Abstract
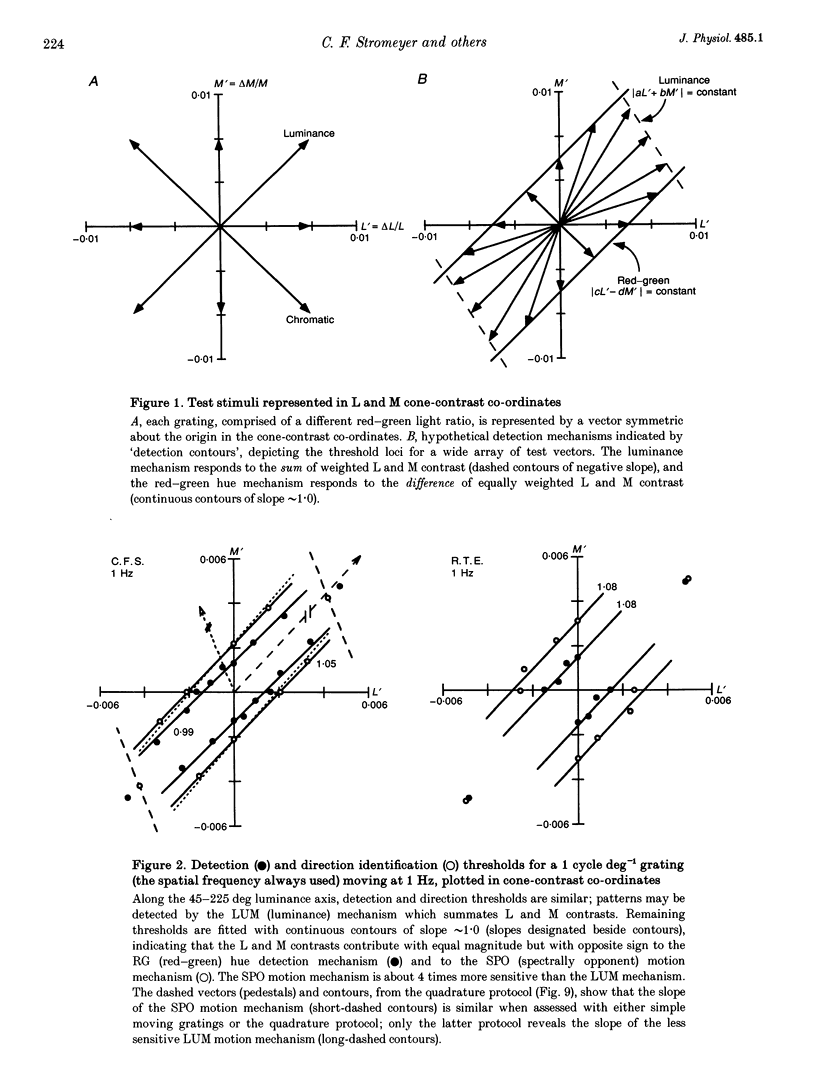
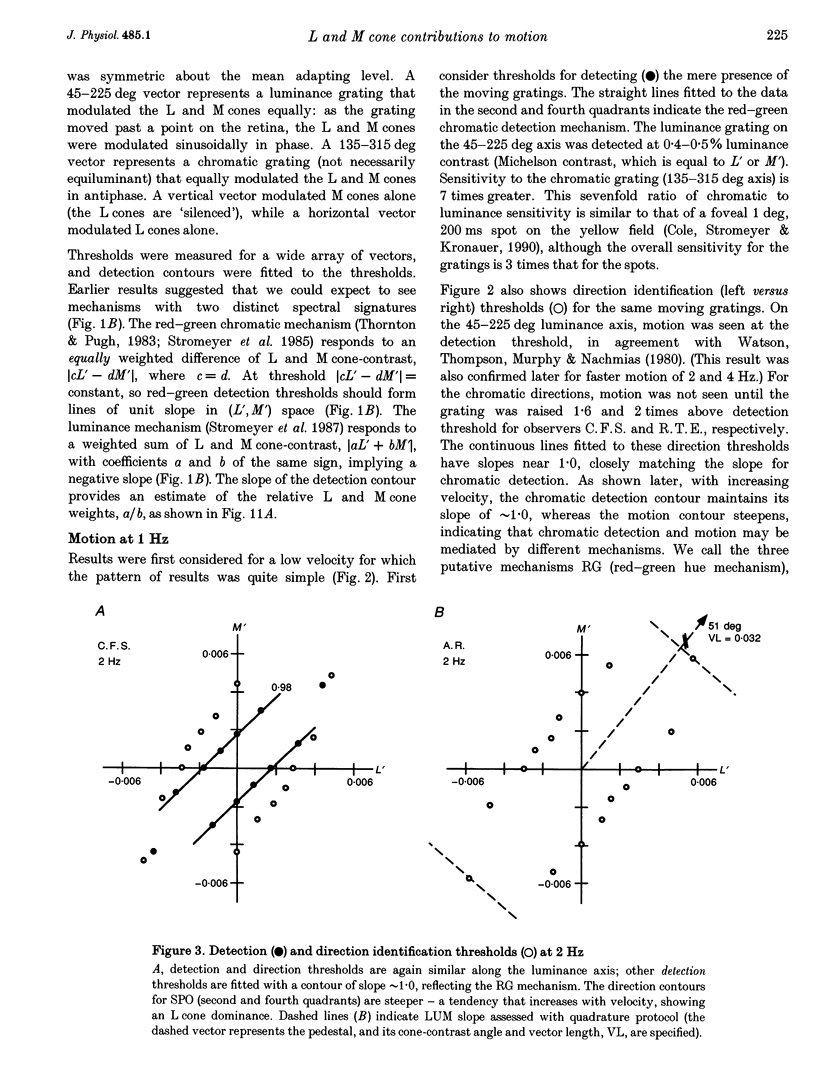
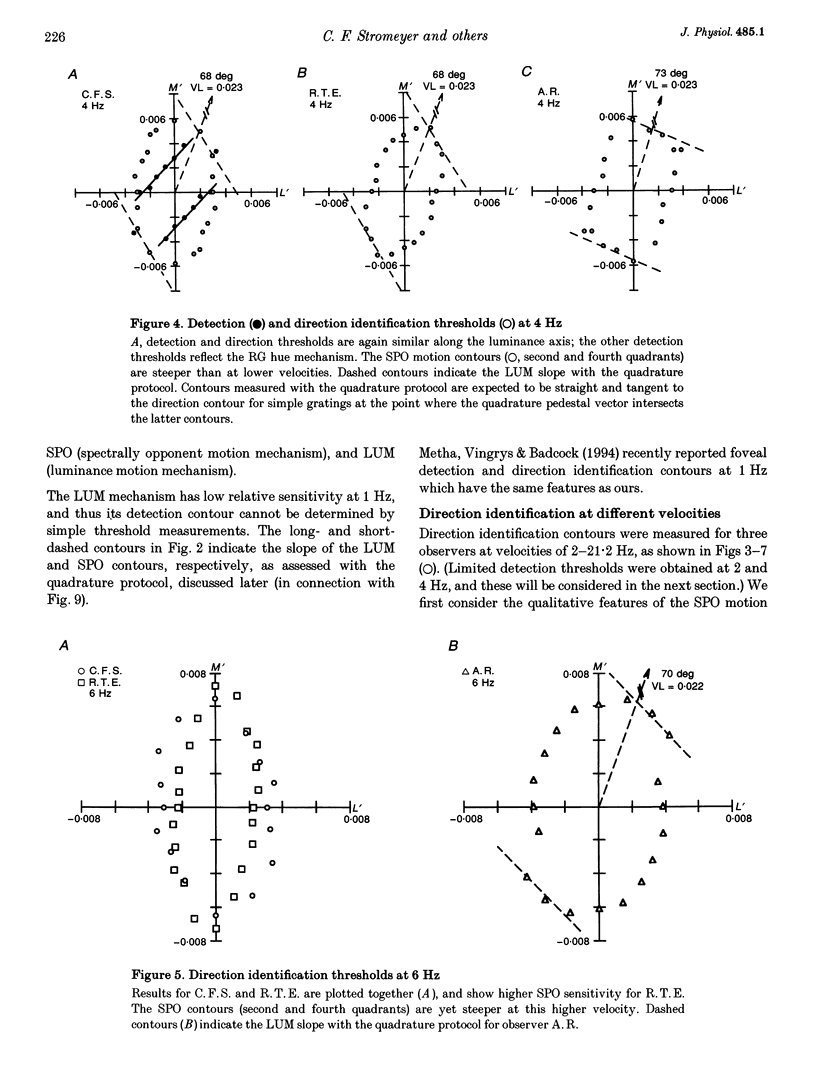
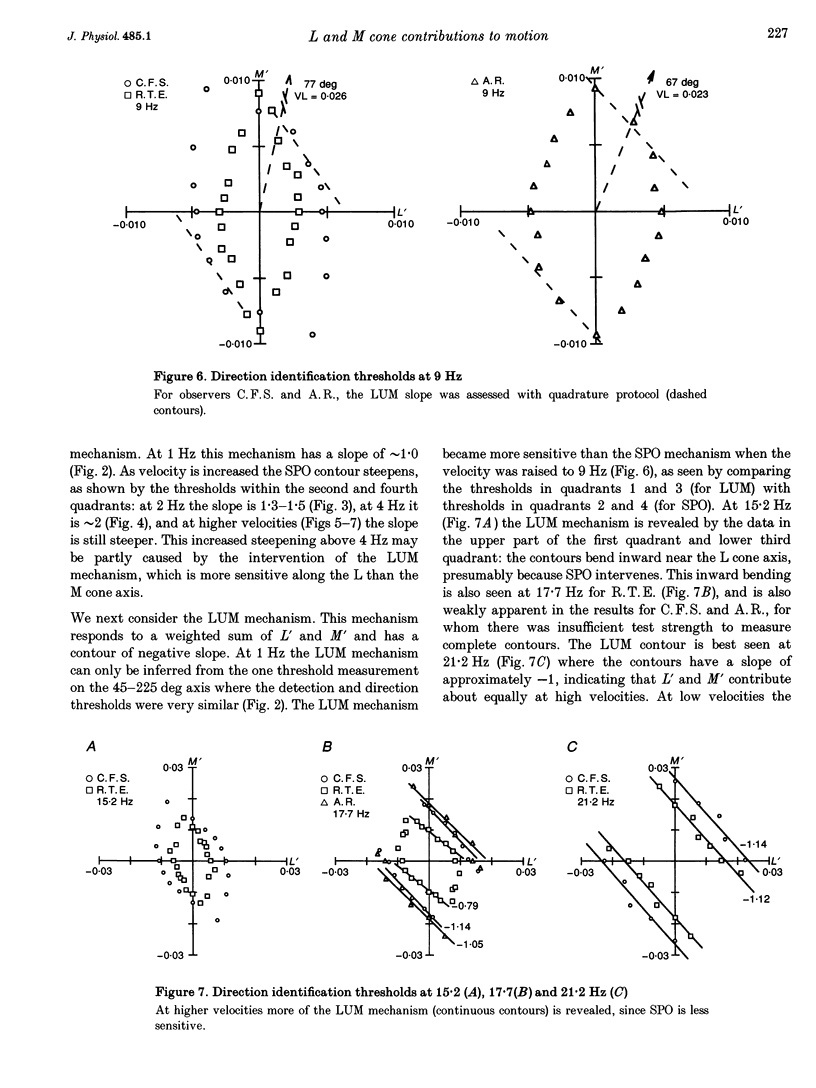
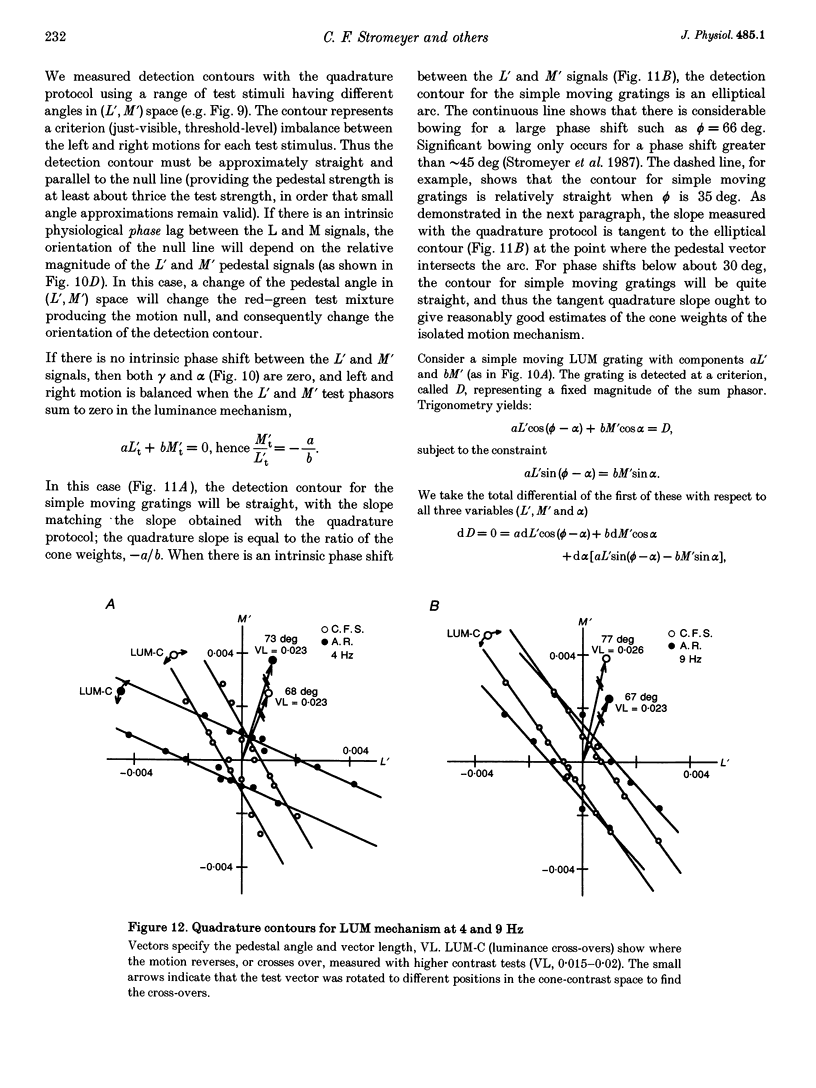
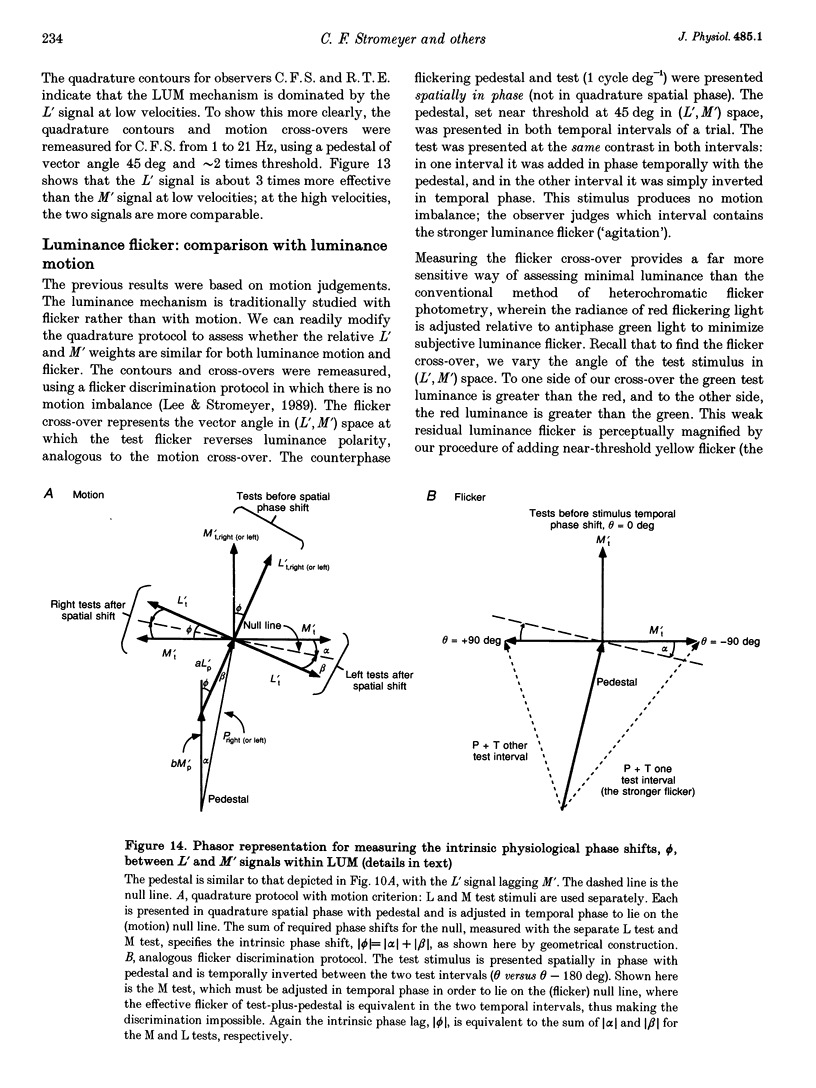
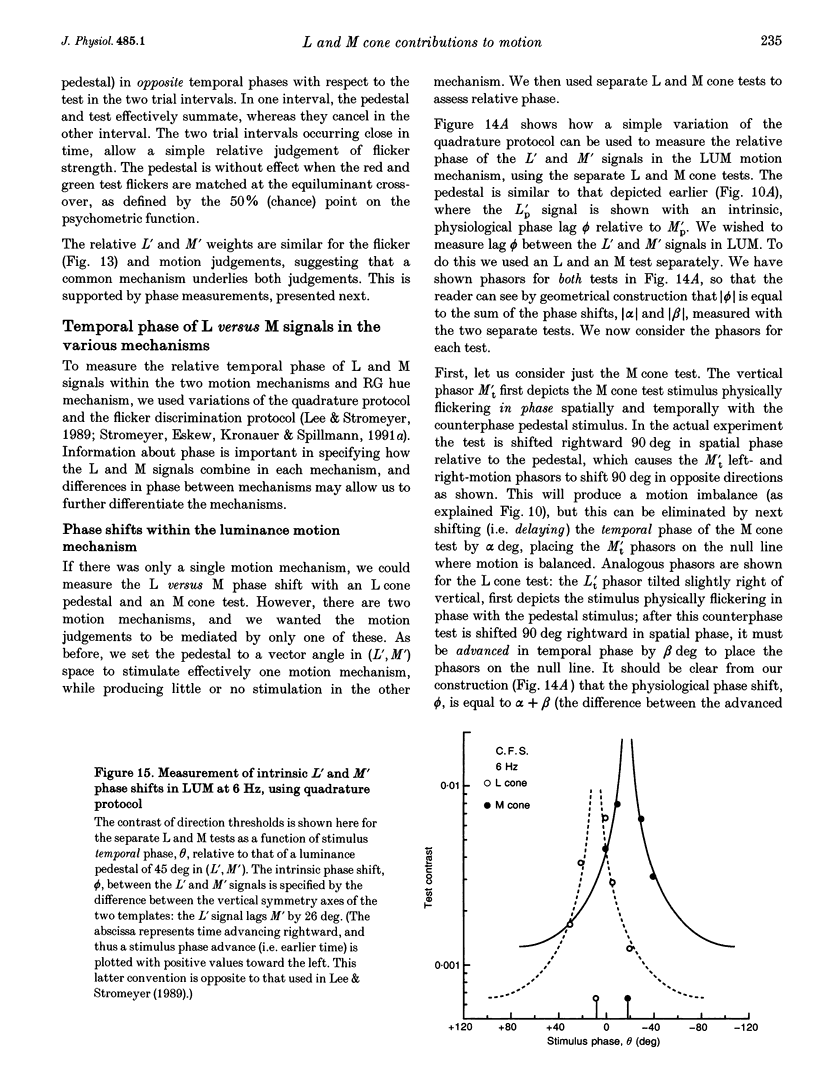
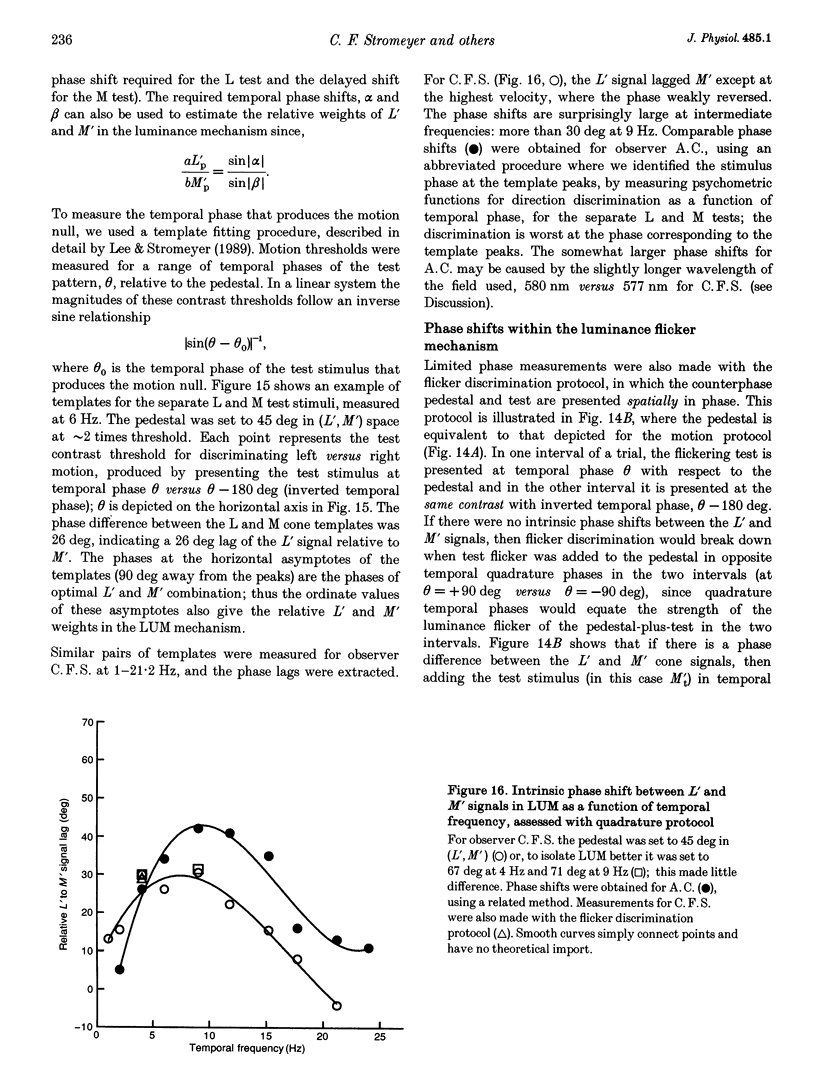
1. It has been suggested that motion may be best detected by the luminance mechanism. If this is the most sensitive mechanism, motion thresholds may be used to isolate the luminance mechanism and study its properties. 2. A moving (1 cycle deg-1), vertical, heterochromatic (red-plus-green), foveal grating was presented on a bright yellow (577 nm wavelength) field. Detection and motion (direction identification: left versus right) thresholds were measured for different amplitude ratios of the red and green components spatially summed in phase or in antiphase. Threshold contours plotted in cone-contrast co-ordinates (L',M') for the long-wave (L) and middle-wave (M) cones, revealed two motion mechanisms: a luminance mechanism that responds to a weighted sum of L and M contrasts, and a spectrally opponent mechanism that responds to a weighted difference. 3. Detection and motion thresholds, measured at 1-4 Hz, were identical for luminance gratings, having equal cone contrasts, L' and M', of the same sign. For chromatic gratings, with L' and M' of opposite sign, motion thresholds were higher than detection thresholds. A red-green hue mechanism may mediate chromatic detection, and a separate spectrally opponent motion mechanism may mediate motion. 4. The red-green hue mechanism was assessed from 1 to 15 Hz with an explicit hue criterion. The detection contour had a constant slope of one, implying equal L' and M' contributions of opposite sign. For motion identification, L' and M' contributed equally at 1 Hz, but the M' contribution was attenuated at higher velocities. 5. The cone-contrast metric provides a physiologically relevant comparison of sensitivities of the two motion mechanisms. At 1 Hz, the spectrally opponent motion mechanism is approximately 4 times more sensitive than the luminance mechanism. As temporal frequency is increased, the relative sensitivities change so that the luminance mechanism is more sensitive above 9 Hz. 6. The less sensitive motion mechanism was isolated with a quadrature phase protocol, using a pair of heterochromatic red-plus-green gratings, counterphase flickering in spatial and temporal quadrature phase with respect to each other. One grating was set slightly suprathreshold and oriented in cone contrast (L',M') so as to potentiate a single motion mechanism, the sensitivity of which was probed with the second grating, which was varied in (L',M'). This allowed us to measure the motion detection contour of the less sensitive luminance mechanism at low velocities.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


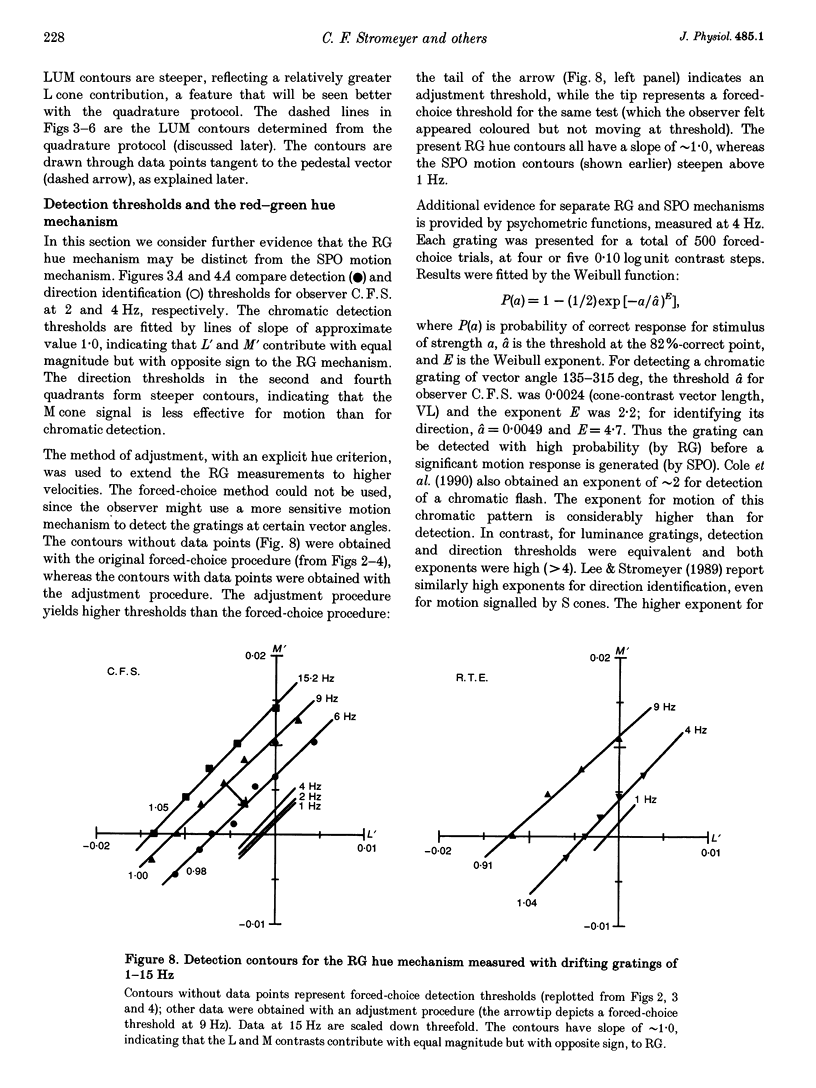
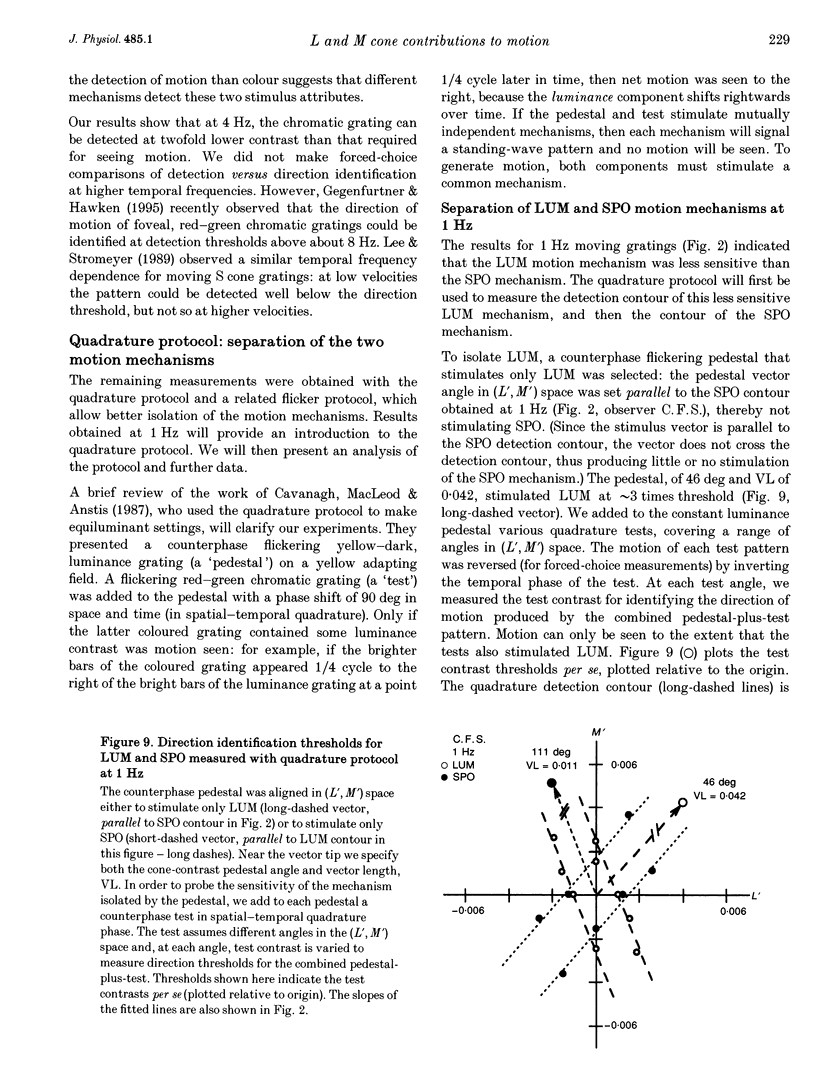
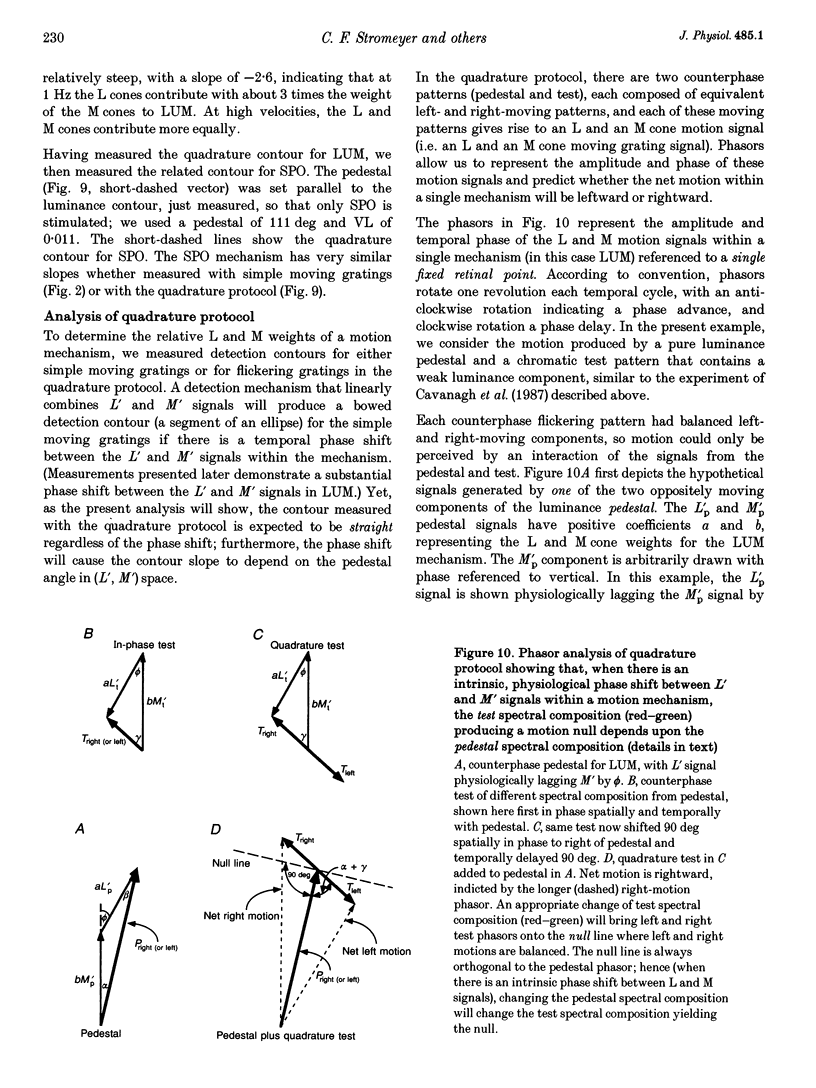
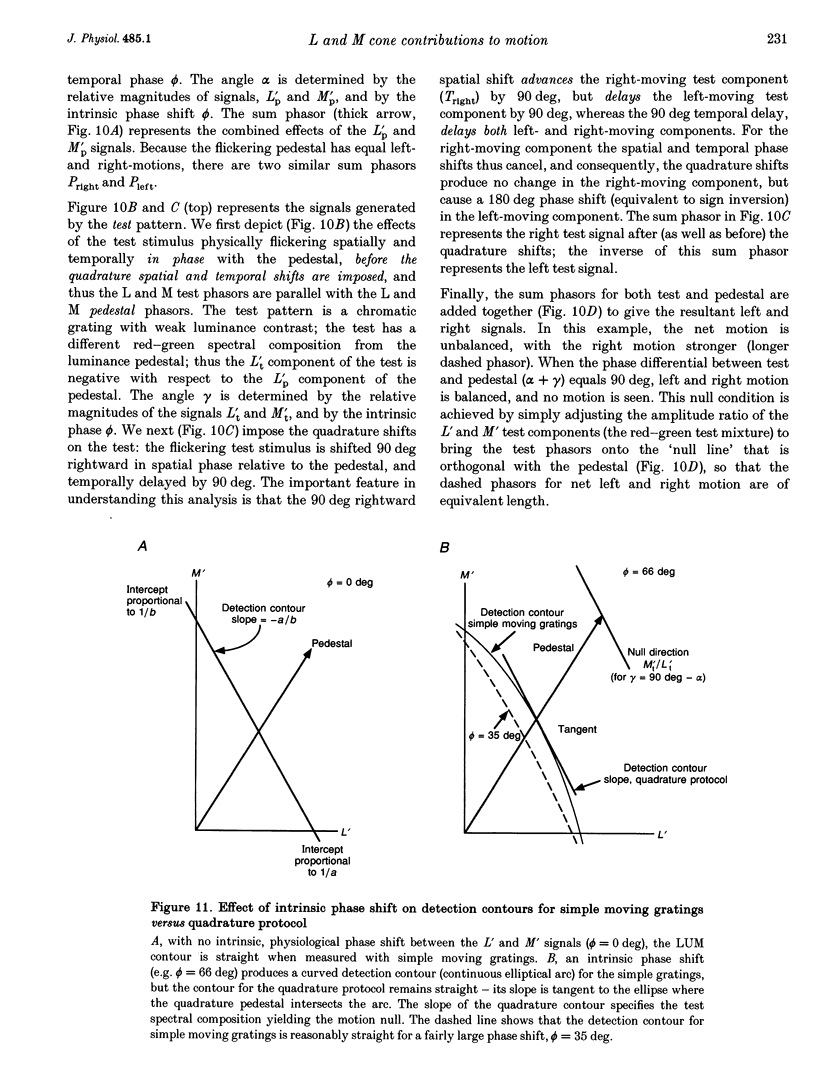






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavanagh P., Anstis S. The contribution of color to motion in normal and color-deficient observers. Vision Res. 1991;31(12):2109–2148. doi: 10.1016/0042-6989(91)90169-6. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Favreau O. E. Color and luminance share a common motion pathway. Vision Res. 1985;25(11):1595–1601. doi: 10.1016/0042-6989(85)90129-4. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., MacLeod D. I., Anstis S. M. Equiluminance: spatial and temporal factors and the contribution of blue-sensitive cones. J Opt Soc Am A. 1987 Aug;4(8):1428–1438. doi: 10.1364/josaa.4.001428. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Tyler C. W., Favreau O. E. Perceived velocity of moving chromatic gratings. J Opt Soc Am A. 1984 Aug;1(8):893–899. doi: 10.1364/josaa.1.000893. [DOI] [PubMed] [Google Scholar]
- Chichilnisky E. J., Heeger D., Wandell B. A. Functional segregation of color and motion perception examined in motion nulling. Vision Res. 1993 Oct;33(15):2113–2125. doi: 10.1016/0042-6989(93)90010-t. [DOI] [PubMed] [Google Scholar]
- Cole G. R., Stromeyer C. F., 3rd, Kronauer R. E. Visual interactions with luminance and chromatic stimuli. J Opt Soc Am A. 1990 Jan;7(1):128–140. doi: 10.1364/josaa.7.000128. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Badcock D. R. The low level motion system has both chromatic and luminance inputs. Vision Res. 1985;25(12):1879–1884. doi: 10.1016/0042-6989(85)90011-2. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Henning G. B. Detecting and discriminating the direction of motion of luminance and colour gratings. Vision Res. 1993 Mar-Apr;33(5-6):799–811. doi: 10.1016/0042-6989(93)90199-7. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Krauskopf J., Lennie P. Chromatic mechanisms in lateral geniculate nucleus of macaque. J Physiol. 1984 Dec;357:241–265. doi: 10.1113/jphysiol.1984.sp015499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenfurtner K. R., Kiper D. C., Beusmans J. M., Carandini M., Zaidi Q., Movshon J. A. Chromatic properties of neurons in macaque MT. Vis Neurosci. 1994 May-Jun;11(3):455–466. doi: 10.1017/s095252380000239x. [DOI] [PubMed] [Google Scholar]
- Krauskopf J., Farell B. Influence of colour on the perception of coherent motion. Nature. 1990 Nov 22;348(6299):328–331. doi: 10.1038/348328a0. [DOI] [PubMed] [Google Scholar]
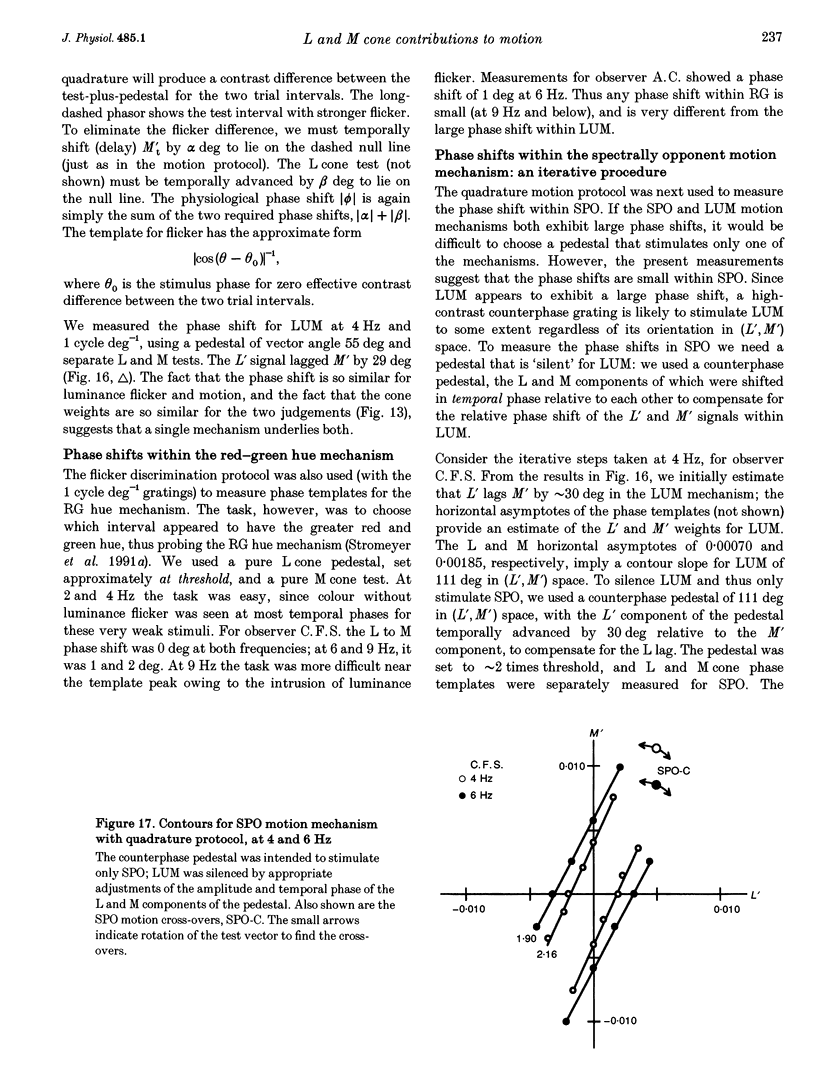
- Lee B. B., Martin P. R., Valberg A. Nonlinear summation of M- and L-cone inputs to phasic retinal ganglion cells of the macaque. J Neurosci. 1989 Apr;9(4):1433–1442. doi: 10.1523/JNEUROSCI.09-04-01433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. Sensitivity of macaque retinal ganglion cells to chromatic and luminance flicker. J Physiol. 1989 Jul;414:223–243. doi: 10.1113/jphysiol.1989.sp017685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. B., Martin P. R., Valberg A. The physiological basis of heterochromatic flicker photometry demonstrated in the ganglion cells of the macaque retina. J Physiol. 1988 Oct;404:323–347. doi: 10.1113/jphysiol.1988.sp017292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Stromeyer C. F., 3rd Contribution of human short-wave cones to luminance and motion detection. J Physiol. 1989 Jun;413:563–593. doi: 10.1113/jphysiol.1989.sp017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P., Krauskopf J., Sclar G. Chromatic mechanisms in striate cortex of macaque. J Neurosci. 1990 Feb;10(2):649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey D. T., Pokorny J., Smith V. C. Phase-dependent sensitivity to heterochromatic flicker. J Opt Soc Am A. 1986 Jul;3(7):921–927. doi: 10.1364/josaa.3.000921. [DOI] [PubMed] [Google Scholar]
- Lindsey D. T., Teller D. Y. Motion at isoluminance: discrimination/detection ratios for moving isoluminant gratings. Vision Res. 1990;30(11):1751–1761. doi: 10.1016/0042-6989(90)90157-g. [DOI] [PubMed] [Google Scholar]
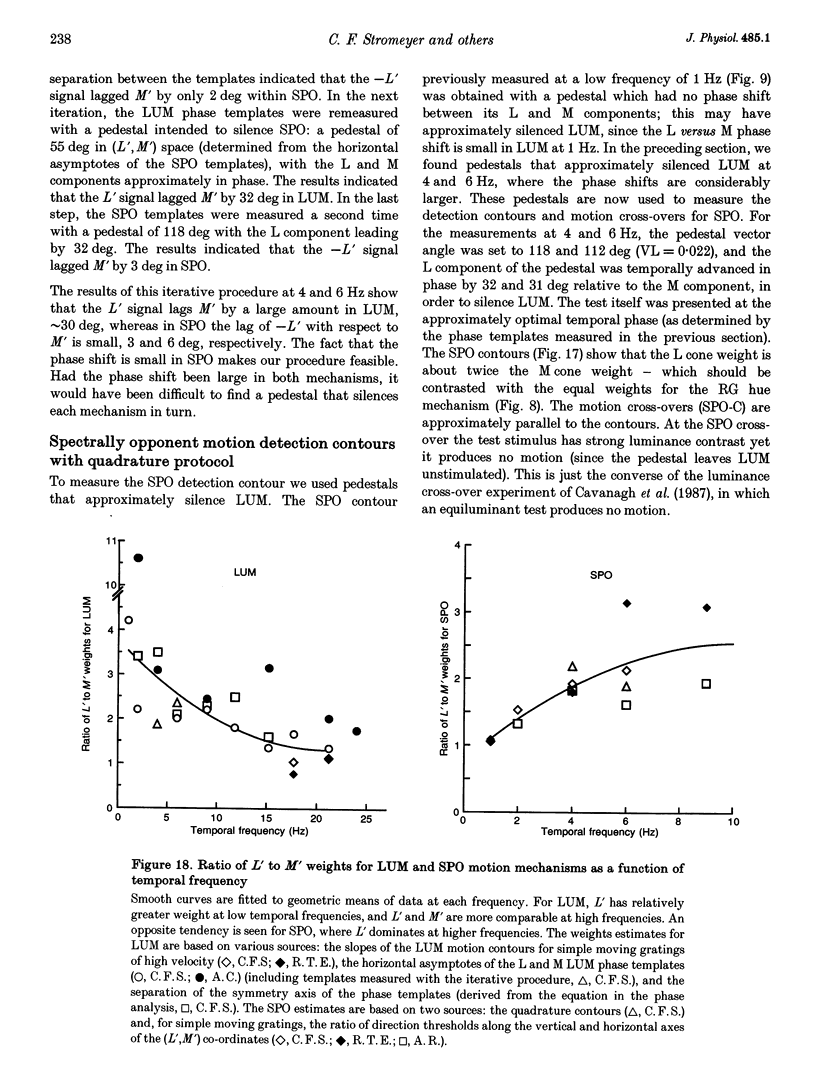
- Livingstone M. S., Hubel D. H. Psychophysical evidence for separate channels for the perception of form, color, movement, and depth. J Neurosci. 1987 Nov;7(11):3416–3468. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J. H., Nealey T. A., DePriest D. D. Magnocellular and parvocellular contributions to responses in the middle temporal visual area (MT) of the macaque monkey. J Neurosci. 1990 Oct;10(10):3323–3334. doi: 10.1523/JNEUROSCI.10-10-03323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan W. H., Byrne C. E., Maunsell J. H. Does primate motion perception depend on the magnocellular pathway? J Neurosci. 1991 Nov;11(11):3422–3429. doi: 10.1523/JNEUROSCI.11-11-03422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan W. H., Katz L. M., Maunsell J. H. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J Neurosci. 1991 Apr;11(4):994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metha A. B., Vingrys A. J., Badcock D. R. Detection and discrimination of moving stimuli: the effects of color, luminance, and eccentricity. J Opt Soc Am A Opt Image Sci Vis. 1994 Jun;11(6):1697–1709. doi: 10.1364/josaa.11.001697. [DOI] [PubMed] [Google Scholar]
- Mullen K. T., Baker C. L., Jr A motion aftereffect from an isoluminant stimulus. Vision Res. 1985;25(5):685–688. doi: 10.1016/0042-6989(85)90174-9. [DOI] [PubMed] [Google Scholar]
- Mullen K. T., Boulton J. C. Absence of smooth motion perception in color vision. Vision Res. 1992 Mar;32(3):483–488. doi: 10.1016/0042-6989(92)90240-j. [DOI] [PubMed] [Google Scholar]
- Mullen K. T., Boulton J. C. Interactions between colour and luminance contrast in the perception of motion. Ophthalmic Physiol Opt. 1992 Apr;12(2):201–205. doi: 10.1111/j.1475-1313.1992.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Palmer J., Mobley L. A., Teller D. Y. Motion at isoluminance: discrimination/detection ratios and the summation of luminance and chromatic signals. J Opt Soc Am A. 1993 Jun;10(6):1353–1362. doi: 10.1364/josaa.10.001353. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Does colour provide an input to human motion perception? Nature. 1978 Sep 7;275(5675):55–56. doi: 10.1038/275055a0. [DOI] [PubMed] [Google Scholar]
- Reid R. C., Shapley R. M. Spatial structure of cone inputs to receptive fields in primate lateral geniculate nucleus. Nature. 1992 Apr 23;356(6371):716–718. doi: 10.1038/356716a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Logothetis N. K., Charles E. R. Role of the color-opponent and broad-band channels in vision. Vis Neurosci. 1990 Oct;5(4):321–346. doi: 10.1017/s0952523800000420. [DOI] [PubMed] [Google Scholar]
- Smith V. C., Lee B. B., Pokorny J., Martin P. R., Valberg A. Responses of macaque ganglion cells to the relative phase of heterochromatically modulated lights. J Physiol. 1992 Dec;458:191–221. doi: 10.1113/jphysiol.1992.sp019413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith V. C., Pokorny J. Spectral sensitivity of the foveal cone photopigments between 400 and 500 nm. Vision Res. 1975 Feb;15(2):161–171. doi: 10.1016/0042-6989(75)90203-5. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Cole G. R., Kronauer R. E. Chromatic suppression of cone inputs to the luminance flicker mechanism. Vision Res. 1987;27(7):1113–1137. doi: 10.1016/0042-6989(87)90026-5. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Cole G. R., Kronauer R. E. Second-site adaptation in the red-green chromatic pathways. Vision Res. 1985;25(2):219–237. doi: 10.1016/0042-6989(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Eskew R. T., Jr, Kronauer R. E., Spillmann L. Temporal phase response of the short-wave cone signal for color and luminance. Vision Res. 1991;31(5):787–803. doi: 10.1016/0042-6989(91)90147-w. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Kronauer R. E., Madsen J. C., Klein S. A. Opponent-movement mechanisms in human vision. J Opt Soc Am A. 1984 Aug;1(8):876–884. doi: 10.1364/josaa.1.000876. [DOI] [PubMed] [Google Scholar]
- Stromeyer C. F., 3rd, Lee J., Eskew R. T., Jr Peripheral chromatic sensitivity for flashes: a post-receptoral red-green asymmetry. Vision Res. 1992 Oct;32(10):1865–1873. doi: 10.1016/0042-6989(92)90047-m. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Pokorny J., Smith V. C. Effects of chromatic adaptation on phase-dependent sensitivity to heterochromatic flicker. J Opt Soc Am A. 1988 Nov;5(11):1976–1982. doi: 10.1364/josaa.5.001976. [DOI] [PubMed] [Google Scholar]
- Thornton J. E., Pugh E. N., Jr Red/Green color opponency at detection threshold. Science. 1983 Jan 14;219(4581):191–193. doi: 10.1126/science.6849131. [DOI] [PubMed] [Google Scholar]
- Watson A. B., Thompson P. G., Murphy B. J., Nachmias J. Summation and discrimination of gratings moving in opposite directions. Vision Res. 1980;20(4):341–347. doi: 10.1016/0042-6989(80)90020-6. [DOI] [PubMed] [Google Scholar]
- Webster M. A., Mollon J. D. Contrast adaptation dissociates different measures of luminous efficiency. J Opt Soc Am A. 1993 Jun;10(6):1332–1340. doi: 10.1364/josaa.10.001332. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]


