the balance of benefits and harms was judged to probably favour not receiving medical cannabis. There is important uncertainty/variability in values and preferences. Resource use, acceptability, and feasibility vary. Medical cannabis may reduce equity, as availability varies and prescription may be limited to specialized centres. As a result of these judgements, the panel formulated a conditional recommendation against use.
Levodopa
Should individuals with CP and generalized dystonia causing interference receive levodopa versus not receiving levodopa?
Recommendation 7
In individuals with CP and generalized dystonia causing interference, we suggest against the use of levodopa (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
The decision to pursue this option will depend on how the individual with CP and their clinician perceive the relative balance between potential benefits and adverse effects. A specific indication where a trial of levodopa may be useful is as part of a diagnostic evaluation (with biochemical and genetic testing) if there is a suspicion that an individual may have a dopa‐responsive dystonia. A withdrawal syndrome with Parkinsonism–hyperpyrexia syndrome can occur with abrupt discontinuation. 21
Levodopa is a dopamine precursor that increases dopamine in the brain. Very low‐certainty direct evidence informed the evidence profile, with indirect evidence specific to dyskinetic CP supplementing tone and pain/comfort outcomes. 19 , 31 The panel judged anticipated desirable and undesirable effects (including exacerbation of dyskinesia, gastrointestinal side effects [e.g. nausea, vomiting], orthostatic hypotension, and psychiatric side effects [e.g. impacts on impulse control, agitation]) to both be trivial, and the balance of benefits and harms to probably favour not receiving levodopa, leading to a conditional recommendation against use.
Injections
Botulinum neurotoxin A
Should individuals with CP and focal or segmental dystonia causing interference receive botulinum neurotoxin A (BoNT‐A) versus not receiving BoNT‐A?
Recommendation 8
In individuals with CP and focal or segmental dystonia causing interference, we suggest the use of BoNT‐A (conditional recommendation, low‐certainty evidence).
Clinical implementation considerations
BoNT‐A is reasonable for individuals with CP and focal or segmental dystonia causing interference who value the potential relief of dystonia symptoms over avoiding the risk of adverse effects (such as transitory weakness, dysphagia in the case of cervical injections 19 ). Those who value avoiding the risks of BoNT‐A over potential improvements may select against BoNT‐A. BoNT‐A may also be considered in combination with other interventions in individuals with generalized dystonia who present with focal postures. Candidates should receive treatment from a clinician with expertise. Pre‐injection analysis and guided injections (e.g. use of ultrasound) are suggested. Consider injecting both flexors and extensors at the single‐joint level to preserve balance over the joint. The use of high doses or frequent injections may increase the risk of adverse effects. Repeated treatments may address different goals and target muscles, given the variability in dystonia over time.
BoNT‐A temporarily inhibits acetylcholine release at the neuromuscular junction, resulting in focal chemodenervation and muscle relaxation. While low‐certainty direct evidence 19 led the panel to judge the anticipated desirable and undesirable effects to both be small, the balance between benefits and harms was judged to probably favour the intervention given improvements in goal achievement, pain, and ease of caregiving. There is possible important uncertainty/variability in values and preferences. Costs were judged to be moderate. BoNT‐A may reduce equity because of variability in access. The panel reached a conditional recommendation for BoNT‐A, driven by improvements in outcomes important to individuals and families.
Neurosurgical interventions
Intrathecal baclofen
Should individuals with CP and severe generalized dystonia who are not responsive to oral/enteral/transdermal dystonia medications receive intrathecal baclofen (ITB) versus not receiving ITB?
Recommendation 9
In individuals with CP and severe generalized dystonia, who are not responsive to oral/enteral/transdermal dystonia medications, we suggest the use of ITB (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
ITB is a reasonable option for individuals with CP and severe generalized dystonia who are not responsive to oral/enteral/transdermal dystonia medications, who value the potential relief of dystonia symptoms over avoiding the moderate risk of complications (including pump infection, catheter‐related problems, and leakage of cerebrospinal fluid 19 ). Those who value avoiding the risks of ITB over potential improvements may select against ITB. A specific situation where ITB may be especially suitable is individuals with severe hypertonia with mixed patterns (both dystonia and spasticity). Lower‐extremity hypertonia may respond more than upper‐extremity hypertonia. Administration of continuous ITB will require surgical intervention, involving implantation of the pump and placement (plus future revisions, if required) of the catheter in the intrathecal space (for dystonia, placement is often in the cervical region). ITB candidates should be carefully selected and managed in specialized centres. Clinicians should screen candidates to ensure understanding of potential risks and willingness to comply with necessary follow‐up for pump refills. Implementation may be challenging in low‐resource settings.
Baclofen is an agonist of GABAB receptors. Intrathecal administration facilitates higher levels of baclofen in the cerebrospinal fluid and allows precise titration. The recommendation was based on very low‐certainty direct evidence. 19 The panel judged anticipated desirable and undesirable effects to be small and moderate respectively, although the evidence is very uncertain. The balance of benefits and harms was judged to probably favour the intervention, given improvements in outcomes of importance to individuals with CP and dystonia and their families. There is possible important uncertainty/variability in values and preferences. Very low‐certainty evidence suggested moderate costs and cost‐effectiveness probably favouring ITB. Implementation may reduce equity and feasibility varies, as ITB is expensive and requires access to quaternary services and urgent care for pump/catheter malfunction. Bearing in mind important considerations related to access and potential risk of complications, the panel reached a conditional recommendation for ITB given evidence supporting desirable effects.
Deep brain stimulation
Should individuals with CP and severe generalized dystonia who are not responsive to oral/enteral/transdermal dystonia medications receive deep brain stimulation (DBS) versus not receiving DBS?
Recommendation 10
In individuals with CP and severe generalized dystonia, who are not responsive to oral/enteral/transdermal dystonia medications, we suggest the use of DBS (conditional recommendation, very low‐certainty evidence).
Clinical implementation considerations
DBS is a reasonable option in individuals with CP and severe generalized dystonia who are not responsive to oral/enteral/transdermal dystonia medications, who value the potential relief of dystonia symptoms over avoiding the small risk of complications (such as infection, battery migration/erosion, stimulation‐induced dysarthria 19 ). Those who value avoiding the risks of DBS over potential improvements may select against DBS. A specific situation where DBS may be especially suitable is individuals with severe dystonia with associated choreoathetosis. While evidence supports a modest improvement in dystonia, clinicians should emphasize to families that functional gains are less pronounced than those observed in primary dystonias. DBS candidates should be managed in specialized centres and carefully selected depending on clinical findings of normal estimates of connectivity in the distributed motor and sensory circuits 42 , 43 (e.g. central motor conduction times, somatosensory evoked potentials); isolated dystonia without spasticity; and a viable target (i.e. globus pallidus or subthalamic nucleus on structural magnetic resonance imaging). 43 DBS is a neurosurgical procedure involving the placement of electrodes and an internal pulse generator. Implementation may be challenging in low‐resource settings.
DBS is a neurosurgical procedure that acts through multifactorial neuromodulatory mechanisms. Direct evidence 19 led the panel to judge anticipated desirable and undesirable effects to both be small, although the evidence is very uncertain. The balance of benefits and harms was judged to probably favour the intervention, given improvements in several outcomes of importance to individuals with CP and dystonia and their families. There is possible important uncertainty/variability in values and preferences. Very low‐certainty evidence suggested moderate costs and cost‐effectiveness probably favouring DBS. Feasibility varies and equity may be reduced, as access varies. Given evidence supporting desirable effects, the panel formulated a conditional recommendation for DBS.
CARE PATHWAY OVERVIEW
An interpretation aid (Figure 1) and practical tools, including a plain language summary and assessment and monitoring tools (available at the AACPDM Care Pathways website [https://www.aacpdm.org/publications/care‐pathways/dystonia‐in‐cerebral‐palsy]), are provided to support implementation. These tools reflect those frequently used in practice. A formal appraisal of their measurement properties is beyond the scope of this paper.
Low‐ to very low‐certainty evidence led to the formulation of conditional recommendations (‘we suggest’), where there is likely to be important variation in decision‐making between individuals. The recommendations of conditional strength support flexibility at the individual level and highlight the importance of a shared decision‐making process where a management plan is developed on the basis of the individual's goals and how the clinician/individual with CP perceive the balance between potential benefits and adverse effects. Response to individual interventions should be monitored by evaluating benefits (i.e. progress made towards individualized goals) and side effects. Pharmacological and neurosurgical interventions are used in conjunction with other therapeutic/rehabilitation approaches.
Oral/enteral/transdermal medications that were evaluated include baclofen, trihexyphenidyl, benzodiazepines, clonidine, gabapentin, levodopa, and medical cannabis. As individuals present with varied benefits and side‐effect profiles in response to these medications, selection of the most suitable option(s) should be guided by their identified goals and response to an individualized trial. Side effects are typically reversible on discontinuation, meaning that a trial can provide a safe and reasonable means of establishing effectiveness. The individual should be carefully weaned in the case of no response or intolerable side effects to avoid symptoms of withdrawal. Depending on individual values and preferences, baclofen is suggested when dystonia is causing interference. While trihexyphenidyl and benzodiazepines are not routinely suggested, they can be reasonably used for those who respond favourably to a trial, with the individual experiencing an outcome aligned with their values and preferences. Medical cannabis is not suggested, as side effects appear to outweigh benefits; however, depending on the values and preferences of the individual with CP and dystonia, an individualized trial may be considered. Levodopa can be considered as part of a diagnostic evaluation (with biochemical and genetic testing) if there is a suspicion that an individual may have dopa‐responsive dystonia. Clonidine or gabapentin are suggested when dystonia is severe. With increasing dystonia severity, multiple medications may be required.
For severe generalized dystonia not responsive to oral/enteral/transdermal dystonia medications, neurosurgical management (i.e. ITB or DBS) is suggested. Future research is required to guide decision‐making about ITB versus DBS; however, ITB may be the favoured option for individuals with mixed dystonia and spasticity, and DBS for individuals with mixed dystonia/choreoathetosis. Periodic BoNT‐A injections are suggested for focal or segmental dystonia causing interference. Individuals with generalized dystonia and persistent focal postures may also consider BoNT‐A, and those with focal or segmental presentations may also consider a trial of oral/enteral/transdermal medications.
RESEARCH PRIORITIES
Intervention‐specific priorities are provided (Table 3). Well‐designed trials evaluating efficacy/comparative effectiveness and safety across interventions are needed. More refined evaluations of efficacy and recommendations tailored to the most suitable subpopulations will be enabled by future studies differentiating between predominant CP subtypes. Available studies are limited by a lack of differentiation between participants with a predominant presentation of dystonia or choreoathetosis. It is therefore challenging to isolate the interventions' efficacy on the hypertonia/slow involuntary movements versus rapid movements. The Dyskinesia Impairment Scale helps to differentiate between the two subtypes of dyskinetic CP by providing a quantitative rating of the severity of both dystonia and choreoathetosis and allowing the determination of the dominant form. 44 , 45 In addition, given the frequency of mixed tone (spastic and dystonic) in spastic CP, the development of a tool to quantify the amount of dystonic and spastic tone present in an individual and incorporating this into reported participant characteristics will help to inform the evidence base.
TABLE 3.
Intervention‐specific research priorities.
| Intervention | Research priorities for dystonia and cerebral palsy |
|---|---|
| Oral/enteral baclofen | Efficacy trials of oral/enteral baclofen to establish direct evidence. |
| Benzodiazepines | Comparative effectiveness studies evaluating the role of benzodiazepines compared with other interventions. |
| Trihexyphenidyl | Clinical research studies evaluating the effectiveness and safety (including a focus on the effect on cognitive function) of trihexyphenidyl in a subgroup of individuals with cerebral palsy and ‘isolated’ dystonia. |
| Clonidine | Prospective studies of clonidine particularly in the subgroup of individuals with CP and severe generalized dystonia. |
| Gabapentin | Prospective studies of gabapentin using standardized outcome measures, including pain. |
| Levodopa | Levodopa was not identified as a research priority. |
| Medical cannabis | Prospective studies comparing medical cannabis to other pain management options for dystonia (e.g. gabapentin). |
| BoNT‐A | Prospective studies evaluating the role of BoNT‐A, using standardized outcome measures, including pain. |
| ITB |
Prospective cohort studies addressing the long‐term impact of ITB and identifying baseline factors that influence outcome. Long‐term (over 2–3 years) comparative effectiveness studies of ITB and DBS. |
| DBS |
Prospective cohort studies addressing the long‐term impact of DBS and identifying baseline factors that influence outcome. Long‐term (over 2–3 years) comparative effectiveness studies of ITB and DBS International collaboration using more refined selection criteria, defining assessment ‘toolkits’ including neurophysiology, imaging and assessment protocols, obtaining blinded assessments, and data handling. |
Abbreviations: BoNT‐A, botulinum neurotoxin A; DBS, deep brain stimulation; ITB, intrathecal baclofen.
There has been renewed discussion around the term ‘spastic dystonia’ defined as ‘stretch and effort‐unrelated sustained involuntary muscle activity following central motor lesion’, 46 which is postulated to ‘be mediated by abnormal tonic supraspinal drive’ 47 in contrast to spasticity which is mediated by sensory afferent 1a input. Further building on consensus around neurological definitions, their underlying pathophysiological underpinnings, and clinical measurement will help move the field forward. Evidence for rehabilitation strategies is also needed before formal rehabilitation recommendations can be developed.
Newer methodologies (e.g. single‐case experimental design with replications across centres, N‐of‐1 studies) could generate international evidence. Important outcomes identified through our stakeholder consultation with individuals with CP and dystonia and their families should be assessed, including dystonia severity, achievement of individualized goals, motor function, pain/comfort, sleep duration and quality, ease of caregiving, quality of life, and adverse events (including emergency care). 48
Researchers can consider the individualized form of the Performance Quality Rating Scale to objectively evaluate goal performance. 49 A consensus on which existing dystonia severity scale to use (e.g. Barry Albright Dystonia Scale, Burke–Fahn–Marsden Dystonia Rating Scale, Dyskinesia Impairment Scale dystonia subscale, Global Dystonia Severity Rating Scale, 50 Dyskinetic CP Functional Impact Scale) could enable pooling, cross‐trial comparisons, and detection of clinically meaningful change. Continued development of instrumented kinematic measures of dystonia severity, with a focus on establishing thresholds for clinically meaningful change, is of interest. 51
Studies will benefit from incorporating health economic and physiological monitoring tools with outputs covering daily activities. Sleep/wake charts for monitoring in home, school, and hospital settings should be studied. Smart technologies should be harnessed for monitoring and possible machine‐learning interpretations of clinical state before, during, and after interventions.
LIMITATIONS
Given the limited evidence base specific to CP and dystonia, some recommendations are based on evidence from other populations, whose response to interventions may differ from those with CP and dystonia. Users of the guideline are encouraged to interpret recommendations with care, especially those informed by indirect evidence. Direct evidence is further limited by a lack of differentiation between contributions of hypertonia and hyperkinesia to dystonic presentations. The evidence‐to‐recommendations process was informed by systematic reviews published up to January 2022. More recent studies 52 , 53 will be considered in the next iteration of the Care Pathway. Despite including best available evidence, included systematic reviews vary in methodological quality and most primary studies included among them are observational, leading to low‐ or very low‐certainty evidence. Estimates of the magnitude of desirable and undesirable effects are, therefore, very uncertain in most cases. As a result, the recommendations are conditional in strength, highlighting the need for consideration of individual values and preferences. Access and resources were considered within the GRADE Evidence‐to‐Decision frameworks when formulating recommendations, and the medical complexity of ITB and DBS were identified as a potential barrier to implementation in low‐ to middle‐income settings.
CONCLUSION
This guideline was developed using GRADE and following AGREE II and AACPDM Care Pathway standards. It is intended to guide clinical decisions related to pharmacological and neurosurgical management aligned with individual values and preferences. Evidence continues to be limited, as illustrated by conditional recommendations. Research addressing identified priorities will contribute to optimizing evidence‐based care for individuals with CP and dystonia.
Supporting information
Appendix S1: Methods.
Appendix S2: GRADE evidence profiles.
Appendix S3: Evidence‐to‐Decision frameworks.
Appendix S4: External review process: stakeholder consultation.
Appendix S5: Internal review process: AACPDM Care Pathways Committee review and public comment.
ACKNOWLEDGEMENTS
We extend our appreciation to participants in the consumer consultation and external review process (Appendix S1). We recognize Mohan Belthur for acting as liaison, and Stacey Miller, Paige Church, Lynne Romeiser‐Logan, Anna McCormick, Kat Kolaski, and other members of the AACPDM Care Pathways Committee for their review and appraisal of the Care Pathway. This research was conducted with the support of the Ontario Brain Institute, the Ward Family to the Holland Bloorview Kids Rehabilitation Hospital Foundation, and the AACPDM. The Ontario Brain Institute is an independent non‐profit corporation, funded partly by the Ontario government.
Fehlings D, Agnew B, Gimeno H, Harvey A, Himmelmann K, Lin J‐P, et al. Pharmacological and neurosurgical management of cerebral palsy and dystonia: Clinical practice guideline update. Dev Med Child Neurol. 2024;66:1133–1147. 10.1111/dmcn.15921
This clinical practice guideline is commented by Lumsden on pages 1116–1117 of this issue.
Plain language summary: https://onlinelibrary.wiley.com/doi/10.1111/dmcn.15979
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28(7):863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanger TD, Delgado MR, Gaebler‐Spira D, Hallett M, Mink JW, Disorders TFoCM . Classification and definition of disorders causing hypertonia in childhood. Pediatrics. 2003;111(1):e89‐e97. [DOI] [PubMed] [Google Scholar]
- 3. Jethwa A, Mink J, Macarthur C, Knights S, Fehlings T, Fehlings D. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Developmental Medicine & Child Neurology. 2010;52(5):e83‐e7. [DOI] [PubMed] [Google Scholar]
- 4. National Institute of Neurological Disorders and Stroke . Dystonia Bethesda, MD: National Institute of Neurological Disorders and Stroke; [updated 2023 Jan 20; cited 2023 Feb 1]. Available from: https://www.ninds.nih.gov/health‐information/disorders/dystonia.
- 5. Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109(suppl 109):8–14. [PubMed] [Google Scholar]
- 6. Cans C, Dolk H, Platt MJ, Colver A. Recommendations from the SCPE collaborative group for defining and classifying cerebral palsy. Developmental medicine and child neurology. 2007;49:35. [DOI] [PubMed] [Google Scholar]
- 7. Rice J, Skuza P, Baker F, Russo R, Fehlings D. Identification and measurement of dystonia in cerebral palsy. Developmental medicine and child neurology. 2017;59(12):1249–55. [DOI] [PubMed] [Google Scholar]
- 8. Penner M, Xie WY, Binepal N, Switzer L, Fehlings D. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. 2013;132(2):e407‐e13. [DOI] [PubMed] [Google Scholar]
- 9. Monbaliu E, De La Peña MG, Ortibus E, Molenaers G, Deklerck J, Feys H. Functional outcomes in children and young people with dyskinetic cerebral palsy. Developmental Medicine & Child Neurology. 2017;59(6):634–40. [DOI] [PubMed] [Google Scholar]
- 10. Fehlings D, Brown L, Harvey A, Himmelmann K, Lin JP, Macintosh A, et al. Pharmacological and neurosurgical interventions for managing dystonia in cerebral palsy: a systematic review. Dev Med Child Neurol. 2018;60(4):356–66. [DOI] [PubMed] [Google Scholar]
- 11. Kolaski K. Instructions for AACPDM Care Pathway Development.: Milwaukeee, WI: The American Academy for Cerebral Palsy and Developmental Mecidine; 2020. [Google Scholar]
- 12. Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Cmaj. 2014;186(3):E123‐E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlaggar BL, Mink JW. Movement disorders in children. Pediatrics in Review. 2003;24(2):39–51. [DOI] [PubMed] [Google Scholar]
- 15. Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Movement Disorders. 2010;25(11):1538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Allen NM, Lin JP, Lynch T, King MD. Status dystonicus: a practice guide. Developmental Medicine & Child Neurology. 2014;56(2):105–12. [DOI] [PubMed] [Google Scholar]
- 17. Scalise R, Sgandurra G, Menici V, Capodagli N, Di Pietro R, Romeo DM, et al. A retrospective longitudinal study in a cohort of children with dyskinetic cerebral palsy treated with tetrabenazine. Frontiers in Neurology. 2021;12:612429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manji H, Howard R, Miller D, Hirsch N, Carr L, Bhatia K, et al. Status dystonicus: the syndrome and its management. Brain: a journal of neurology. 1998;121(2):243–52. [DOI] [PubMed] [Google Scholar]
- 19. Bohn E, Goren K, Switzer L, Falck‐Ytter Y, Fehlings D. Pharmacological and neurosurgical interventions for individuals with cerebral palsy and dystonia: a systematic review update and meta‐analysis. Developmental Medicine and Child Neurology. 2021;63(9):1038–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lexicomp Online, Pediatric and Neonatal Lexi‐Drugs Online Waltham, MA: UptoDate, Inc.: Lexicomp, Inc.; [updated accessed 2022 Nov 4]. Available from: http://online.lexi.com.
- 22. Harvey A, Bear N, Rice J, Antolovich G, Waugh MC. National surveillance of oral medication prescription for children with dystonic cerebral palsy. Journal of Paediatrics and Child Health. 2021. [DOI] [PubMed] [Google Scholar]
- 23. Lumsden DE, Crowe B, Basu A, Amin S, Devlin A, DeAlwis Y, et al. Pharmacological management of abnormal tone and movement in cerebral palsy. Arch Dis Child. 2019;104(8):775–80. [DOI] [PubMed] [Google Scholar]
- 24. Delgado MR, Hirtz D, Aisen M, Ashwal S, Fehlings DL, McLaughlin J, et al. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2010;74(4):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Navarrete‐Opazo AA, Gonzalez W, Nahuelhual P. Effectiveness of oral baclofen in the treatment of spasticity in children and adolescents with cerebral palsy. Archives of physical medicine and rehabilitation. 2016;97(4):604–18. [DOI] [PubMed] [Google Scholar]
- 26. Balash Y, Giladi N. Efficacy of pharmacological treatment of dystonia: evidence‐based review including meta‐analysis of the effect of botulinum toxin and other cure options. European Journal of Neurology. 2004;11(6):361–70. [DOI] [PubMed] [Google Scholar]
- 27. Goyal V, Laisram N, Wadhwa RK, Kothari SY. Prospective randomized study of oral diazepam and baclofen on spasticity in cerebral palsy. Journal of clinical and diagnostic research: JCDR. 2016;10(6):RC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Scheinberg A, Hall K, Lam LT, O'Flaherty S. Oral baclofen in children with cerebral palsy: A double‐blind cross‐over pilot study. Journal of paediatrics and child health. 2006;42(11):715–20. [DOI] [PubMed] [Google Scholar]
- 29. Carranza‐del Rio J, Clegg NJ, Moore A, Delgado MR. Use of trihexyphenidyl in children with cerebral palsy. Pediatr Neurol. 2011;44(3):202–6. [DOI] [PubMed] [Google Scholar]
- 30. Tsoi WSE, Zhang LA, Wang WY, Tsang KL, Lo SK. Improving quality of life of children with cerebral palsy: a systematic review of clinical trials. Child: Care Health Dev. 2011; 38(1): 21–31. [DOI] [PubMed] [Google Scholar]
- 31. Masson R, Pagliano E, Baranello G. Efficacy of oral pharmacological treatments in dyskinetic cerebral palsy: a systematic review. Dev Med Child Neurol. 2017;59(12):1237–48. [DOI] [PubMed] [Google Scholar]
- 32. Fasano A, Ricciardi L, Bentivoglio AR, Canavese C, Zorzi G, Petrovic I , et al. Status dystonicus: Predictors of outcome and progression patterns of underlying disease. Mov Disord. 2012; 27(6): 783–788. [DOI] [PubMed] [Google Scholar]
- 33. Nakou V, Williamson K, Arichi T, Lumsden DE, Tomlin S, Kaminska M, et al. Safety and efficacy of high‐dose enteral, intravenous, and transdermal clonidine for the acute management of severe intractable childhood dystonia and status dystonicus: An illustrative case‐series. European Journal of Paediatric Neurology. 2017;21(6):823–32. [DOI] [PubMed] [Google Scholar]
- 34. Ostojic K, Paget SP, Morrow AM. Management of pain in children and adolescents with cerebral palsy: a systematic review. Developmental Medicine & Child Neurology. 2019;61(3):315–21. [DOI] [PubMed] [Google Scholar]
- 35. Liow NY‐K, Gimeno H, Lumsden DE, Marianczak J, Kaminska M, Tomlin S, et al. Gabapentin can significantly improve dystonia severity and quality of life in children. european journal of paediatric neurology. 2016;20(1):100–7. [DOI] [PubMed] [Google Scholar]
- 36. Egunsola O, Wylie CE, Chitty KM, Buckley NA. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesthesia & Analgesia. 2019;128(4):811–9. [DOI] [PubMed] [Google Scholar]
- 37. Arya R, Glauser TA. Pharmacotherapy of focal epilepsy in children: a systematic review of approved agents. CNS drugs. 2013;27(4):273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fairhurst C, Kumar R, Checketts D, Tayo B, Turner S. Efficacy and safety of nabiximols cannabinoid medicine for paediatric spasticity in cerebral palsy or traumatic brain injury: a randomized controlled trial. Dev Med Child Neurol. 2020. [DOI] [PubMed] [Google Scholar]
- 39. Nielsen S, Murnion B, Campbell G, Young H, Hall W. Cannabinoids for the treatment of spasticity. Developmental Medicine & Child Neurology. 2019;61(6):631–8. [DOI] [PubMed] [Google Scholar]
- 40. Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. Cannabis‐based products for pediatric epilepsy: A systematic review. Epilepsia. 2019;60(1):6–19. [DOI] [PubMed] [Google Scholar]
- 41. Lim K, See YM, Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin Psychopharmacol Neurosci. 2017;15(4):301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McClelland VM, Fialho D, Flexney‐Briscoe D, Holder GE, Elze MC, Gimeno H, et al. Somatosensory Evoked Potentials and Central Motor Conduction Times in children with dystonia and their correlation with outcomes from Deep Brain Stimulation of the Globus pallidus internus. Clinical Neurophysiology. 2018;129(2):473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shah SA, Brown P, Gimeno H, Lin J‐P, McClelland VM. Application of Machine Learning Using Decision Trees for Prognosis of Deep Brain Stimulation of Globus Pallidus Internus for Children With Dystonia. Frontiers in neurology. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Monbaliu E, Ortibus E, De Cat J, Dan B, Heyrman L, Prinzie P, et al. The Dyskinesia Impairment Scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Developmental Medicine & Child Neurology. 2012;54(3):278–83. [DOI] [PubMed] [Google Scholar]
- 45. Vanmechelen I, Danielsson A, Lidbeck C, Tedroff K, Monbaliu E, Krumlinde‐Sundholm L. The Dyskinesia Impairment Scale: Development, construct validity, and reliability. Developmental Medicine & Child Neurology. 2022. [DOI] [PubMed] [Google Scholar]
- 46. Lorentzen J, Pradines M, Gracies J‐M, Nielsen JB. On Denny‐Brown's ‘spastic dystonia’–What is it and what causes it? Clinical Neurophysiology. 2018;129(1):89–94. [DOI] [PubMed] [Google Scholar]
- 47. Lumsden DE. Spastic dystonia: Still a valid term. Developmental Medicine & Child Neurology. 2023. [DOI] [PubMed] [Google Scholar]
- 48. Lin JP, Nardocci N. Recognizing the Common Origins of Dystonia and the Development of Human Movement: A Manifesto of Unmet Needs in Isolated Childhood Dystonias. Front Neurol. 2016;7:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gimeno H, Farber J, Thornton J, Polatajko H. The Relative Merits of an Individualized Versus a Generic Approach to Rating Functional Performance in Childhood Dystonia. Children. 2021;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Comella CL, Leurgans S, Wuu J, Stebbins GT, Chmura T, Group DS . Rating scales for dystonia: a multicenter assessment. Movement disorders. 2003;18(3):303–12. [DOI] [PubMed] [Google Scholar]
- 51. Haberfehlner H, Goudriaan M, Bonouvrié LA, Jansma EP, Harlaar J, Vermeulen RJ, et al. Instrumented assessment of motor function in dyskinetic cerebral palsy: a systematic review. Journal of neuroengineering and rehabilitation. 2020;17(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bonouvrie LA, Haberfehlner H, Becher JG, Vles JS, Vermeulen RJ, Buizer AI, et al. Attainment of personal goals in the first year of intrathecal baclofen treatment in dyskinetic cerebral palsy: a prospective cohort study. Disabil Rehabil. 2023; 45(8): 1315–22. [DOI] [PubMed] [Google Scholar]
- 53. Koy A, Kuehn AA, Huebl J, Schneider GH, van Riesen AK, Eckenweiler M, et al. Quality of Life After Deep Brain Stimulation of Pediatric Patients with Dyskinetic Cerebral Palsy: A Prospective, Single‐Arm, Multicenter Study with a Subsequent Randomized Double‐Blind Crossover (STIM‐CP). Mov Disord. 2022; 37(4): 799–811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Methods.
Appendix S2: GRADE evidence profiles.
Appendix S3: Evidence‐to‐Decision frameworks.
Appendix S4: External review process: stakeholder consultation.
Appendix S5: Internal review process: AACPDM Care Pathways Committee review and public comment.
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


