Abstract
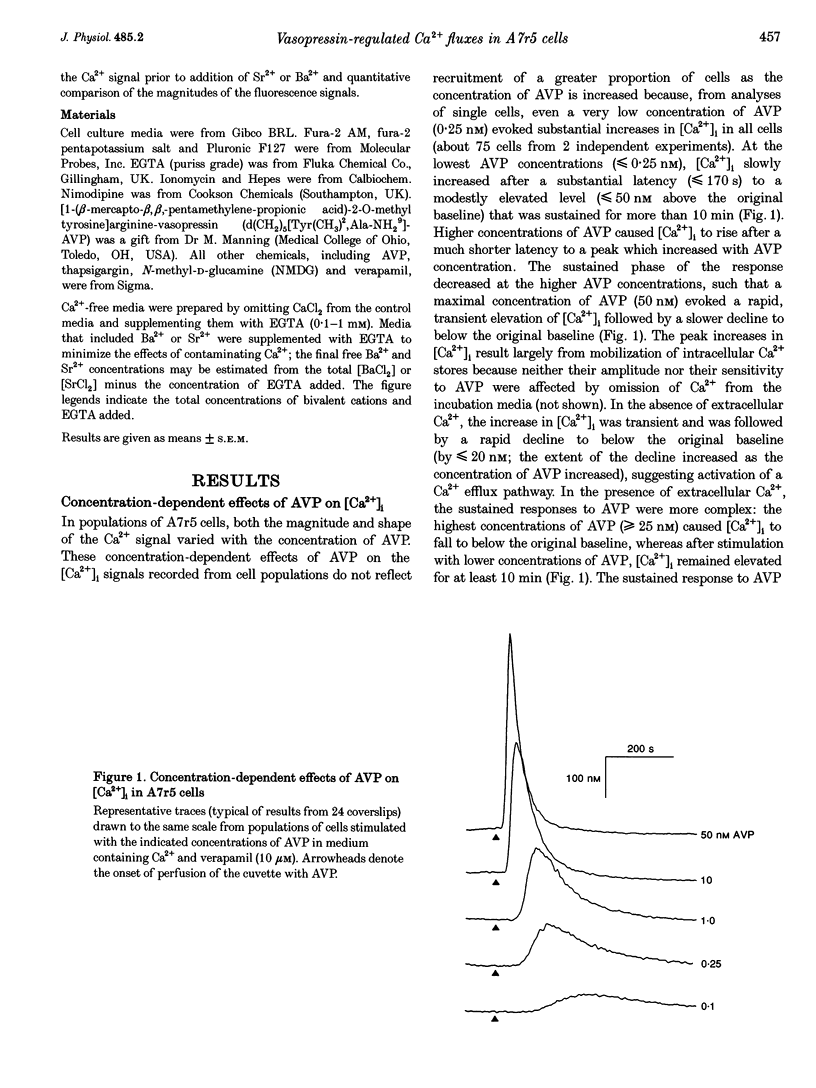
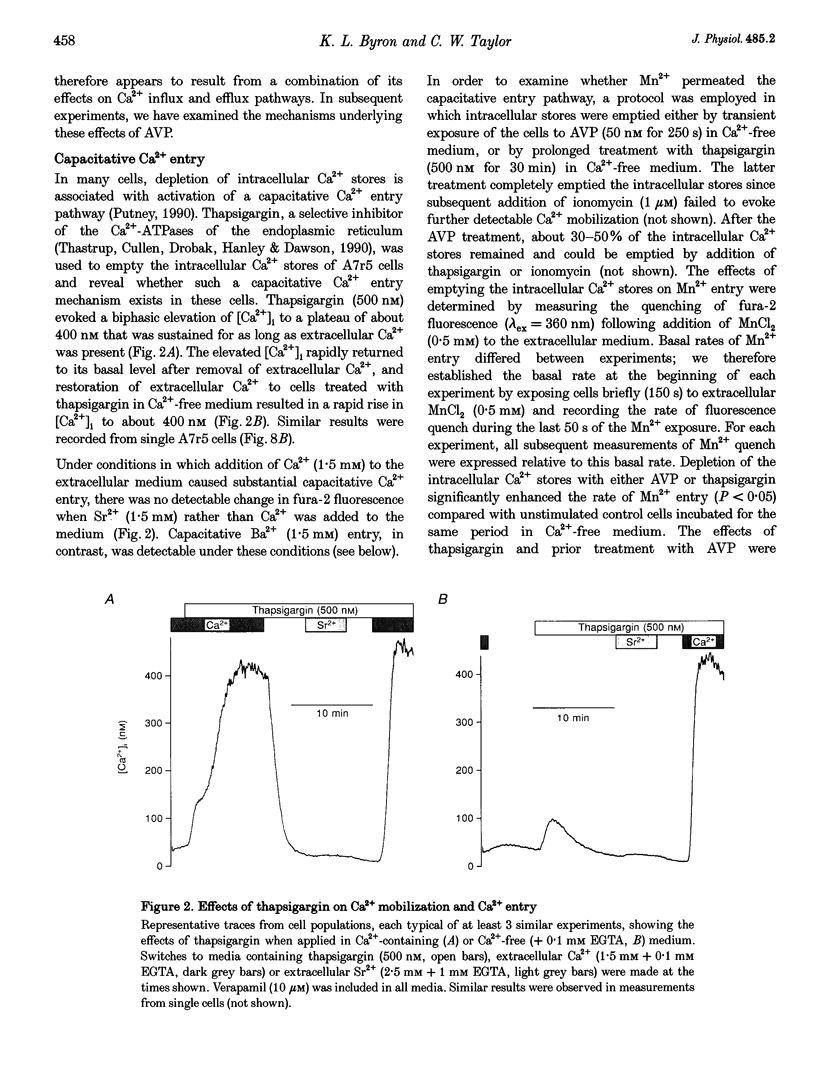
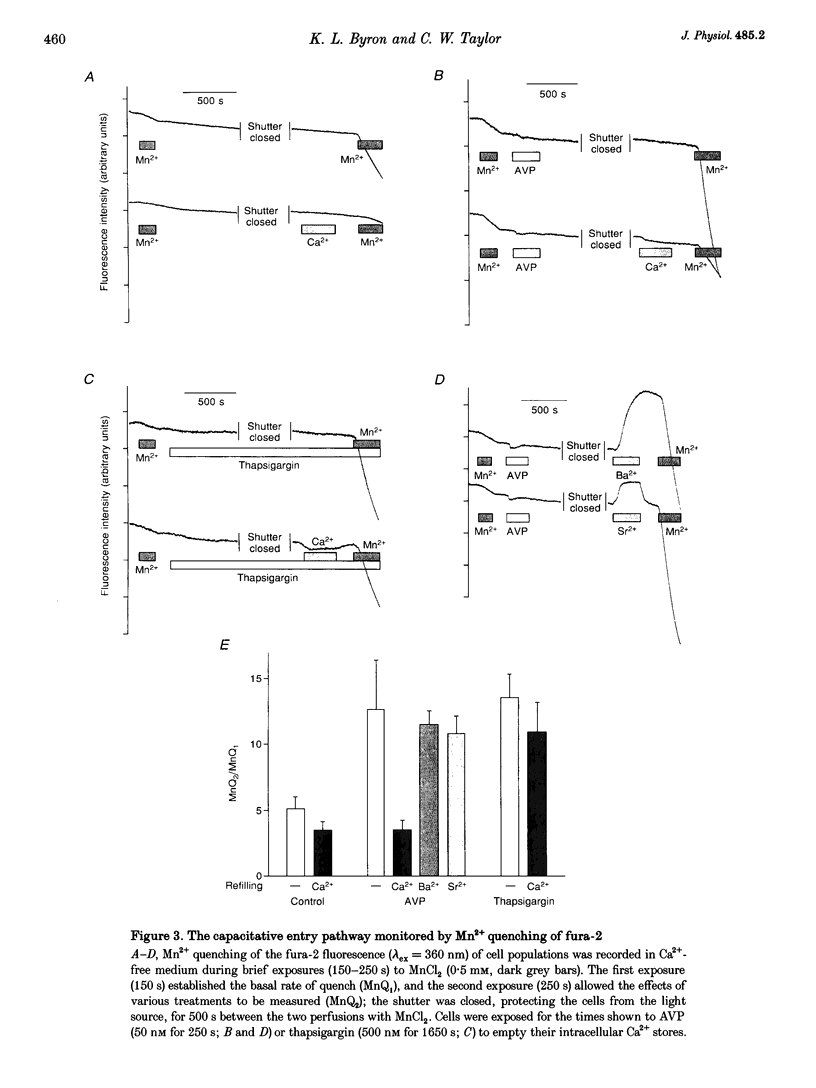
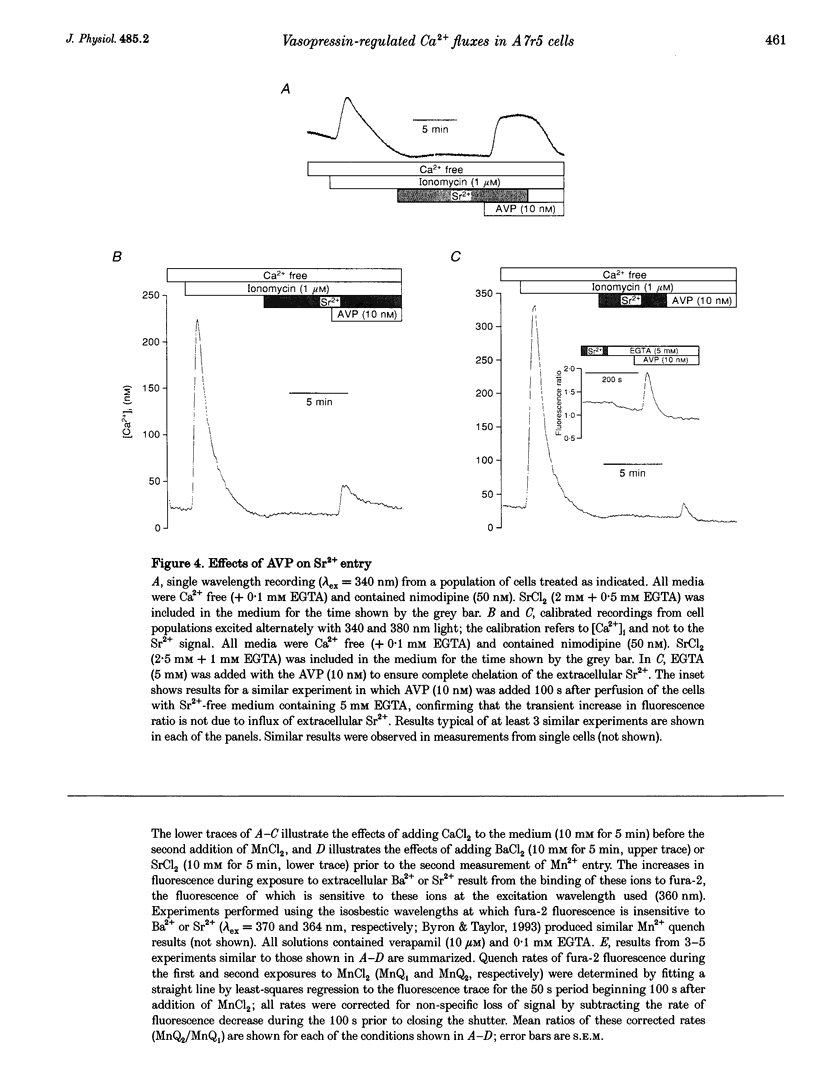
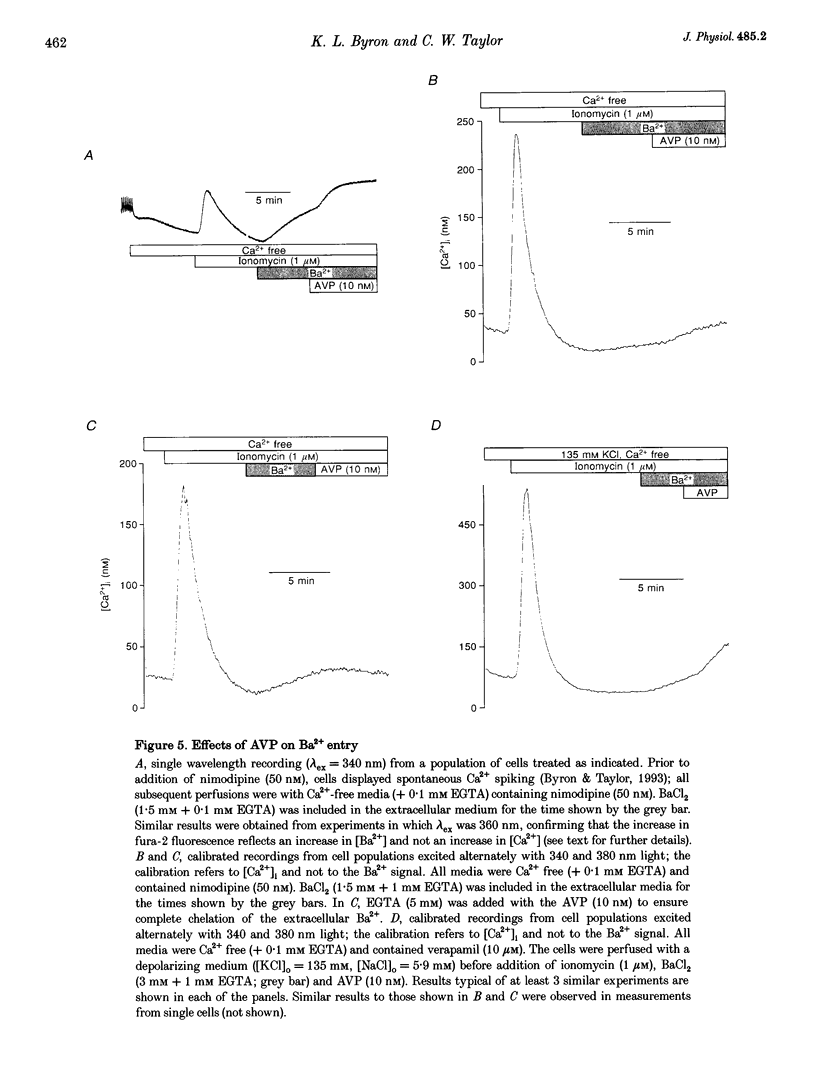
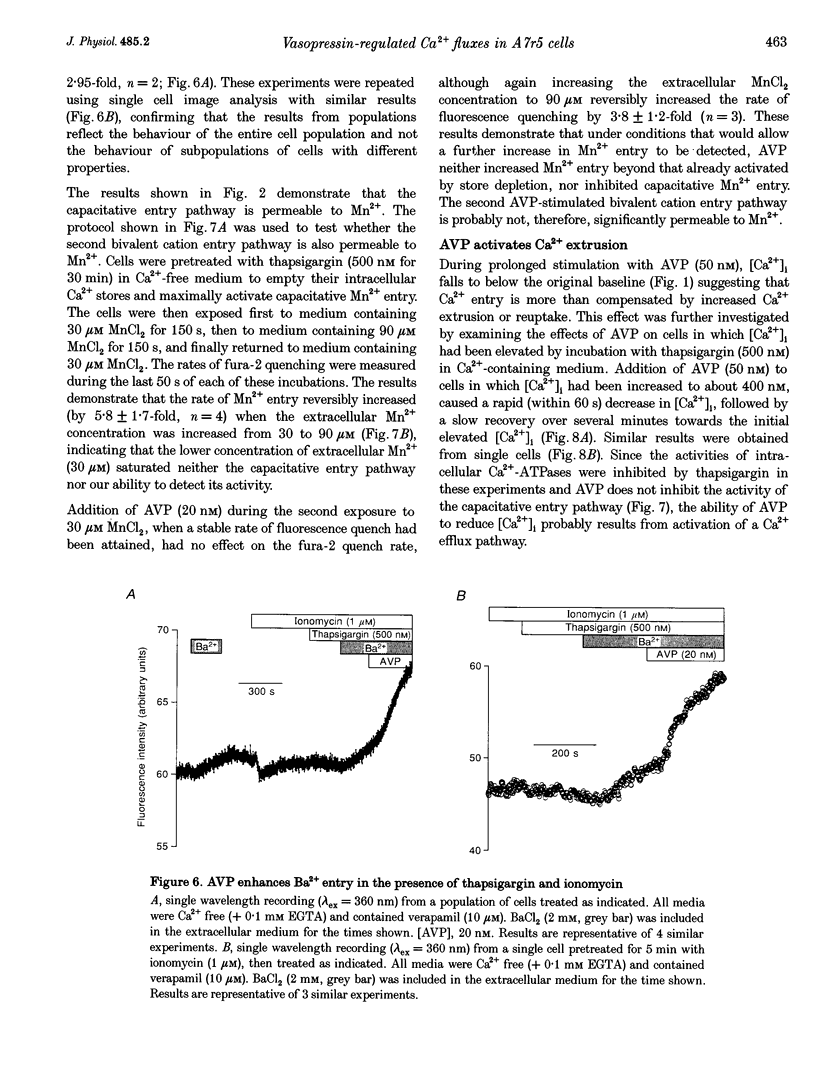
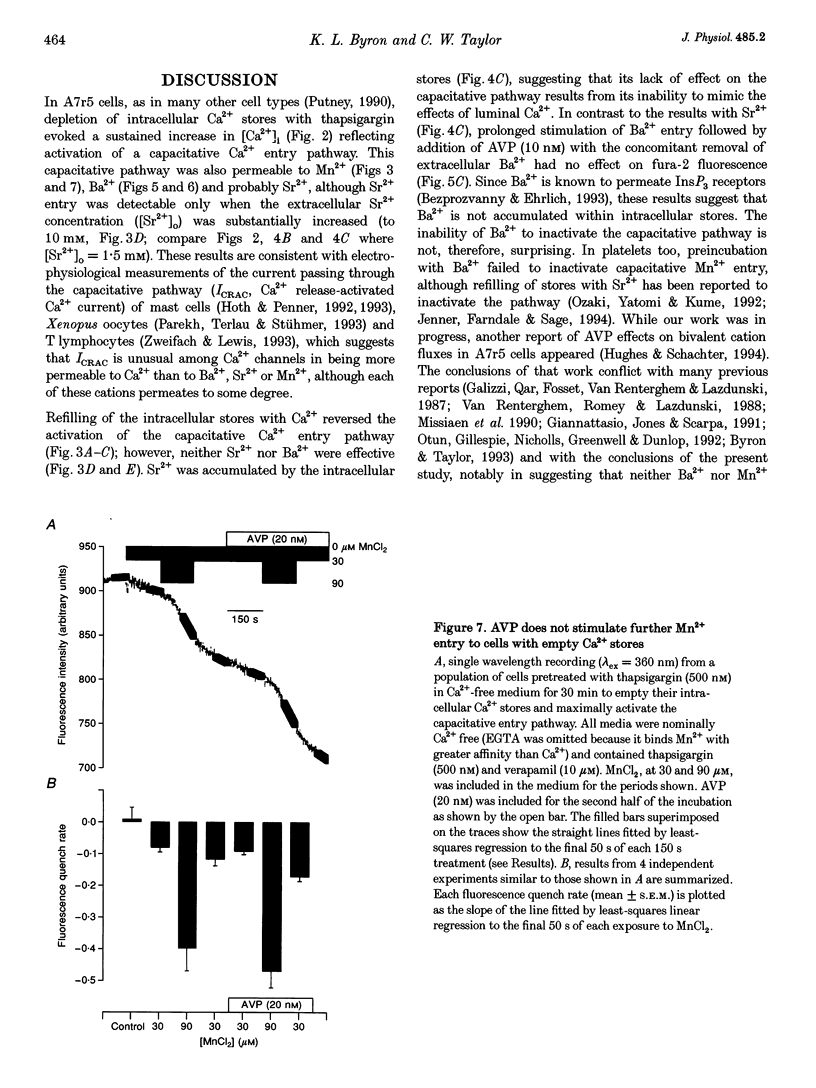
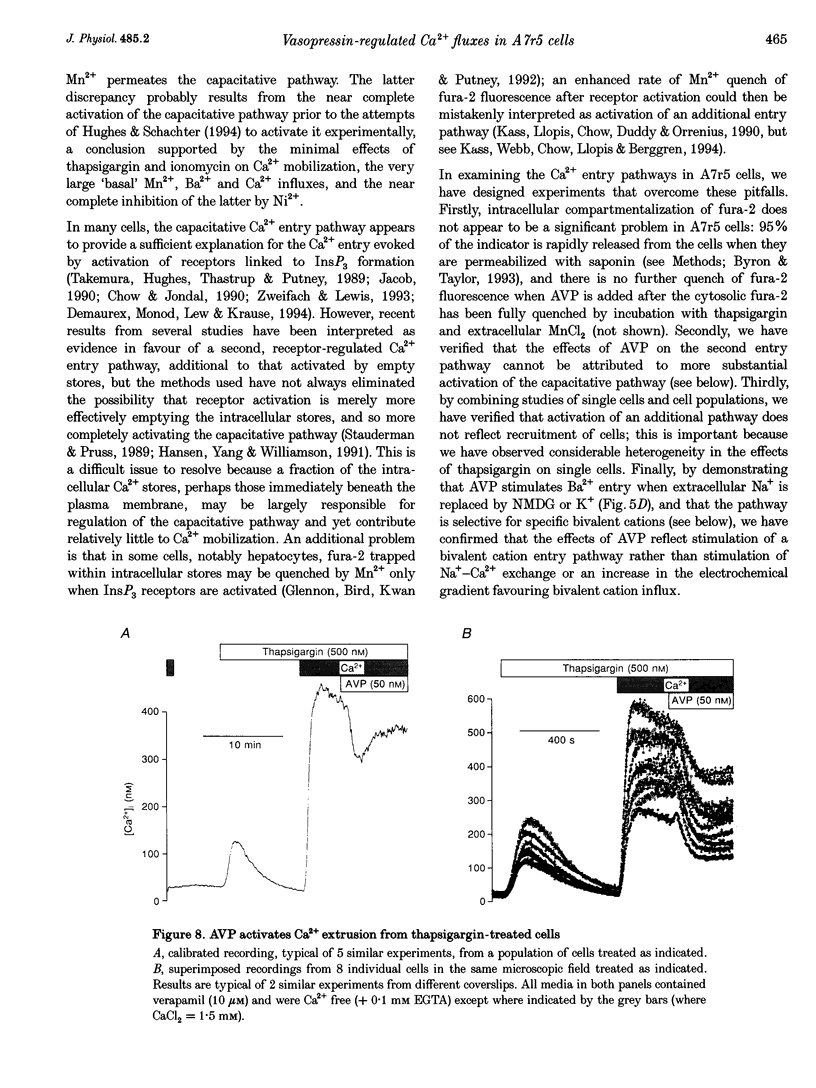
1. Arg8-vasopressin (AVP)-regulated Ca2+ transport were investigated in fura-2-loaded A7r5 cells using both single cell and population measurements. 2. AVP evokes an initial concentration-dependent rise in cytosolic free Ca2+ concentration ([Ca2+ ]i) to a peak which is independent of extracellular Ca2+, and a sustained Ca2+ signal that results from a balance between stimulation of Ca2+ entry and efflux. 3. Depletion of intracellular Ca2+ stores with thapsigargin, ionomycin, or prior treatment with AVP in Ca2(+)-free medium activates 'capacitative' entry of Ca2+, Ba2+ or Mn2+. Capacitative Mn2+ entry is inhibited by refilling stores with Ca2+; neither Sr2+ nor Ba2+ substitute for Ca2+ to give this effect. 4. In cells with empty stores, AVP stimulates further bivalent cation entry, and the effect persists when extracellular Na+ is replaced by N-methyl-D-glucamine or under depolarizing condition (extracellular KCl concentration ([KCl]o), 135 mM). This effect of AVP is not therefore merely a consequence of AVP causing membrane hyperpolarization or stimulation of Na(+)-Ca2+ exchange, but results from opening of a bivalent cation influx pathway. 5. Several lines of evidence indicate that AVP-stimulated bivalent cation entry is not a consequence of more complete emptying of the intracellular stores and consequent further activation of the capacitative pathway. AVP stimulates Ba2+ entry when the intracellular Ca2+ stores have been both emptied by ionomycin and prevented from refilling by thapsigargin. Mn2+ permeates the capacitative pathway, but AVP does not further increase Mn2+ entry, confirming that AVP does not further activate the capacitative pathway and that the two pathways differ in their permeability to Mn2+. When the extracellular [Sr2+] is low, empty stores do not stimulate detectable Sr2+ entry, but addition of AVP causes substantial Sr2+ entry. 6. A decrease in [Ca2+]i occurs when 50 nM AVP is added during a sustained elevation of [Ca2+]i evoked by thapsigargin. Since AVP does not inhibit the capacitative pathway, this result suggests that AVP stimulates Ca2+ extrusion. 7. We conclude that stimulation of Ca2+ mobilization, two modes of bivalent cation entry, and Ca2+ efflux all contribute to the complex concentration-dependent effects of AVP in A7r5 smooth muscle cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bird G. S., Rossier M. F., Hughes A. R., Shears S. B., Armstrong D. L., Putney J. W., Jr Activation of Ca2+ entry into acinar cells by a non-phosphorylatable inositol trisphosphate. Nature. 1991 Jul 11;352(6331):162–165. doi: 10.1038/352162a0. [DOI] [PubMed] [Google Scholar]
- Byron K. L., Taylor C. W. Spontaneous Ca2+ spiking in a vascular smooth muscle cell line is independent of the release of intracellular Ca2+ stores. J Biol Chem. 1993 Apr 5;268(10):6945–6952. [PubMed] [Google Scholar]
- Byron K. L., Villereal M. L. Mitogen-induced [Ca2+]i changes in individual human fibroblasts. Image analysis reveals asynchronous responses which are characteristic for different mitogens. J Biol Chem. 1989 Oct 25;264(30):18234–18239. [PubMed] [Google Scholar]
- Chow S. C., Jondal M. Ca2+ entry in T cells is activated by emptying the inositol 1,4,5-triphosphate sensitive Ca2+ pool. Cell Calcium. 1990 Nov-Dec;11(10):641–646. doi: 10.1016/0143-4160(90)90018-p. [DOI] [PubMed] [Google Scholar]
- Clementi E., Scheer H., Zacchetti D., Fasolato C., Pozzan T., Meldolesi J. Receptor-activated Ca2+ influx. Two independently regulated mechanisms of influx stimulation coexist in neurosecretory PC12 cells. J Biol Chem. 1992 Feb 5;267(4):2164–2172. [PubMed] [Google Scholar]
- Demaurex N., Monod A., Lew D. P., Krause K. H. Characterization of receptor-mediated and store-regulated Ca2+ influx in human neutrophils. Biochem J. 1994 Feb 1;297(Pt 3):595–601. doi: 10.1042/bj2970595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Poulter M. O., Wess J. Muscarinic receptor-operated Ca2+ influx in transfected fibroblast cells is independent of inositol phosphates and release of intracellular Ca2+. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):509–513. doi: 10.1073/pnas.89.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizzi J. P., Qar J., Fosset M., Van Renterghem C., Lazdunski M. Regulation of calcium channels in aortic muscle cells by protein kinase C activators (diacylglycerol and phorbol esters) and by peptides (vasopressin and bombesin) that stimulate phosphoinositide breakdown. J Biol Chem. 1987 May 25;262(15):6947–6950. [PubMed] [Google Scholar]
- Giannattasio B., Jones S. W., Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Calcium-dependent and voltage-dependent inactivation. J Gen Physiol. 1991 Nov;98(5):987–1003. doi: 10.1085/jgp.98.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon M. C., Bird G. S., Kwan C. Y., Putney J. W., Jr Actions of vasopressin and the Ca(2+)-ATPase inhibitor, thapsigargin, on Ca2+ signaling in hepatocytes. J Biol Chem. 1992 Apr 25;267(12):8230–8233. [PubMed] [Google Scholar]
- Hansen C. A., Yang L. J., Williamson J. R. Mechanisms of receptor-mediated Ca2+ signaling in rat hepatocytes. J Biol Chem. 1991 Oct 5;266(28):18573–18579. [PubMed] [Google Scholar]
- Hoth M., Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993 Jun;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hughes A. D., Schachter M. Multiple pathways for entry of calcium and other divalent cations in a vascular smooth muscle cell line (A7r5). Cell Calcium. 1994 Apr;15(4):317–330. doi: 10.1016/0143-4160(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Cullen P. J. Will the real IP4 receptor please stand up? Curr Biol. 1993 Aug 1;3(8):540–543. doi: 10.1016/0960-9822(93)90052-p. [DOI] [PubMed] [Google Scholar]
- Jacob R. Agonist-stimulated divalent cation entry into single cultured human umbilical vein endothelial cells. J Physiol. 1990 Feb;421:55–77. doi: 10.1113/jphysiol.1990.sp017933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner S., Farndale R. W., Sage S. O. The effect of calcium-store depletion and refilling with various bivalent cations on tyrosine phosphorylation and Mn2+ entry in fura-2-loaded human platelets. Biochem J. 1994 Oct 15;303(Pt 2):337–339. doi: 10.1042/bj3030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kass G. E., Webb D. L., Chow S. C., Llopis J., Berggren P. O. Receptor-mediated Mn2+ influx in rat hepatocytes: comparison of cells loaded with Fura-2 ester and cells microinjected with Fura-2 salt. Biochem J. 1994 Aug 15;302(Pt 1):5–9. doi: 10.1042/bj3020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Klein M. G., Schneider M. F., Snyder S. H. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992 Aug 7;257(5071):815–818. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976 Mar 15;98(2):349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Liu C., Hermann T. E. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978 Sep 10;253(17):5892–5894. [PubMed] [Google Scholar]
- Lückhoff A., Clapham D. E. Inositol 1,3,4,5-tetrakisphosphate activates an endothelial Ca(2+)-permeable channel. Nature. 1992 Jan 23;355(6358):356–358. doi: 10.1038/355356a0. [DOI] [PubMed] [Google Scholar]
- Manning M., Stoev S., Bankowski K., Misicka A., Lammek B., Wo N. C., Sawyer W. H. Synthesis and some pharmacological properties of potent and selective antagonists of the vasopressor (V1-receptor) response to arginine-vasopressin. J Med Chem. 1992 Jan 24;35(2):382–388. doi: 10.1021/jm00080a027. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oike M., Kitamura K., Kuriyama H. Protein kinase C activates the non-selective cation channel in the rabbit portal vein. Pflugers Arch. 1993 Jul;424(2):159–164. doi: 10.1007/BF00374607. [DOI] [PubMed] [Google Scholar]
- Otun H., Gillespie J. I., Nicholls J. A., Greenwell J. R., Dunlop W. Transients in intracellular free calcium in subconfluent and confluent cultures of a rat smooth muscle cell line. Exp Physiol. 1992 Sep;77(5):749–756. doi: 10.1113/expphysiol.1992.sp003641. [DOI] [PubMed] [Google Scholar]
- Ozaki Y., Yatomi Y., Kume S. Evaluation of platelet calcium ion mobilization by the use of various divalent ions. Cell Calcium. 1992 Jan;13(1):19–27. doi: 10.1016/0143-4160(92)90026-o. [DOI] [PubMed] [Google Scholar]
- Palade P., Dettbarn C., Brunder D., Stein P., Hals G. Pharmacology of calcium release from sarcoplasmic reticulum. J Bioenerg Biomembr. 1989 Apr;21(2):295–320. doi: 10.1007/BF00812074. [DOI] [PubMed] [Google Scholar]
- Parekh A. B., Terlau H., Stühmer W. Depletion of InsP3 stores activates a Ca2+ and K+ current by means of a phosphatase and a diffusible messenger. Nature. 1993 Aug 26;364(6440):814–818. doi: 10.1038/364814a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Fasolato C., Hoth M. Calcium influx and its control by calcium release. Curr Opin Neurobiol. 1993 Jun;3(3):368–374. doi: 10.1016/0959-4388(93)90130-q. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sill J. C., Eskuri S., Nelson R., Tarara J., Van Dyke R. A. The volatile anesthetic isoflurane attenuates Ca++ mobilization in cultured vascular smooth muscle cells. J Pharmacol Exp Ther. 1993 Apr;265(1):74–80. [PubMed] [Google Scholar]
- Stauderman K. A., Pruss R. M. Dissociation of Ca2+ entry and Ca2+ mobilization responses to angiotensin II in bovine adrenal chromaffin cells. J Biol Chem. 1989 Nov 5;264(31):18349–18355. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibonnier M., Bayer A. L., Simonson M. S., Kester M. Multiple signaling pathways of V1-vascular vasopressin receptors of A7r5 cells. Endocrinology. 1991 Dec;129(6):2845–2856. doi: 10.1210/endo-129-6-2845. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Romey G., Lazdunski M. Vasopressin modulates the spontaneous electrical activity in aortic cells (line A7r5) by acting on three different types of ionic channels. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9365–9369. doi: 10.1073/pnas.85.23.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A., Lewis R. S. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]