Abstract
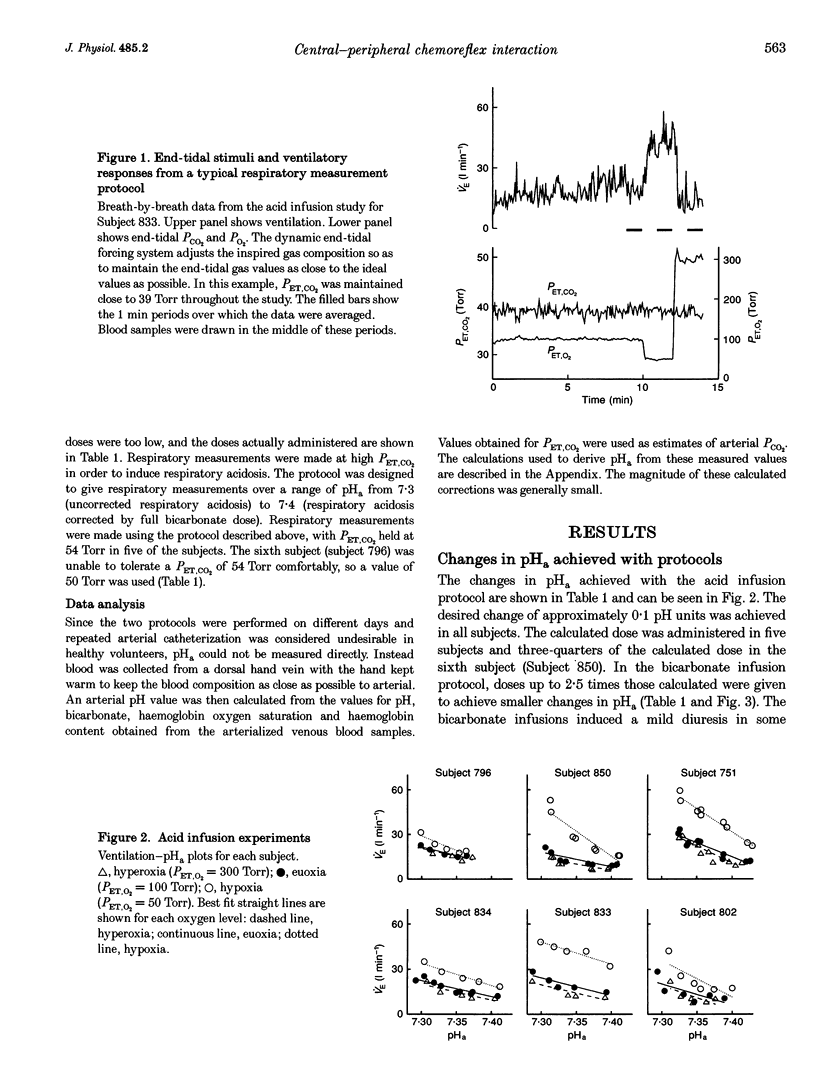
1. The object of this study was to investigate the effect of central chemoreceptor stimulation on the ventilatory responses to peripheral chemoreceptor stimulation. 2. The level of central chemoreceptor stimulation was varied by performing experiments at two different levels of end-tidal CO2 pressure (PCO2). Variations in peripheral chemoreceptor stimulus were achieved by varying arterial pH (at constant end-tidal PCO2) and by varying end-tidal O2 pressure (PO2). 3. Two protocols were each performed on six human subjects. In one protocol ventilatory measurements were made during eucapnia, when the arterial pH was lowered from 7.4 to 7.3. The variation in pH was achieved by the progressive infusion of acid (0.1 M HCl). In the other protocol ventilatory measurements were made during hypercapnia, when the arterial pH was increased from 7.3 to 7.4. The variation in pH was achieved by the progressive infusion of 1.26% NaHCO3. In each protocol ventilatory responses were measured during euoxia (end-tidal PO2, 100 Torr), hypoxia (end-tidal PO2, 50 Torr) and hyperoxia (end-tidal PO2, 300 Torr), with end-tidal PCO2 held constant. 4. The increase in ventilatory sensitivity to arterial pH induced by hypoxia (50 Torr) was not significantly different between protocols (acid protocol, -104 +/- 31 l min-1 (pH unit)-1 vs. bicarbonate protocol, -60 +/- 44 l min-1 (pH unit)-1; mean +/- S.E.M.; not significant (n.s.)). The ventilatory sensitivity to hypoxia at an arterial pH of 7.35 was not significantly different between protocols (acid protocol, 14.7 +/- 3.3 l min-1 vs. bicarbonate protocol, 15.6 +/- 2.4 l min-1; mean +/- S.E.M.; n.s.). The results provide no evidence to suggest that peripheral chemoreflex ventilatory responses are modulated by central chemoreceptor stimulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellville J. W., Whipp B. J., Kaufman R. D., Swanson G. D., Aqleh K. A., Wiberg D. M. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol Respir Environ Exerc Physiol. 1979 Apr;46(4):843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- Clement I. D., Bascom D. A., Conway J., Dorrington K. L., O'Connor D. F., Painter R., Paterson D. J., Robbins P. A. An assessment of central-peripheral ventilatory chemoreflex interaction in humans. Respir Physiol. 1992 Apr-May;88(1-2):87–100. doi: 10.1016/0034-5687(92)90031-q. [DOI] [PubMed] [Google Scholar]
- Dahan A., DeGoede J., Berkenbosch A., Olievier I. C. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol. 1990 Sep;428:485–499. doi: 10.1113/jphysiol.1990.sp018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly D. F., Smith E., Dutton R. E. Carbon dioxide versus H ion as a chemoreceptor stimulus. Brain Res. 1982 Aug 5;245(1):136–138. doi: 10.1016/0006-8993(82)90347-x. [DOI] [PubMed] [Google Scholar]
- Eldridge F. L., Kiley J. P., Millhorn D. E. Respiratory responses to medullary hydrogen ion changes in cats: different effects of respiratory and metabolic acidoses. J Physiol. 1985 Jan;358:285–297. doi: 10.1113/jphysiol.1985.sp015551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamnei S., Robbins P. A. Hypoxic depression of ventilation in humans: alternative models for the chemoreflexes. Respir Physiol. 1990 Jul;81(1):117–134. doi: 10.1016/0034-5687(90)90074-9. [DOI] [PubMed] [Google Scholar]
- Knill R. L., Clement J. L. Ventilatory responses to acute metabolic acidemia in humans awake, sedated, and anesthetized with halothane. Anesthesiology. 1985 Jun;62(6):745–753. doi: 10.1097/00000542-198506000-00008. [DOI] [PubMed] [Google Scholar]
- LAMBERTSEN C. J., SEMPLE S. J., SMYTH M. G., GELFAND R. H and pCO2 as chemical factors in respiratory and cerebral circulatory control. J Appl Physiol. 1961 May;16:473–484. doi: 10.1152/jappl.1961.16.3.473. [DOI] [PubMed] [Google Scholar]
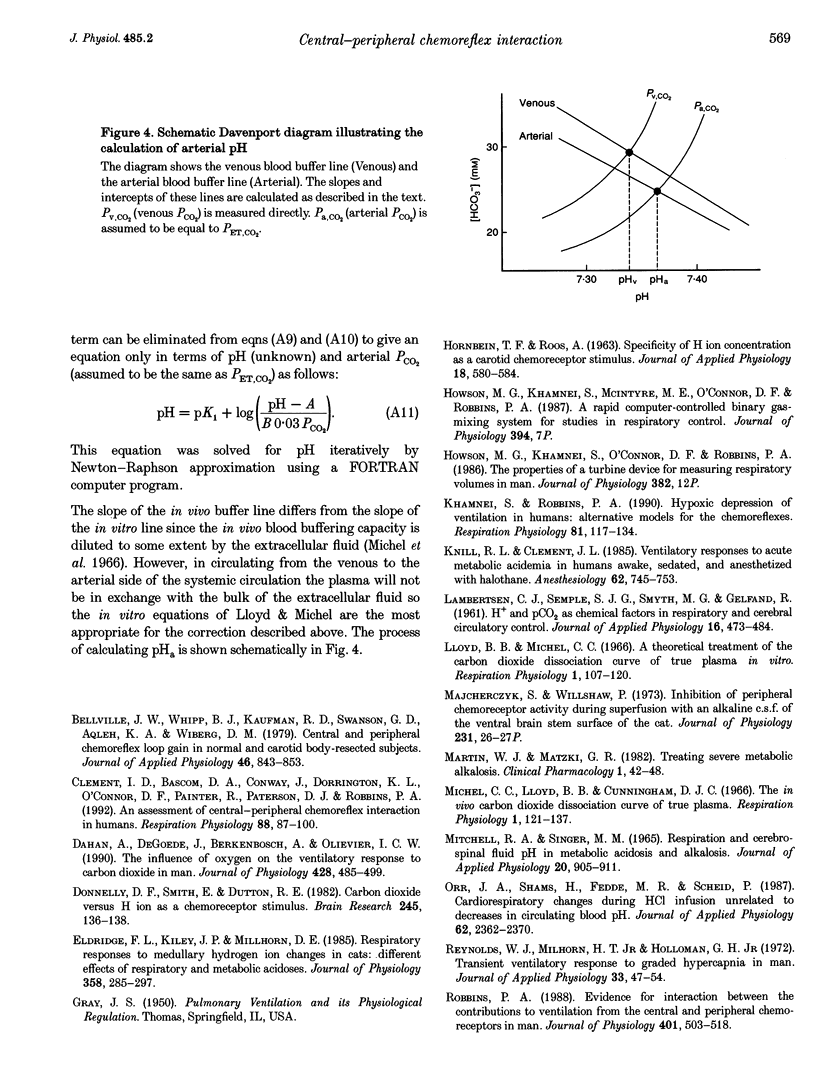
- Lloyd B. B., Michel C. C. A theoretical treatment of the carbon dioxide dissociation curve of true plasma in vitro. Respir Physiol. 1966;1(2):107–120. doi: 10.1016/0034-5687(66)90010-7. [DOI] [PubMed] [Google Scholar]
- Majcherczyk S., Willshaw P. Inhibition of peripheral chemoreceptor activity during superfusion with an alkaline c.s.f. of the ventral brain stem surface of the cat. J Physiol. 1973 May;231(1):26P–27P. [PubMed] [Google Scholar]
- Martin W. J., Matzke G. R. Treating severe metabolic alkalosis. Clin Pharm. 1982 Jan-Feb;1(1):42–48. [PubMed] [Google Scholar]
- Michel C. C., Lloyd B. B., Cunningham D. J. The in vivo carbon dioxide dissociation curve of true plasma. Respir Physiol. 1966;1(2):121–137. doi: 10.1016/0034-5687(66)90011-9. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Singer M. M. Respiration and cerebrospinal fluid pH in metabolic acidosis and alkalosis. J Appl Physiol. 1965 Sep;20(5):905–911. doi: 10.1152/jappl.1965.20.5.905. [DOI] [PubMed] [Google Scholar]
- Orr J. A., Shams H., Fedde M. R., Scheid P. Cardiorespiratory changes during HCl infusion unrelated to decreases in circulating blood pH. J Appl Physiol (1985) 1987 Jun;62(6):2362–2370. doi: 10.1152/jappl.1987.62.6.2362. [DOI] [PubMed] [Google Scholar]
- Reynolds W. J., Milhorn H. T., Jr, Holloman G. H., Jr Transient ventilatory response to graded hypercapnia in man. J Appl Physiol. 1972 Jul;33(1):47–54. doi: 10.1152/jappl.1972.33.1.47. [DOI] [PubMed] [Google Scholar]
- Robbins P. A. Evidence for interaction between the contributions to ventilation from the central and peripheral chemoreceptors in man. J Physiol. 1988 Jul;401:503–518. doi: 10.1113/jphysiol.1988.sp017175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. A., Swanson G. D., Howson M. G. A prediction-correction scheme for forcing alveolar gases along certain time courses. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1353–1357. doi: 10.1152/jappl.1982.52.5.1353. [DOI] [PubMed] [Google Scholar]
- Shams H., Peskar B. A., Scheid P. Acid infusion elicits thromboxane A2-mediated effects on respiration and pulmonary hemodynamics in the cat. Respir Physiol. 1988 Feb;71(2):169–183. doi: 10.1016/0034-5687(88)90014-x. [DOI] [PubMed] [Google Scholar]
- Teppema L. J., Barts P. W., Folgering H. T., Evers J. A. Effects of respiratory and (isocapnic) metabolic arterial acid-base disturbances on medullary extracellular fluid pH and ventilation in cats. Respir Physiol. 1983 Sep;53(3):379–395. doi: 10.1016/0034-5687(83)90127-5. [DOI] [PubMed] [Google Scholar]
- van Beek J. H., Berkenbosch A., de Goede J., Olievier C. N. Influence of peripheral O2 tension on the ventilatory response to CO2 in cats. Respir Physiol. 1983 Mar;51(3):379–390. doi: 10.1016/0034-5687(83)90030-0. [DOI] [PubMed] [Google Scholar]


