Abstract
Whether vitamin D deficiency causes atrial fibrillation and ischemic stroke of young onset was unknown. We derived a Genetic Risk Score for vitamin D from 3,922 subjects in Hong Kong and applied it in an independent sample (n = 1,297) for clinical outcomes. Primary endpoint was a composite of atrial fibrillation and/or ischemic stroke. A second study was performed in the UK Biobank (n = 392,010; 46% men; 14,878 atrial fibrillation and 4,050 ischemic stroke cases, vs 374,102 controls). After 76 ± 46 months, 240 primary endpoints (18.5%) were adjudicated. Higher genetically-predicted vitamin D independently predicted reduced primary endpoint [odds ratio = 0.83 (0.72 to 0.95), p = 0.008]. Mendelian randomization analyses indicated vitamin D was causally protective against the primary endpoint [odds ratio = 0.81 (95% CI: 0.65 to 0.98)]. Independent analyses in the UK Biobank revealed that vitamin D was protective against young-onset ischemic stroke <50 years and atrial fibrillation combined [odds ratio = 0.36 (95% CI 0.14 to 0.94)], with predominant effect amongst men [odds ratio = 0.28 (95% CI 0.09 to 0.91)] compared to women [odds ratio = 0.60 (95% CI: 0.11 to 3.22)]. In conclusion, vitamin D may protect against young-onset ischemic stroke through preventing atrial fibrillation. Investigating the sex-specifc effects of vitamin D deficiency may elucidate sex disparities of atrial fibrillation in the young.
Keywords: vitamin D, young-onset ischemic stroke, atrial fibrillation, sex disparity, Mendelian randomization
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia, carries high clinical burdens of morbidity and mortalities, resulting mainly from its cardio-embolic and ischemic stroke sequealae.(1) A proportion of young-onset ischemic stroke, which might otherwise be categorized as cryptogenic, could be secondary to undiagnosed paroxysmal AF. Young-onset stroke, in particular, is disabling and confers heavy sufferings to patients and quality-adjusted life years loss from the societal perspective. Despite knowledge advances, a large etiological fraction of AF has remained elusive,(2) and the reason for AF to exhibit marked sex disparities remained poorly understood.(3)
Prior experimental studies revealed that Vitamin D (Vit-D) has direct electromechanical effects on the atria and produces dose-dependent lengthening of atrial action potential. It also ameliorates inter-atrial electromechanical delay, thus altering arrhythmic substrate for AF development.(4,5) Indeed, Vit-D deficiency was more commonly observed in patients with non-valvular AF,(6) with similar relations seen in patients with hypertension(7) and heart failure.(8,9) In a meta-analysis, Vit-D deficiency was associated with 31% excess risk of AF,(10) with a dose-response relationship observed.(11) Although a recent landmark trial, the VITAL Rhythm Study,(12) failed to show any effect of Vit-D on incident AF in healthy middle-aged or elder persons, its effect on AF together with ischemic stroke complications in younger persons aged ≤50 years had never been studied.
Increasing evidence suggests that Vit-D is linked to sex hormone levels in the human body.(13) A Mendelian randomization study showed that genetically predicted Vit-D was associated with increased total testosterone in men,(14) while clinical trial results were inconclusive.(15) Given that low testosterone level is a known risk factor for AF in men but protective in women,(8) the effect of Vit-D in sex hormone modulation through testosterone may potentially contribute to differential risks of AF and its complications between sexes.
Here we primarily hypothesized that Vit-D may protect against AF and ischemic stroke, with a particular focus on young-onset events in the population aged ≤50 years. We further tested another secondary hypothesis that the protective effects of Vit-D against AF and ischemic stroke may exhibit a sex-specific pattern. To test these hypotheses, we performed a series of two Mendelian randomization studies. Firstly, we investigated the effects of genetically-predicted Vit-D on AF and ischemic stroke in a relatively high-risk Chinese population. Secondly, we performed another Mendelian randomization study in a large independent cohort, the UK Biobank, to assess the general effects of genetically-predicted Vit-D on AF and young-onset ischemic stroke, as well as according to sexes.
Methods
Study subjects and cohort platforms
The study was performed based on the existing platform of the University of Hong Kong Theme-Based Research Scheme Cohort (HKU-TRS), as detailed elsewhere,(16) which recruited a total of 6,048 Southern Chinese subjects from the Hong Kong Chinese Coronary Artery Disease (CAD) Cohort,(17–19) the Hong Kong West Diabetes Registry (HKWDR),(20) as well as the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS/CRISPS2).(21) As prior described, subjects underwent Exome chip-genotyping with individual-level quality control relevant to biological relatedness, any duplication, sample contamination, or gender mismatch (n = 288 samples excluded), such that 5,760 samples passed quality control, comprising cases (known CAD; n = 2,372) and controls (no CAD; n = 3,388) for the HKU-TRS study on genetics of cardiovascular (CV) diseases in Chinese (recruitment period: Mar 2003 to December 2014). In the methodological structure of current analyses, subjects were respectively included in the Derivation Cohort and Translation Cohort samples. The 3,922 subjects in the Derivation Cohort who met the quality control criteria and had both serum and genetic Vit-D measurements were included solely for derivation of the genetic instrument for Vit-D pathways. The genetic instrument constructed was then applied for Mendelian randomization analyses in the Translation Cohort of 1,297 subjects (The Hong Kong Chinese CAD Cohort), who had been prospectively studied for development of incident AF and ischemic stroke in an extended follow-up (Supplemental Fig. 1*). Written informed consent was obtained for all subjects. The study was approved by the Institutional Review Board, University of Hong Kong/Hong Kong West Cluster, Hospital Authority.
The UK Biobank
For verification and generalizability analyses, findings from the HKU-TRS were studied in parallel with that from the UK Biobank. Cohort details of the UK Biobank had been extensively described.(22,23) The UK Biobank is an ongoing large prospective cohort study. The UK Biobank recruited 502,713 individuals aged 40–69 years, with a mean age of 56.5 years and 45.6% men, from England, Scotland and Wales between 2006 and 2010. Ninety four percent of subjects had self-reported European ancestry. The median follow-up time was 7.1 years. Disease outcomes were obtained from hospital episode records, an interview with nurses, and death certificates, with ongoing follow-up via record linkage to all health service encounters and deaths.(24) Genotyping was assessed using two very similar arrays, i.e., the UK BiLEVE array and UK Biobank Axiom array. To control for population stratification, we restricted our analysis to participants with self-reported and genetic validated ethnicity of white British. For quality control, we also excluded participants with 1) excess relatedness (more than 10 putative third-degree relatives) or 2) mismatched information on sex between genotyping and self-report, or 3) sex-chromosomes not XX or XY, or 4) poor-quality genotyping based on heterozygosity and missing rates. After the quality control, we identified 392,010 white British in the UK Biobank, including 17,908 cases with combined endpoint of AF and ischemic stroke.
This current research has been conducted using the UK Biobank Resource under application number 42468. No original data were collected for the current study. Ethical approval for each of the studies included in the investigation can be found in the original publications (including informed consent from each participant). The UK Biobank has already received ethical approval from the Research Ethics Committee, and participants provided written informed consent.
Baseline clinical assessments and definitions
Baseline demographic data, cardiovascular risk factors and medications were studied in the HKU-TRS. Hypertension was defined as either resting systolic/diastolic blood pressure (BP) ≥140/90 mmHg at two different clinic visits or on medications. Diabetes mellitus was defined by serum fasting glucose ≥7.0 mmol/L or on medications. Hypercholesterolemia was defined by a fasting total plasma cholesterol level of >200 mg/dl or on cholesterol-lowering medications. Diagnosis of ischemic stroke was made on the basis of clinical examinations and computed tomography or magnetic resonance brain imaging.(25) AF was defined as a supraventricular arrhythmia with uncoordinated atrial activity that was characterized by fibrillatory waves variable in magnitude, morphology, and timing and were associated with irregular ventricular response in the background of intact atrioventricular conduction. An AF episode was defined by duration lasting for more than 30s, with or without presence of clinical symptoms warranting physician attendance. The definition of AF entails paroxysmal, persistent or permanent AF, that had been detailed elsewhere.(19) Smoking status was recorded as ever or never smoker. Body-mass index (BMI) was calculated as weight in kilograms divided by square of height in meters. Fasting blood was collected for biochemical analysis of serum low-density/high-density lipoprotein (LDL/HDL)-cholesterol, triglycerides, glucose and creatinine.
Serological Vit-D measurements and validation
Serum 25-hydroxyvitamin D level was measured in the HKU-TRS using a validated enzyme immunoassay (Abbott Diagnostics, Lake Forest, IL). We first performed a preliminary inter-assay variability study, which showed a small inter-assay variability between different validated enzyme immunoassays (correlation coefficient of 0.86, p<0.001, using Abbott vs IDS Diagnostics (Tyne & Wear, UK). We thus performed all measurements using a single assay (Abbott) to enhance internal validity. Prior reliability studies showed good intra-assay variability of the Abbott assay, with coefficient of variations for serum 25-hydroxyvitamin D between 4.2–6.9% for the low 25-hydroxyvitamin D pool [mean 13.6 ng/ml (range 11.6–14.8)] and 1.9–2.5% for the high 25-hydroxyvitamin D pool [mean 36.8 ng/ml (range: 35.6 to 38.4)],(26) with similar results shown in different populations.(27) Vit-D deficiency was defined by serum 25-hydroxyvitamin D <20 ng/ml. Vit-D status was categorized as: sufficient (≥30 ng/ml), insufficient (≥20 to <30 ng/ml), or deficient (<20 ng/ml). Taking into account potential seasonal variations of Vit-D status, seasonality of blood sampling was categorized as taken in the spring (March to April), summer (May to August), autumn (September to October) and winter (November to February) periods.
Genotyping and genetic instrument for Mendelian randomization
Genotyping was performed in the HKU-TRS using a specially designed high-throughput Exome chip array (Illumina HumanExome BeadChip, Asian exome-chip), as prior described.(16,28) 12 single nucleotide polymorphisms (SNPs) involved in the Vit-D mechanistic pathways and detected to have significant associations with serum 25-hydroxyvitamin D from prior genome-wide associations studies (GWAS), including those with subgenome-wide significance, were interrogated (Biosynthetic: rs4646536, rs10877012, rs3829251, rs1790349; Activation: rs2060793, rs1993116; Vitamin D-Binding Protein (VBP)/GC: rs4588, rs7041, rs2282679, rs1155563; and Vit-D Receptor: rs1544410, rs10735810). We thus constructed a simple point-scale 6-alleles Genetic Risk Score (GRS) (linear continuous: 0–6) as an estimate of genetic Vit-D exposure, in an approach similar to previously reported studies.(29,30) Concisely, the final choice of SNPs in the GRS was based on this sole criterion: The multi-loci combination of SNPs that results in the greatest instrumental strength for prediction of serological Vit-D (in terms of R2 and F-Statistic) (Supplemental Table 1*), but without significant link disequilibrium (Supplemental Table 2*). A constituent score of 1 was given for each risk allele present that confers to higher serological Vit-D, where the reference allele was always associated with lower serological Vit-D level. An overall summative GRS (based on 3 SNPs, CYP2R1: rs2060793; GC: rs4588 and rs7041) ranging from 0 to 6 was derived, with GRS = 0 being the reference score that was equivalent to the lowest genetically predicted Vit-D level. GRS was in crude analyses [B = 0.60 (95% CI 0.44 to 0.77), p<0.001] as well as independently associated with serological Vit-D [B = 0.62 (95% CI 0.46 to 0.78), p<0.001], after adjustment for potential confounders (including sex, age, body-mass index, history of smoking, diabetes and hypertension, systolic and diastolic BP, serum levels of glucose, LDL- and HDL-cholesterol and triglycerides, creatinine, lipid-lowering therapy, and serum sampling seasonality). Variance of serological Vit-D explained by GRS was 1.3%, with F-statistic of 50.5 (p<0.001) indicating a strong instrument.
As shown in Supplemental Table 2*, as constituent genetic variants of the GRS, rs2060793 and rs4588 showed no linkage disequilibrium (R2 = 0). It was noted that rs7041 had only weak linkage disequilibrium with rs4588 (R2 = 0.17). Hardy-Weinberg equilibrium was tested by X2 against each SNP locus to exclude genotype-dependent ascertainment bias in the HKU-TRS, using a prior validated equilibrium assessment tool.(31) Analyses revealed no violation (all SNPs X2 0–1.5), suggesting the absence of genotype-dependent ascertainment bias and coherent with the principle of intention-to-Mendelian randomization.
As for verification cohort analyses using data from the UK Biobank, we estimated genetically predicted Vit-D using a genetic score that comprised 6 genetic variants (CYP2R1: rs10741657, AMDHDL: rs10745742, DHCR7: rs12785878, CYP24A1: rs17216707, GC: rs3755967, and SEC23A: rs8018720). This genetic score had been prior validated in a study by another group.(32)
Clinical outcome assessment
During the HKU-TRS prospective follow-up, we adjudicated new onset clinical events based on the International Classification of Diseases, Ninth Revision (ICD-9), consisting of AF and flutter (ICD-9, 427.3), and ischemic stroke (ICD-9 433, 434, 435, 436). These data were retrieved and ascertained from the medical records and the territory-wide computerized clinical data network of the Hong Kong Hospital Authority, and from directly called back follow-ups. Follow-up rate for this HKU-TRS study reached 100%. The primary endpoint was the Mendelian randomization-inferred causal effect of genetically predicted Vit-D on the composite endpoint of prevalent/incident AF and/or any incident ischemic stroke.
For The UK Biobank, we performed analyses for the pre-specified primary endpoint of Mendelian randomization-inferred causal effect of genetically predicted Vit-D on AF and flutter (ICD-9, 427.3; and ICD-10 I48, as well as self-reported cases), and its composite with ischemic stroke (ICD-9 433, 434, 435, 436) at young-onset (defined as participant age <50 years),(33–35) with general and sex-specific estimates.
Statistical analysis
Association between serum Vit-D and primary endpoint of AF and ischemic stroke was assessed by logistic regression model with age and gender adjustment. Crude and multivariable logistic regression models were used to assess the relation of serum Vit-D and GRS with the primary endpoint of AF and/or ischemic stroke. Potential confounders were defined based on prior reported associations, specified a priori. Multivariable regression model was used to adjust for potential confounders including age, gender, smoking, history of hypertension, diabetes mellitus, serum creatinine, and seasonal variation of serum Vit-D sampling. To test for linearity assumptions, we repeated analyses with dichotomous models and categorized exposure variables as appropriate.
We obtained Mendelian randomization estimates for the associations of genetically predicted Vit-D with the combined primary endpoint of AF and/or ischemic stroke using two-sample instrumental variable analysis. Specifically, we obtained SNP-specific Wald’s estimates (quotient of genetic association on AF and/or ischemic stroke divided by genetic association on serological Vit-D) and then an overall estimate under an additive genetic model of the GRS (Wald’s estimate: βxy = βzy divided by βzx), and the ratio 95% confidence interval based upon Fieller’s theorem (E. C. Fieller, 1940), with similar approaches prior described.(23) Briefly, to meet the three basic assumptions in Mendelian randomization, i.e., including relevance, independence, as well as exclusions-restriction principle, we incorporated SNPs that strongly and independently predicted Vit-D levels. We sought to ascertain whether the genetic variants were independent of potential confounders from their crude associations with these variables in the HKU-TRS, including smoking history, use of lipid-lowering therapy, and BMI, and found that there was no significant association (all p>0.20). To strengthen the assumption that such included SNPs were associated with AF and/or ischemic stroke only via Vit-D, we further checked for known direct effects of the genetic variants on AF and ischemic stroke outcomes (i.e., horizontal pleiotropy and therefore violating the exclusions-restriction principle) in three extensive, curated reference databases that record well-established associations between genotypes and phenotypes:
i. PhenoScanner (www.phenoscanner.medschl.cam.ac.uk);
ii. GWAS catalog (https://www.ebi.ac.uk/gwas/);
iii. Ensembl (http://www.ensembl.org/index.html).
Searching through all these databases, the investigators found no relation of these SNPs of interests with potential confounders, including tobacco or alcohol use, physical activity, BMI, as well as socioeconomic position or Townsend index. As there was a limited number of SNPs included in our instrument, MR-Egger was not used because it was based on the Instrument Strength Independent of Direct Effect (InSIDE) assumption and potentially sensitive to outliers. We deliberately considered variants from different genetic loci regions separately to avoid a single region operating through unknown pleiotropic effects towards the clinical outcomes of interest.
A two-sided p value less than 0.05 was considered statistically significant. Any missing value was excluded from the analyses. The statistical software packages IBM SPSS (ver. 21) (SPSS, Chicago, IL), STATA (ver. 14.0), GraphPad/PRISM Statistical calculator, and R-programming language (ver. 3.4.3) were used.
Sample size calculation
The samples included for analyses in the HKU-TRS cohort and the UK Biobank were both determined on a pragmatic basis based on cohort sample availability.
Results
Effects of Vit-D on AF and ischemic stroke in Chinese
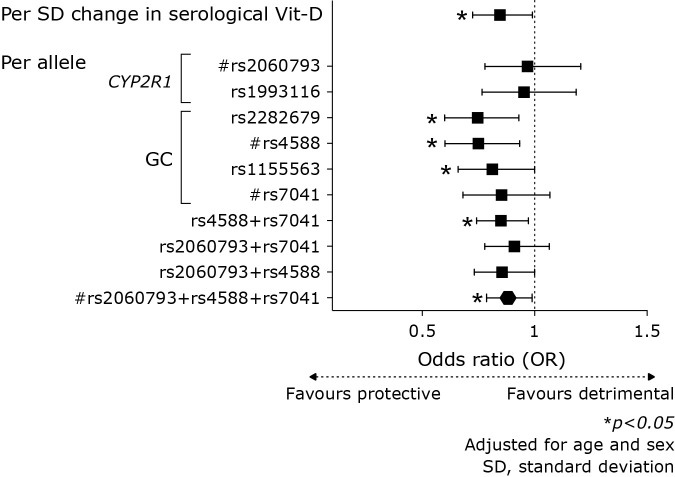
Baseline clinical characteristics of HKU-TRS subjects in the Derivation Cohort and the Translation Cohort (Hong Kong Chinese CAD Cohort) subsamples are shown in Table 1, the latter stratified by presence of the primary endpoint of AF and/or ischemic stroke. The study flowchart is shown in Supplemental Fig. 1*. Clinical events were prospectively studied in 1,297 subjects in the Translation Cohort. Prevalence of Vit-D deficiency (defined as serum 25-hydroxyvitamin D <20 ng/ml) in this sample was 36%. After a follow-up period of 76 ± 46 months, 240 primary endpoints (18.5%) of AF and/or ischemic stroke were adjudicated, comprising 232 prevalent or incident AF, and 41 incident ischemic stroke. Serological Vit-D deficiency was independently associated with AF and/or ischemic stroke [OR = 1.77 (95% CI 1.24 to 2.52), p = 0.002]. Higher genetically predicted Vit-D, denoted by GRS, independently predicted reduced risk of AF and/or ischemic stroke [OR = 0.83 (0.72 to 0.95), p = 0.008, Table 2 and Fig. 1]. MR analyses revealed that Vit-D is causally protective against the primary combined endpoint of AF and/or ischemic stroke [Wald’s estimate: OR = 0.81 (95% CI: 0.65 to 0.98)].
Table 1.
Baseline clinical characteristics of HKU-TRS subjects with or without atrial fibrillation (AF) and/or ischemic stroke
| Derivation cohort | Translation cohort | ||||
|---|---|---|---|---|---|
| n = 3,922 | No AF/ischemic stroke n = 1,057 |
AF and/or ischemic stroke n = 240 |
p value† | ||
| Male [n (%)] | 2,179 (55.6%) | 781 (75.2%) | 171 (71.3%) | 0.21 | |
| Age (years) | 61.8 ± 12.6 | 66.2 ± 10.7 | 69.8 ± 9.4 | <0.001* | |
| Ever smoking [n (%)] | 1,286 (32.8%) | 517 (48.9%) | 117 (48.8%) | 0.96 | |
| Body mass index (kgm−2) | 25.4 ± 4.1 | 25.0 ± 3.5 | 25.8 ± 4.2 | 0.021* | |
| Diabetes mellitus [n (%)] | 2,968 (75.7%) | 354 (33.5%) | 105 (43.8%) | 0.003* | |
| Hypertension [n (%)] | 2,875 (73.3%) | 696 (65.8%) | 172 (71.7%) | 0.084 | |
| Systolic blood pressure (mmHg) | 135.2 ± 21.4 | 130.6 ± 17.3 | 129.3 ± 16.9 | 0.31 | |
| Diastolic blood pressure (mmHg) | 74.4 ± 10.5 | 73.0 ± 10.8 | 70.7 ± 11.6 | 0.003* | |
| LDL-cholesterol (mmol/L) | 2.5 ± 0.9 | 3.4 ± 1.0 | 3.2 ± 1.0 | 0.084 | |
| HDL-cholesterol (mmol/L) | 1.3 ± 0.4 | 1.2 ± 0.7 | 1.1 ± 0.4 | 0.23 | |
| Triglycerides (mmol/L) | 1.5 ± 1.0 | 1.7 ± 1.4 | 1.7 ± 1.1 | 0.92 | |
| Fasting glucose (mmol/L) | 7.1 ± 2.5 | 6.2 ± 1.9 | 6.3 ± 2.0 | 0.3 | |
| Creatinine (μmol/L) | 93.0 ± 63.2 | 104.0 ± 89.4 | 137.0 ± 148.2 | 0.001* | |
| Season of recruitment | <0.001* | ||||
| Spring [n (%)] | 660 (16.8%) | 139 (13.2%) | 32 (13.3%) | ||
| Summer [n (%)] | 1,362 (34.7%) | 168 (15.9%) | 66 (27.5%) | ||
| Autumn [n (%)] | 616 (15.7%) | 211 (20.0%) | 33 (13.8%) | ||
| Winter [n (%)] | 1,284 (32.7%) | 539 (51.0%) | 109 (45.4%) | ||
| Vitamin D deficiency [n (%)] | 1,924 (49.1%) | 358 (33.9%) | 104 (43.3%) | 0.006* | |
| Vitamin D GRS‡ | 2.7 ± 1.2 | 2.7 ± 1.2 | 2.5 ± 1.2 | 0.025* | |
†Comparison between persons with versus without the primary endpoint of AF and/or ischemic stroke. *p<0.05. ‡Genetic Risk Score (GRS) (0–6) based on combined allele score summation from 3 constituent single nucleotide polymorphisms (rs2060793, rs4588, rs7041).
Table 2.
Serological and genetically-predicted vitamin D level for prediction of the combined primary endpoint of atrial fibrillation (AF) and/or ischemic stroke in the HKU-TRS†
| Crude model‡ | Multivariable model§ | ||||
|---|---|---|---|---|---|
| OR (95% CI)2 | p value | OR (95% CI)2 | p value | ||
| Age (years) | 1.04 (1.02 to 1.05) | <0.001* | 1.06 (1.04 to 1.08) | <0.001* | |
| Male | 0.83 (0.61 to 1.14) | 0.25 | — | — | |
| Ever smoking | 0.99 (0.75 to 1.32) | 0.96 | — | — | |
| Body-mass index | 1.06 (1.01 to 1.10) | 0.011* | 1.11 (1.06 to 1.16) | <0.001* | |
| Diabetes mellitus | 1.55 (1.16 to 2.05) | 0.003* | 1.33 (0.92 to 1.91) | 0.13 | |
| Hypertension | 1.31 (0.96 to 1.79) | 0.08 | 0.68 (0.45 to 1.02) | 0.06 | |
| Lipid-lowering therapy | 0.38 (0.23 to 0.63) | <0.001* | 0.36 (0.18 to 0.70) | 0.003* | |
| Serum creatinine (μmol/L) | 1.002 (1.001 to 1.003) | <0.001* | 1.002 (1.001 to 1.003) | 0.006* | |
| Recruitment season | <0.001* | 0.005* | |||
| Spring | Reference | Reference | |||
| Summer | 1.71 (1.06 to 2.75) | 1.63 (0.94 to 2.84) | |||
| Autumn | 0.68 (0.40 to 1.16) | 0.64 (0.34 to 1.20) | |||
| Winter | 0.88 (0.57 to 1.36) | 0.88 (0.53 to 1.47) | |||
| Serological vitamin D deficiency (25-hydroxyvitamin D <25 ng/ml) |
1.49 (1.12 to 1.99) | 0.006* | 1.77 (1.24 to 2.52) | 0.002* | |
| Genetic vitamin D exposure Genetic risk score (GRS)# |
0.88 (0.78 to 0.98) | 0.025* | 0.83 (0.72 to 0.95) | 0.008* | |
*Statistically significant at 95% confidence (p<0.05). †Odds ratio (OR) prediction estimates and 95% confidence interval for AF and/or ischemic stroke explained by variable of interest as shown by univariable and multivariable logistic regression analyses; ‡Crude model: univariable estimate with no adjustment; §Multivariable model: adjusted for sex and age plus potential confounders with p value ≤0.10 in crude model; #Combined linear genetic score (0–6) based on aggregated allele score summation from 3 constituent single nucleotide polymorphisms (rs2060793, rs4588, rs7041).
Fig. 1.
Serological Vit-D and per-allele risk estimates in odds ratio (OR) for the primary endpoint of AF and/or ischemic stroke. Serological Vit-D deficiency independently predicted the primary endpoint of AF and/or ischemic stroke [OR = 1.77 (95% CI 1.24 to 2.52), p = 0.002] in the HKU-TRS. Higher genetically predicted Vit-D, denoted by GRS, independently predicted reduced risk of AF and/or ischemic stroke [OR = 0.83 (0.72 to 0.95), p = 0.008]. Predictive estimates of other candidate genetic variants or combinations provided sensitivity analyses which corroborated similar findings.
Vit-D protects against young-onset AF and ischemic stroke in the European population: The UK Biobank
Analysing the large UK Biobank as an external verification cohort with 392,010 subjects (46% male) that included 14,878 AF cases and 4,050 ischemic stroke, Mendelian randomization analyses shows that genetically-predicted Vit-D as denoted by genetic score comprising 6 genetic variants (CYP2R1: rs10741657, AMDHDL: rs10745742, DHCR7: rs12785878, CYP24A1: rs17216707, GC: rs3755967, and SEC23A: rs8018720) is causally linked to reduced risk of young-onset ischemic stroke in subjects <50 years and AF combined [OR = 0.36 (95% CI 0.14 to 0.94), Table 3]. Furthermore, genetically-predicted Vit-D is also protective against AF alone in this group of subjects [OR = 0.24 (95% CI 0.08 to 0.72)]. Nevertheless, any protective effect against AF alone [OR = 1.01 (0.79 to 1.30)], or the combined endpoint of AF and ischemic stroke [OR = 1.05 (95% CI 0.84 to 1.33)] are not observed in subjects ≥50 years old.
Table 3.
General and sex-specific effects of genetically predicted vitamin D on atrial fibrillation (AF) and ischemic stroke in the UK Biobank (n = 392,010)
| Clinical endpoint | Demographic stratification | Mendelian randomization estimates | |||
|---|---|---|---|---|---|
| Age | Sex | Odds ratio [OR (95% CI)] | p value | ||
| AF and ischemic stroke (n = 17,908, out of 392,010) |
All people | Both | 0.99 (0.79 to 1.24) | 0.96 | |
| Men | 1.04 (0.79 to 1.37) | 0.77 | |||
| Women | 0.91 (0.62 to 1.32) | 0.61 | |||
| <50 years old | Both | 0.36 (0.14 to 0.94) | 0.04* | ||
| Men | 0.28 (0.09 to 0.91) | 0.03* | |||
| Women | 0.60 (0.11 to 3.22) | 0.55 | |||
| ≥50 years old | Both | 1.05 (0.84 to 1.33) | 0.66 | ||
| Men | 1.12 (0.97 to 1.30) | 0.42 | |||
| Women | 0.93 (0.76 to 1.13) | 0.71 | |||
| AF (n = 14,878, out of 392,010) |
All people | Both | 0.94 (0.74 to 1.19) | 0.63 | |
| Men | 0.95 (0.71 to 1.29) | 0.76 | |||
| Women | 0.91 (0.60 to 1.40) | 0.61 | |||
| <50 years old | Both | 0.24 (0.08 to 0.72) | 0.01* | ||
| Men | 0.25 (0.07 to 0.92) | 0.04* | |||
| Women | 0.22 (0.03 to 1.71) | 0.15 | |||
| ≥50 years old | Both | 1.01 (0.79 to 1.30) | 0.95 | ||
| Men | 1.02 (0.75 to 1.39) | 0.88 | |||
| Women | 0.97 (0.63 to 1.50) | 0.9 | |||
*p<0.05. Based on the genetic score comprising of 6 genetic variants (rs10741657 in CYP2R1, rs10745742 in AMDHDL, rs12785878 in DHCR7, rs17216707 in CYP24A1, rs3755967 in GC and rs8018720 in SEC23A) from a genome-wide association study of Vit-D, consistent with a previous MR study.
Sex-specific analyses in the UK Biobank
A predominant effect of Vit-D protection against the primary endpoint of AF and young-onset ischemic stroke was seen amongst men [OR = 0.28 (95% CI 0.09 to 0.91)], compared to women [OR = 0.60 (95% CI: 0.11 to 3.22)]. Similarly, genetically-predicted Vit-D had a protective effect against AF alone in young men <50 years [OR = 0.25 (95% CI: 0.07 to 0.92)], but such protection was not apparent in women [OR = 0.22 (95% CI 0.03 to 1.71)].
Discussion
To our knowledge, this is the first Mendelian randomization study or series that investigated the effect of genetically predicted Vit-D on AF and its composite endpoint with ischemic stroke. Our study series provided novel data showing that genetically predicted Vit-D is causally protective against AF/flutter and ischemic stroke, in particular those of young onset <50 years. Such protective effects are predominantly seen in men. Of note, our analyses series had included the Asian as well as European populations from different continents, substantiating study generalizability.
The novel findings from this Mendelian randomization series need to be cautiously interpreted in the context of current literature. The single largest randomized controlled study to date that investigated Vit-D for the prevention of AF was the VITAL Rhythm study. In VITAL Rhythm,(12) an embedded ancillary trial using the 2 × 2 factorial design, neither marine omega-3 fatty acids nor Vit-D was found to impact on incident AF amongst men and women aged respectively ≥50 years and ≥55 years. Consequentially, any effect in the younger persons had remained unknown. Furthermore, in VITAL Rhythm, while most subjects had normal Vit-D levels, subgroup analyses amongst those with Vit-D deficiency indeed trended towards a potential (albeit statistically insignificant) benefit. In our study, by inclusion of the younger group of subjects <50 years old and a wider spectrum of Vit-D serological status (Vit-D deficiency had 45% prevalence of in the HKU-TRS), as well as incorporation of the robust and pathophysiological-linked endpoint of ischemic stroke in the primary analyses, our data provided a unique and complimentary perspective that was unexplored by prior studies.
Although reasons were not entirely clear, the findings of Vit-D having protective effects against AF and ischemic stroke in the younger persons indeed corroborated with prior observations such as the ARIC study,(36) in which Vit-D deficiency was found to be associated with AF in younger subjects (<58 years old). It is known that hypertension, amongst other risk factors, contributes the largest fraction of population attributable risk (up to >40%) to the development of AF.(2) While the incidence of hypertension and other CV risk factors increases with age, it may be possible that the role of Vit-D deficiency in the pathogenesis of AF is only apparent in younger persons free from any strong influences from hypertension or other established CV risk factors. As ageing occurs, emergence of hypertension and other CV risk factors then may predominate and overtake the influences on AF development.
Mechanistically, Vit-D may interfere with the pathogenesis of AF through its direct electrophysiological effects on the atrial myocardium, or indirectly through altering systemic or micro-environmental influences.(19) For instance, Vit-D has been shown in animal experimental models to have direct electromechanical effects on the atria, resulting in dose-dependent lengthening of atrial action potential duration, thus altering arrhythmic substrate for AF. It was shown experimentally to prevent or terminate AF.(4) Canpolat et al.(5) further showed in clinical studies that patients with Vit-D deficiency had increased P wave dispersion, as well as exaggerated inter-atrial and intra-atrial electromechanical delay which is ameliorated following Vit-D replacement therapy. Furthermore, reduced Vit-D was found to be linked to worsened left atrial fibrosis in patients undergoing cryoballoon ablation, a hallmark of arrhythmogenic substrate for AF,(37) which may potentiate risk for recurrence post-ablation. Moreover, Vit-D negatively regulates the renin-angiotensin-aldosterone system and mediates calcium homeostasis, both having effects on AF pathogenesis. It may also modulate oxidative stress and reduce free radicals formation in the atria, dampening the inflammatory cytokine cascades which are pro-arrhythmogenic.(38) Interestingly, a study showed that the treatment benefit of renin-angiotensin inhibition was attenuated in patients with Vit-D deficiency, suggesting that deficiency of Vit-D may be a permissive cofactor in the neurohormonal promulgation of AF pathogenesis.(39) Other indirect mechanisms through which Vit-D may alter AF risk may include its indirect impact on vascular endothelial function,(40) progenitor cell expression/activity,(41) inhibition of foam cell formation and suppression of macrophage cholesterol uptake,(42) as well as vascular regeneration.(43)
The finding of a predominant effect of Vit-D on AF and stroke protection amongst men, compared to women, is intriguing and should warrant further investigation. Epidemiology of AF exhibits marked sex differences.(3) While exact mechanisms for sex disparity are not fully understood, the role of hormonal influences is indispensible. Experimental studies showed that deficiency of testosterone increased atrial arrhythmic substrate.(44) In humans, testosterone level is negatively associated with risk of AF in men, where the association is paradoxically reversed in women.(45) Correspondingly, deficiency of plasma levels of androgen in the young, not an uncommon problem, may underlie an under-represented aetiological fraction of AF risk in the young.(46)
Recent Mendelian randomization analyses suggested that Vit-D may increase testosterone level,(14) although results from randomized controlled trials were conflicting or inconclusive.(15,47) It is therefore possible that genetically predicted Vit-D may alter the risk of AF and ischemic stroke through modulating the androgen profiles, thus providing a plausible explanation for the observed sex-specific risk estimates of genetically predicted Vit-D in men vs women. These observations will need to be further investigated with detailed mechanistic studies.
With extensive studies of Vit-D in recent years, it has become clear that non-discriminate supplementation of Vit-D for the primary prevention of atherosclerotic CV diseases in healthy subjects with normal Vit-D levels is unlikely to result in any benefit.(48) Nevertheless, studies which targeted at those with confirmed serological Vit-D deficiency or high-risk group,(49) as well as important CV risk factors(50,51) suggested that targeting the “right” group of subjects might be a crucial factor in assessing for a benefit. Here, our study unveiled a group of vulnerable subjects with young-onset ischemic stroke and AF whose risk is altered with genetic Vit-D exposure. This observation ought to be tested in a randomized controlled trial. Given the disabilities, heavy sufferings and quality-adjusted life years loss from AF and consequential young-onset stroke, such study findings may carry huge implications for stroke prevention in the young. Future research should focus on assessing the therapeutic effects of reversing confirmed Vit-D deficiency for AF and stroke prevention, particularly in the young. The potential role of Vit-D on sex disparity of AF and moderation of hormonal changes will also need further mechanistic studies (Conceptual Paradigm, Fig. 2).
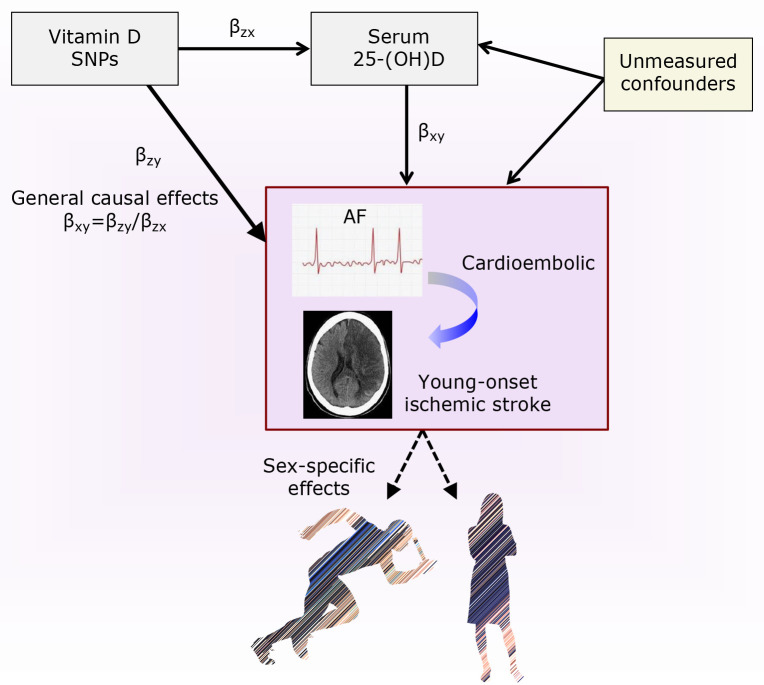
Fig. 2.
Series of Mendelian randomization. Effects of vitamin D (Vit-D) on atrial fibrillation (AF) and young-onset ischemic stroke. Conceptual paradigm illustrating the causal inference paths of Mendelian randomization on the general and sex-specific effects of Vit-D against AF and the pathophysiologically-linked clinical sequela of ischemic stroke.
Despite the strengths of our study described above, it has a number of limitations. These include the short duration of follow-up and a lack of detailed mechanistic studies, as well as possibility of unknown or undetected biases (in both directions) and confounding inherent to non-experimental controlled study. The possibility of vertical or horizontal pleiotropy cannot be totally excluded. Nevertheless, the external verification and sensitivity analyses helped to alleviate the mentioned limitations.
We conclude that Vit-D may protect against young-onset ischemic stroke through preventing AF. Investigating the sex-specifc effects of Vit-D deficiency, a worldwide pandemic, may elucidate sex disparities of AF in the young. The results need to be confirmed a in randomized controlled trial and further mechanistic studies are required.
Acknowledgments
YHC is supported by the Li Shu Fan Medical Fellowship for Internal Medicine, The University of Hong Kong. Analyses of data from the UK Biobank have been conducted using the UK Biobank resource (https://www.ukbiobank.ac.uk) under application number #42468, and that the authors would like to thank the UK Biobank for approving our application.
We sincerely thank all participants in the HKU-TRS and the UK-Biobank for their contribution and devotion to the advancement of science and medicine. Without their support, this endeavour would have never been possible.
Abbreviations
- AF
atrial fibrillation
- BP
blood pressure
- CV
cardiovascular
- GWAS
genome-wide association studies
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- Vit-D
vitamin D
Funding
This study was funded by the Health and Medical Research Fund, HKSAR (Project no. 10111531) and the Hong Kong Research Grants Council Theme-Based Research Scheme (T12-705/11), General Research Fund (777511M, 776412M, 776513M, and 17128515) and Innovation and Technology Support Programme (Tier 3) (ITS/303/12).
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet 2012; 379: 648–661. [DOI] [PubMed] [Google Scholar]
- 2.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation 2011; 123: 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volgman AS, Bairey Merz CN, Benjamin EJ, et al. Sex and race/ethnicity differences in atrial fibrillation. J Am Coll Cardiol 2019; 74: 2812–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanafy DA, Chang SL, Lu YY, et al. Electromechanical effects of 1,25-dihydroxyvitamin D with antiatrial fibrillation activities. J Cardiovasc Electrophysiol 2014; 25: 317–323. [DOI] [PubMed] [Google Scholar]
- 5.Canpolat U, Yayla Ç, Akboğa MK, et al. Effect of vitamin D replacement on atrial electromechanical delay in subjects with vitamin D deficiency. J Cardiovasc Electrophysiol 2015; 26: 649–655. [DOI] [PubMed] [Google Scholar]
- 6.Demir M, Uyan U, Melek M. The effects of vitamin D deficiency on atrial fibrillation. Clin Appl Thromb Hemost 2014; 20: 98–103. [DOI] [PubMed] [Google Scholar]
- 7.Ozcan OU, Gurlek A, Gursoy E, Gerede DM, Erol C. Relation of vitamin D deficiency and new-onset atrial fibrillation among hypertensive patients. J Am Soc Hypertens 2015; 9: 307–312. [DOI] [PubMed] [Google Scholar]
- 8.Belen E, Aykan AC, Kalaycioglu E, Sungur MA, Sungur A, Cetin M. Low-level vitamin D is associated with atrial fibrillation in patients with chronic heart failure. Adv Clin Exp Med 2016; 25: 51–57. [DOI] [PubMed] [Google Scholar]
- 9.Nolte K, Herrmann-Lingen C, Platschek L, et al. Vitamin D deficiency in patients with diastolic dysfunction or heart failure with preserved ejection fraction. ESC Heart Fail 2019; 6: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Yang Y, Ng CY, et al. Meta-analysis of vitamin D deficiency and risk of atrial fibrillation. Clin Cardiol 2016; 39: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Wang W, Tan Z, et al. The relationship between vitamin D and risk of atrial fibrillation: a dose-response analysis of observational studies. Nutr J 2019; 18: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert CM, Cook NR, Pester J, et al. Effect of marine omega-3 fatty acid and vitamin D supplementation on incident atrial fibrillation: a randomized clinical trial. JAMA 2021; 325: 1061–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Andrea S, Martorella A, Coccia F, et al. Relationship of vitamin D status with testosterone levels: a systematic review and meta-analysis. Endocrine 2021; 72: 49–61. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Zhai H, Cheng J, et al. Causal link between vitamin D and total testosterone in men: a mendelian randomization analysis. J Clin Endocrinol Metab 2019; 104: 3148–3156. [DOI] [PubMed] [Google Scholar]
- 15.Hosseini Marnani E, Mollahosseini M, Gheflati A, Ghadiri-Anari A, Nadjarzadeh A. The effect of vitamin D supplementation on the androgenic profile in men: a systematic review and meta-analysis of clinical trials. Andrologia 2019; 51: e13343. [DOI] [PubMed] [Google Scholar]
- 16.Tang CS, Zhang H, Cheung CY, et al. Exome-wide association analysis reveals novel coding sequence variants associated with lipid traits in Chinese. Nat Commun 2015; 6: 10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong YK, Cheung CYY, Tang CS, et al. Age-biomarkers-clinical risk factors for prediction of cardiovascular events in patients with coronary artery disease. Arterioscler Thromb Vasc Biol 2018; 38: 2519–2527. [DOI] [PubMed] [Google Scholar]
- 18.Chan YH, Yiu KH, Lau KK, et al. The CHADS2 and CHA2DS2-VASc scores predict adverse vascular function, ischemic stroke and cardiovascular death in high-risk patients without atrial fibrillation: role of incorporating PR prolongation. Atherosclerosis 2014; 237: 504–513. [DOI] [PubMed] [Google Scholar]
- 19.Chan YH, Yiu KH, Hai JJ, et al. Genetically deprived vitamin D exposure predisposes to atrial fibrillation. Europace 2017; 19 (Suppl 4): iv25–iv31. [DOI] [PubMed] [Google Scholar]
- 20.Hui E, Yeung CY, Lee PC, et al. Elevated circulating pigment epithelium-derived factor predicts the progression of diabetic nephropathy in patients with type 2 diabetes. J Clin Endocrinol Metab 2014; 99: E2169–E2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow WS, Tso AW, Xu A, et al. Elevated circulating adipocyte-fatty acid binding protein levels predict incident cardiovascular events in a community-based cohort: a 12-year prospective study. J Am Heart Assoc 2013; 2: e004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo S, Au Yeung SL, Zhao JV, Burgess S, Schooling CM. Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ 2019; 364: l476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Au Yeung SL, Luo S, Schooling CM. The impact of glycated hemoglobin (HbA1c) on cardiovascular disease risk: a Mendelian randomization study using UK Biobank. Diabetes Care 2018; 41: 1991–1997. [DOI] [PubMed] [Google Scholar]
- 24.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams H, Adams R, Del Zoppo G, Goldstein LB; Stroke Council of the American Heart Association. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke 2005; 36: 916–923. [DOI] [PubMed] [Google Scholar]
- 26.Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem 2012; 58: 531–542. [DOI] [PubMed] [Google Scholar]
- 27.Koivula MK, Matinlassi N, Laitinen P, Risteli J. Four automated 25-OH total vitamin D immunoassays and commercial liquid chromatography tandem-mass spectrometry in Finnish population. Clin Lab 2013; 59: 397–405. [DOI] [PubMed] [Google Scholar]
- 28.Cheung CYY, Tang CS, Xu A, et al. An exome-chip association analysis in Chinese subjects reveals a functional missense variant of GCKR that regulates FGF21 levels. Diabetes 2017; 66: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 29.Afzal S, Brøndum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ 2014; 349: g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brøndum-Jacobsen P, Benn M, Afzal S, Nordestgaard BG. No evidence that genetically reduced 25-hydroxyvitamin D is associated with increased risk of ischaemic heart disease or myocardial infarction: a Mendelian randomization study. Int J Epidemiol 2015; 44: 651–661. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009; 169: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong J, Gharahkhani P, Chow WH, et al. No association between vitamin D status and risk of Barrett’s esophagus or esophageal adenocarcinoma: a Mendelian randomization study. Clin Gastroenterol Hepatol 2019; 17: 2227–2235.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dongen MME, Aarnio K, Martinez-Majander N, et al. Use of statins after ischemic stroke in young adults and its association with long-term outcome. Stroke 2019; 50: 3385–3392. [DOI] [PubMed] [Google Scholar]
- 34.Ekker MS, Verhoeven JI, Vaartjes I, van Nieuwenhuizen KM, Klijn CJM, de Leeuw FE. Stroke incidence in young adults according to age, subtype, sex, and time trends. Neurology 2019; 92: e2444–e2454. [DOI] [PubMed] [Google Scholar]
- 35.Kivioja R, Pietilä A, Martinez-Majander N, et al. Risk factors for early-onset ischemic stroke: a case-control study. J Am Heart Assoc 2018; 7: e009774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso A, Misialek JR, Michos ED, et al. Serum 25-hydroxyvitamin D and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Europace 2016; 18: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Canpolat U, Aytemir K, Hazirolan T, Özer N, Oto A. Relationship between vitamin D level and left atrial fibrosis in patients with lone paroxysmal atrial fibrillation undergoing cryoballoon-based catheter ablation. J Cardiol 2017; 69: 16–23. [DOI] [PubMed] [Google Scholar]
- 38.Thompson J, Nitiahpapand R, Bhatti P, Kourliouros A. Vitamin D deficiency and atrial fibrillation. Int J Cardiol 2015; 184: 159–162. [DOI] [PubMed] [Google Scholar]
- 39.Turin A, Bax JJ, Doukas D, et al. Interactions among vitamin D, atrial fibrillation, and the renin-angiotensin-aldosterone system. Am J Cardiol 2018; 122: 780–784. [DOI] [PubMed] [Google Scholar]
- 40.Chan YH, Lau KK, Yiu KH, et al. Vascular protective effects of statin-related increase in serum 25-hydroxyvitamin D among high-risk cardiac patients. J Cardiovasc Med (Hagerstown) 2015; 16: 51–58. [DOI] [PubMed] [Google Scholar]
- 41.Yiu YF, Chan YH, Yiu KH, et al. Vitamin D deficiency is associated with depletion of circulating endothelial progenitor cells and endothelial dysfunction in patients with type 2 diabetes. J Clin Endocrinol Metab 2011; 96: E830–E835. [DOI] [PubMed] [Google Scholar]
- 42.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation 2009; 120: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong MS, Leisegang MS, Kruse C, et al. Vitamin D promotes vascular regeneration. Circulation 2014; 130: 976–986. [DOI] [PubMed] [Google Scholar]
- 44.Tsuneda T, Yamashita T, Kato T, et al. Deficiency of testosterone associates with the substrate of atrial fibrillation in the rat model. J Cardiovasc Electrophysiol 2009; 20: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 45.Zeller T, Schnabel RB, Appelbaum S, et al. Low testosterone levels are predictive for incident atrial fibrillation and ischaemic stroke in men, but protective in women - results from the FINRISK study. Eur J Prev Cardiol 2018; 25: 1133–1139. [DOI] [PubMed] [Google Scholar]
- 46.Guerra F, Ciliberti G, Capucci A. Sex differences in atrial fibrillation: the case of testosterone. Eur J Prev Cardiol 2018; 25: 1131–1132. [DOI] [PubMed] [Google Scholar]
- 47.Rockwell MS, Frisard MI, Rankin JW, et al. Effects of seasonal vitamin D3 supplementation on strength, power, and body composition in college swimmers. Int J Sport Nutr Exerc Metab 2020; 30: 165–173. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K, Ao M, Tamaru J, Kuwabara A. Vitamin D insufficiency and disease risk in the elderly. J Clin Biochem Nutr 2024; 74: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witte KK, Byrom R, Gierula J, et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol 2016; 67: 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a Mendelian randomisation study. Lancet Diabetes Endocrinol 2014; 2: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afzal S, Nordestgaard BG. Vitamin D, hypertension, ischemic stroke in 116 655 individuals from the general population: a genetic study. Hypertension 2017; 70: 499–507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.