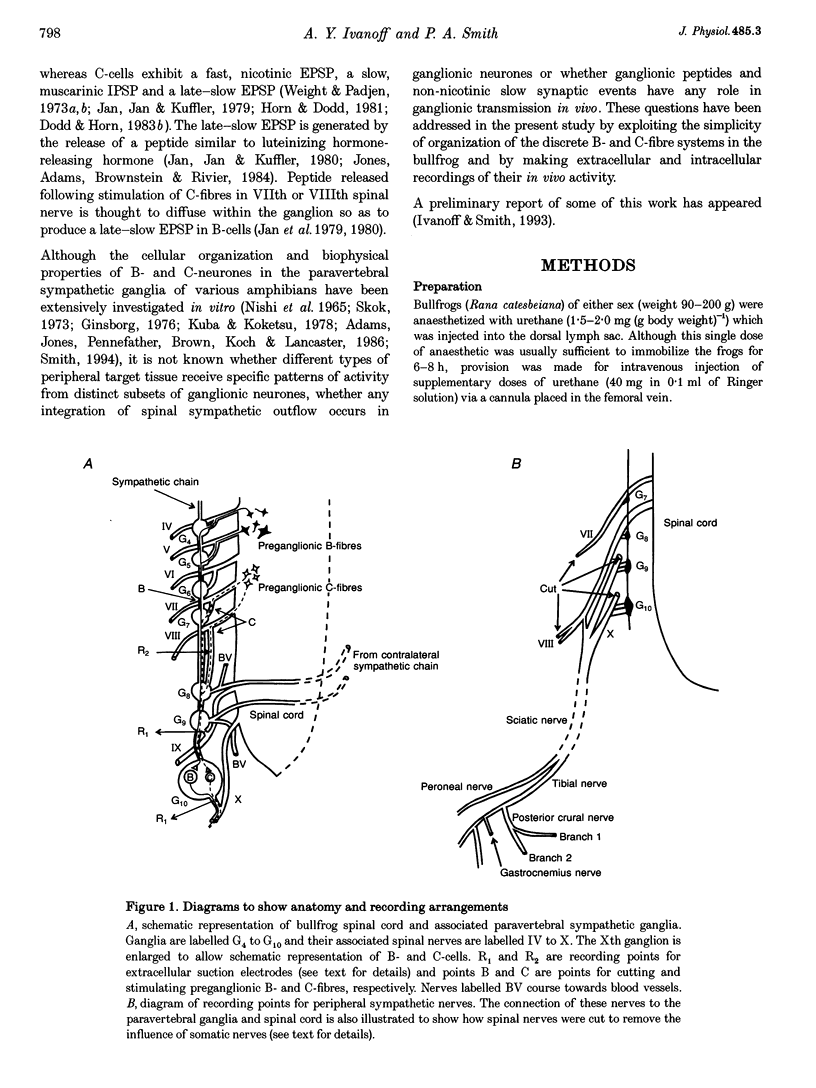
Abstract
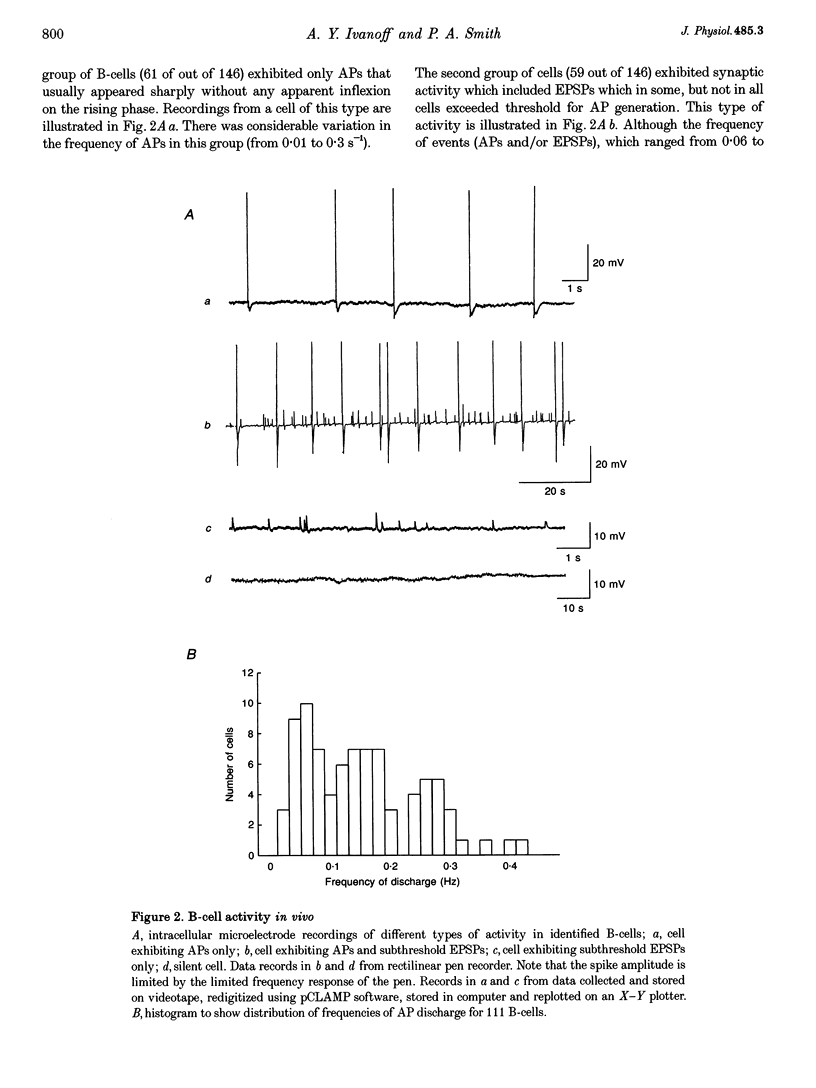
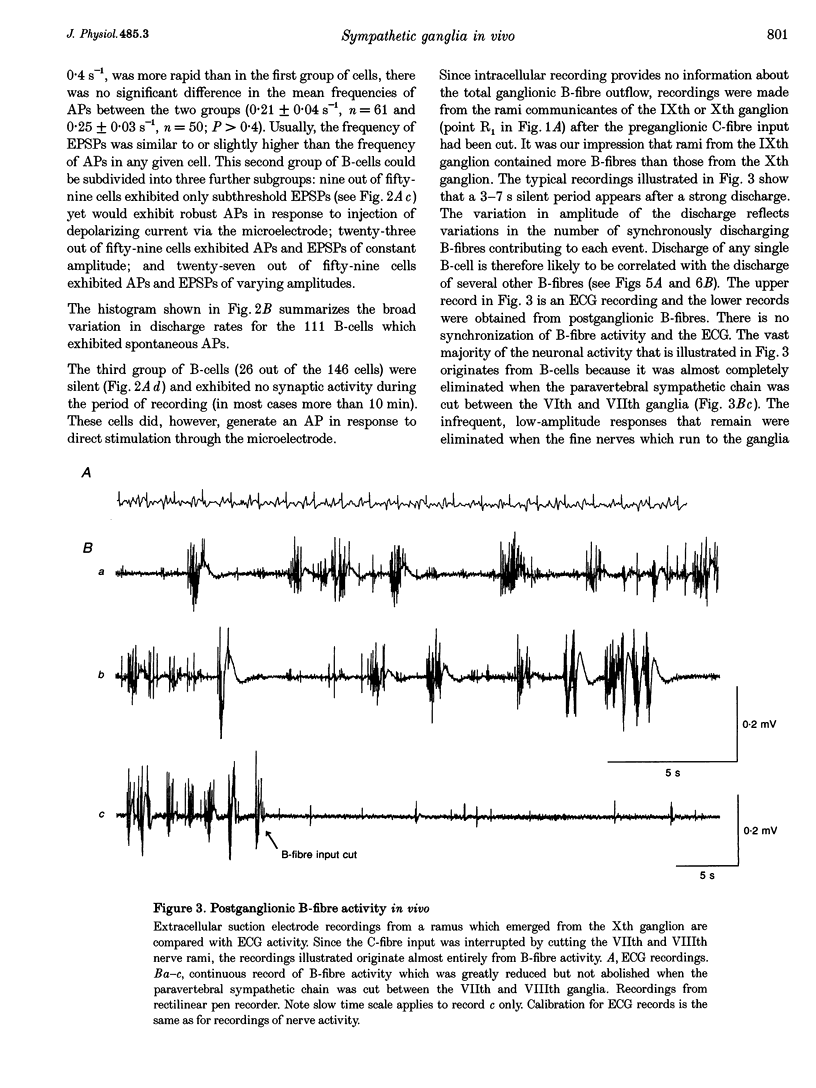
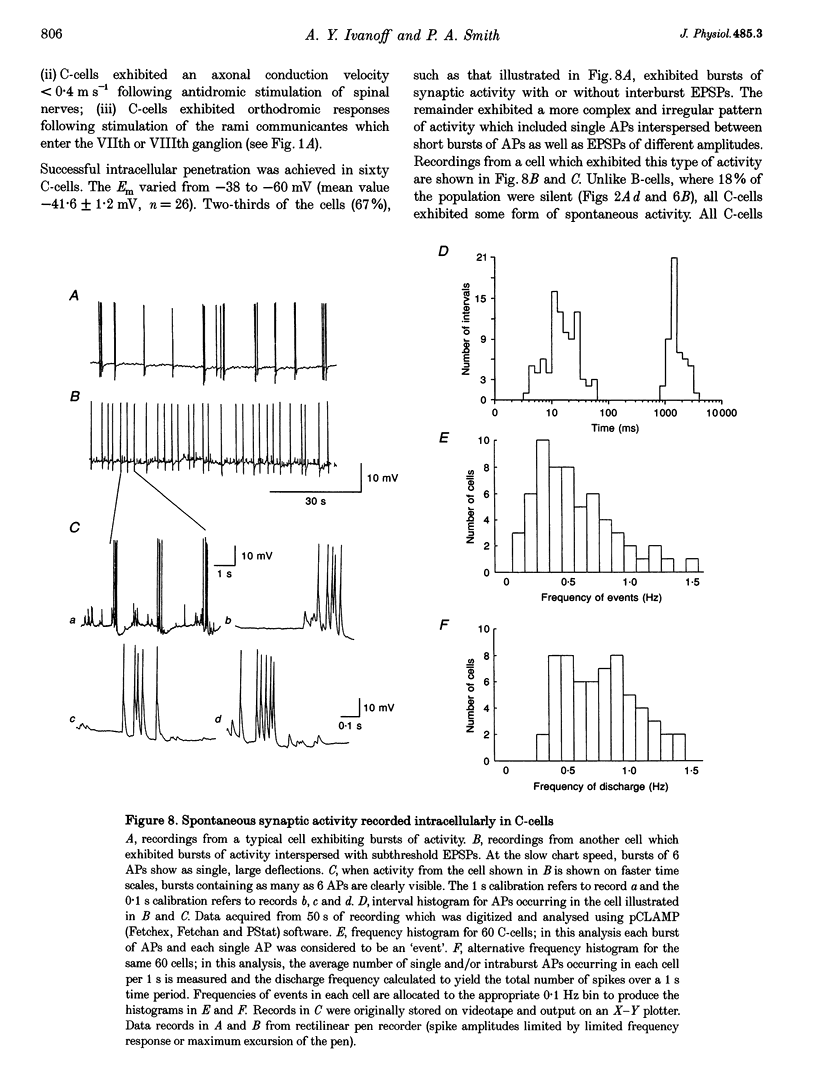
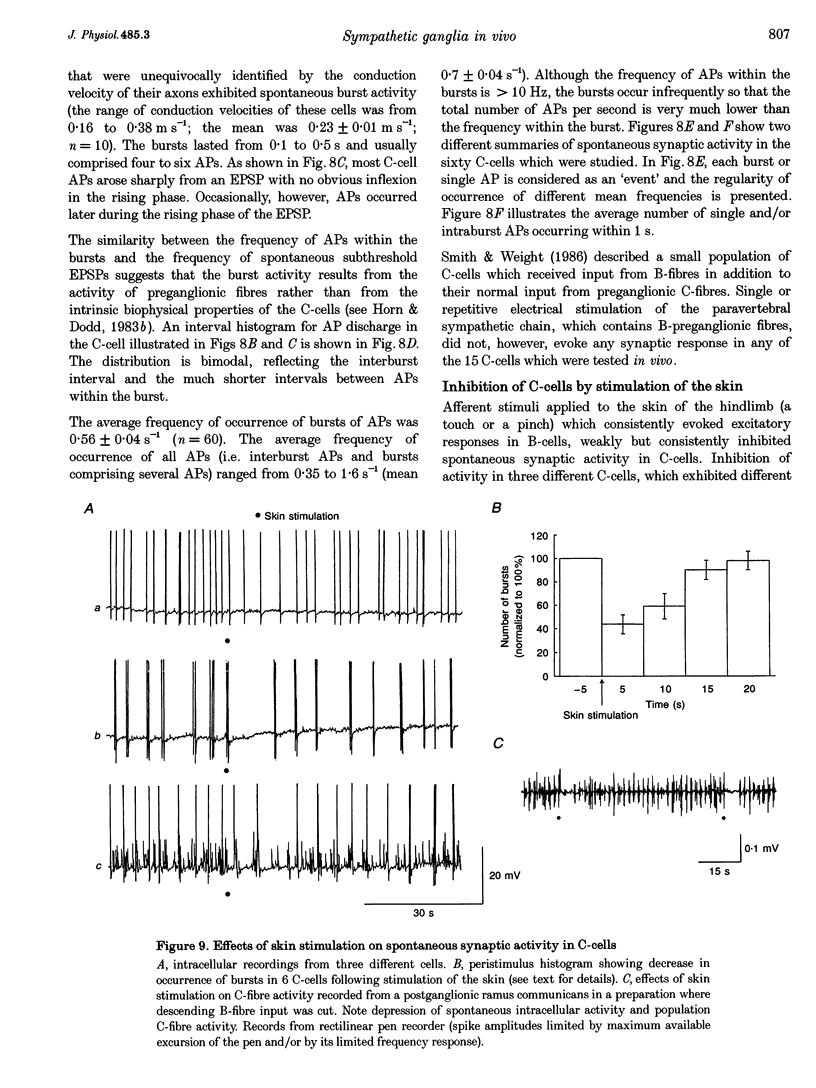
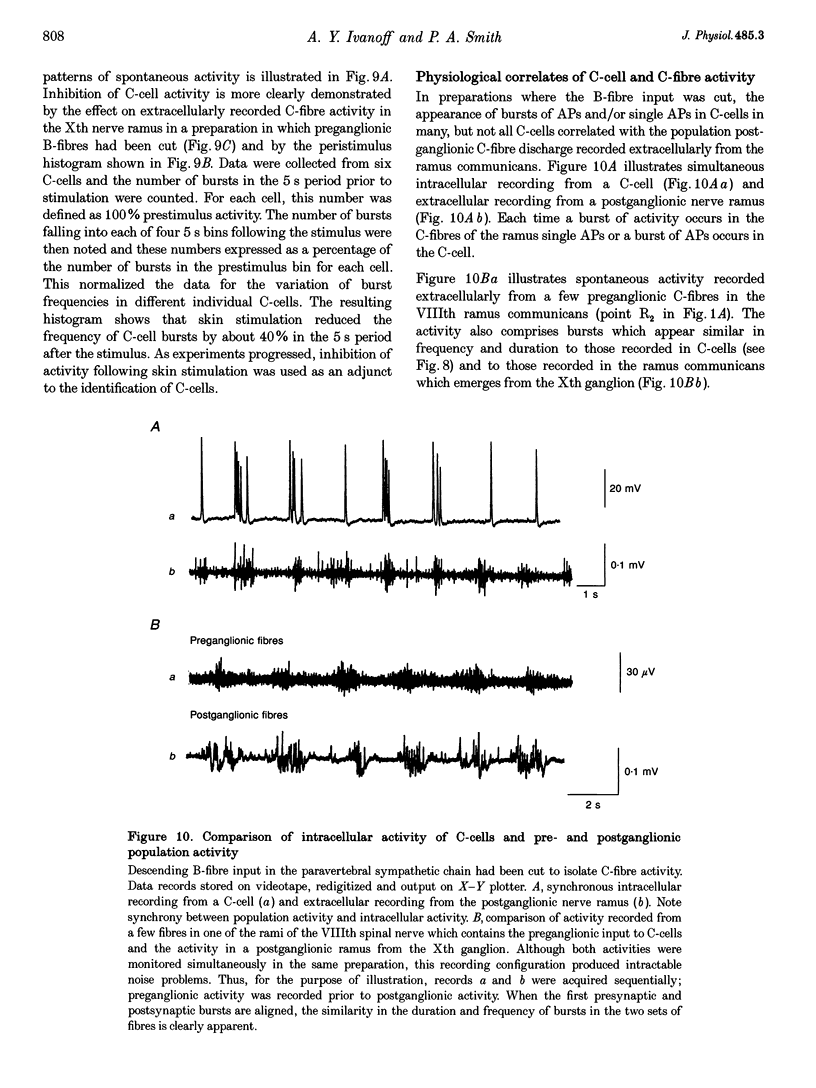
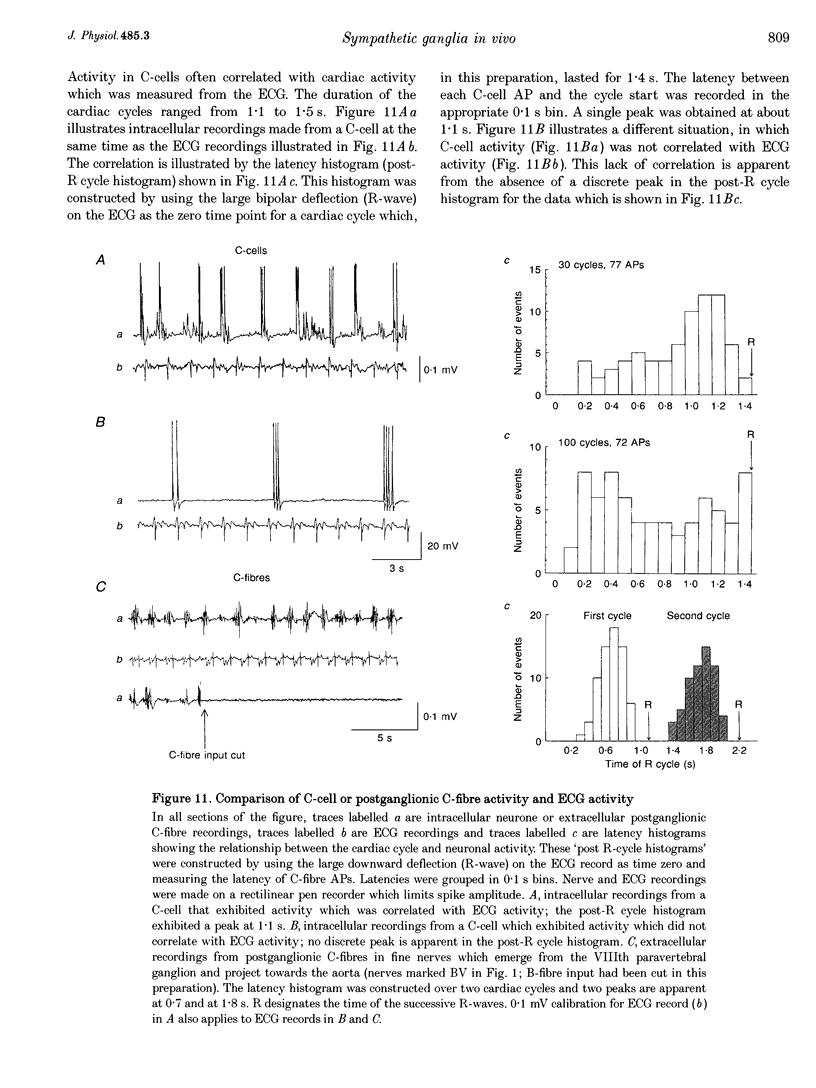
1. Spontaneous, in vivo synaptic activity was recorded from 146 B-cells and 60 C-cells in the IXth and Xth paravertebral sympathetic ganglia of the urethane-anaesthetized bullfrog. Sympathetic outflow to the blood vessels, which are innervated by C-cells, is different from that received by targets in the skin, which are innervated by B-cells. 2. B-cells were divided into three groups: the first (61 cells) exhibited only action potentials (APs) at 0.01-0.3 s-1; the second (59 cells) exhibited APs and EPSPs and the third (26 cells) were silent. In addition to their usual suprathreshold input from the ipsilateral sympathetic chain, 53% of B-cells received subthreshold input which probably arose from fibres in the contralateral chain. 'Slow' B-cells exhibited less subthreshold activity and a slightly higher AP frequency than 'fast' B-cells. All B-cells are involved in a sympathetic reflex which is activated by tactile stimulation of the skin of the hindlimb. Activation of this reflex increased AP frequency without promoting long-lasting depolarization. 3. Sixty-seven per cent of C-cells exhibited rhythmic bursting activity with or without small intraburst EPSPs. Bursts tended to correlate with electrocardiographic (ECG) activity. The remainder exhibited an irregular pattern of activity which was not correlated with ECG activity and which included one to three APs and EPSPs interspersed between the bursts. Activity of both types of C-cell was inhibited following stimulation of the skin. 4. An average of twenty-three B-cells and twenty-one C-cells discharge simultaneously in vivo. This reflects branching of preganglionic fibres and results in synchrony of discharge in both postganglionic B- and C-fibres.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

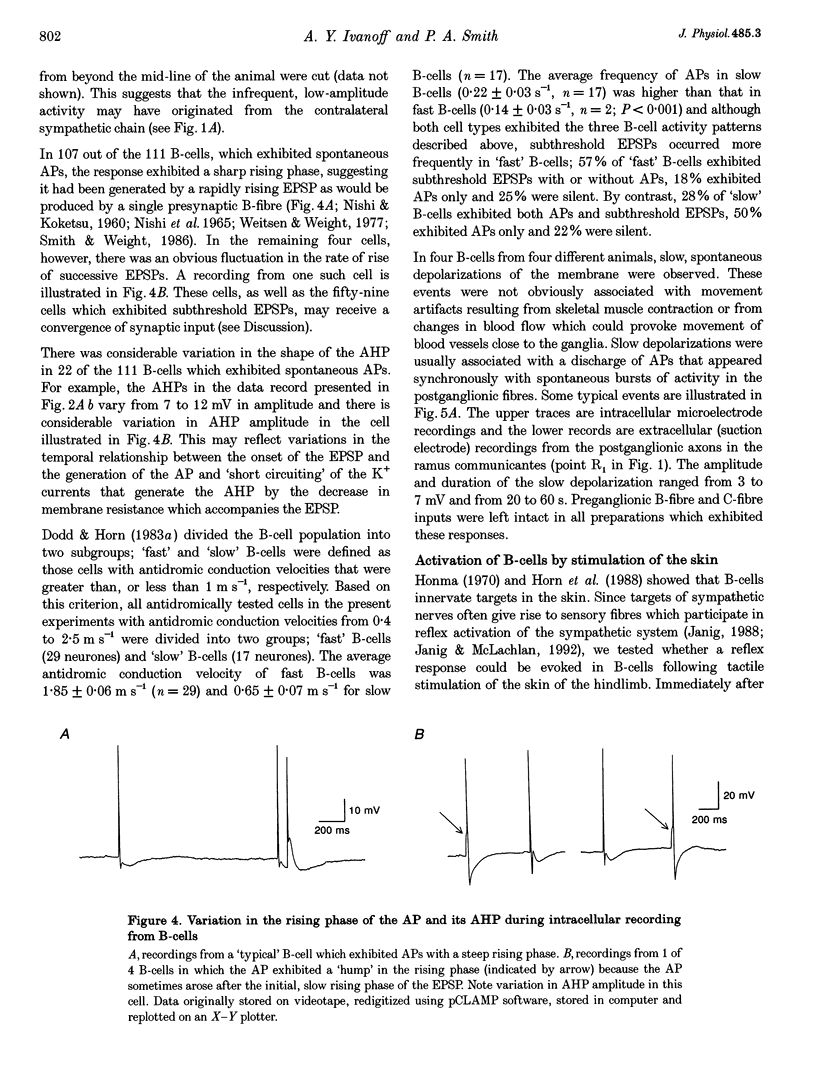
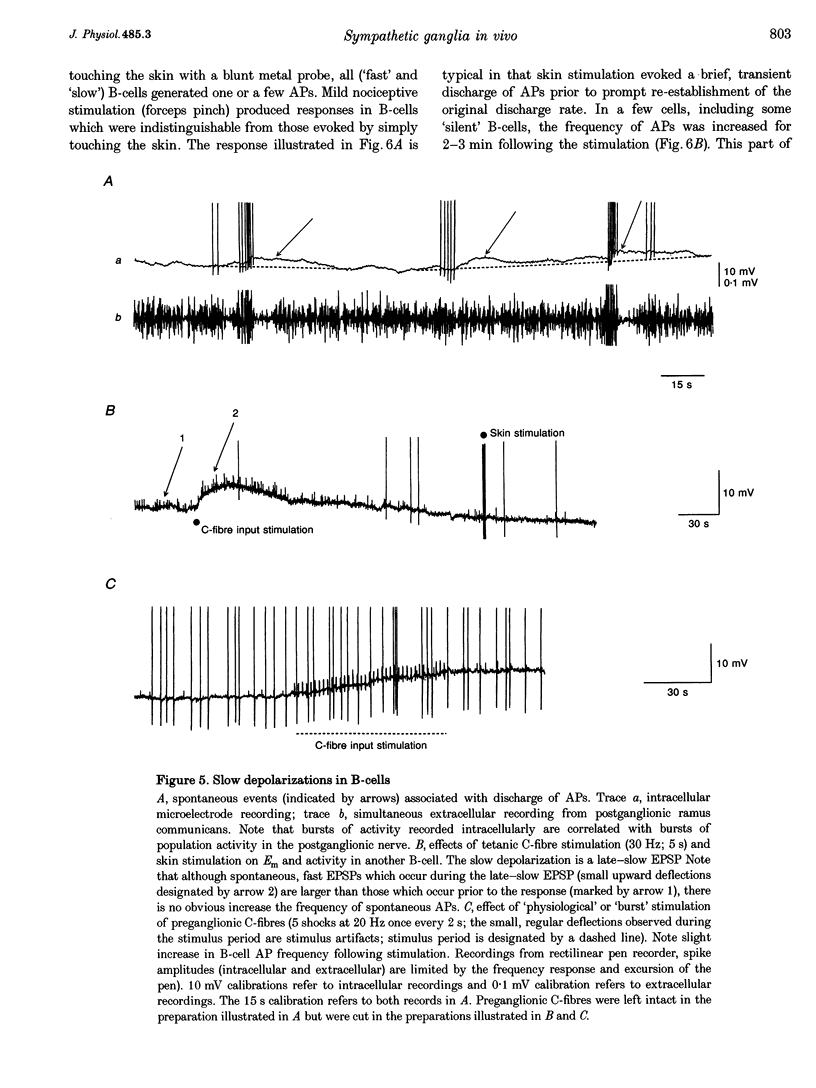
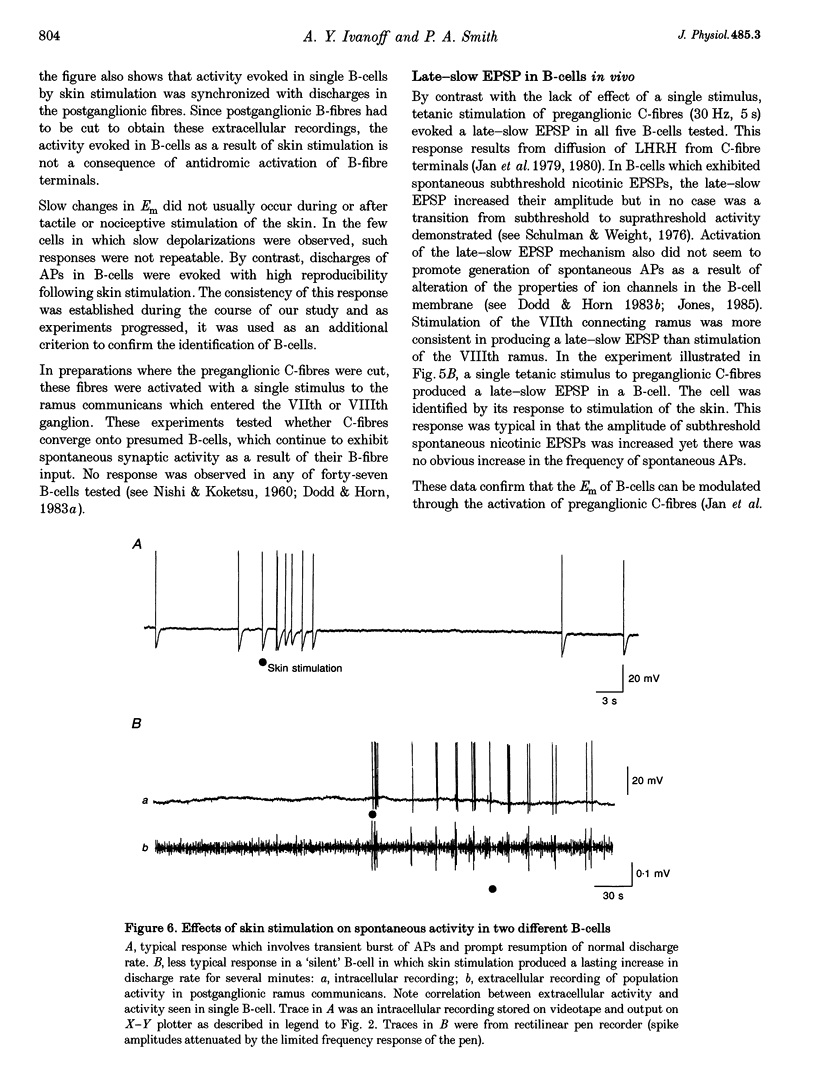
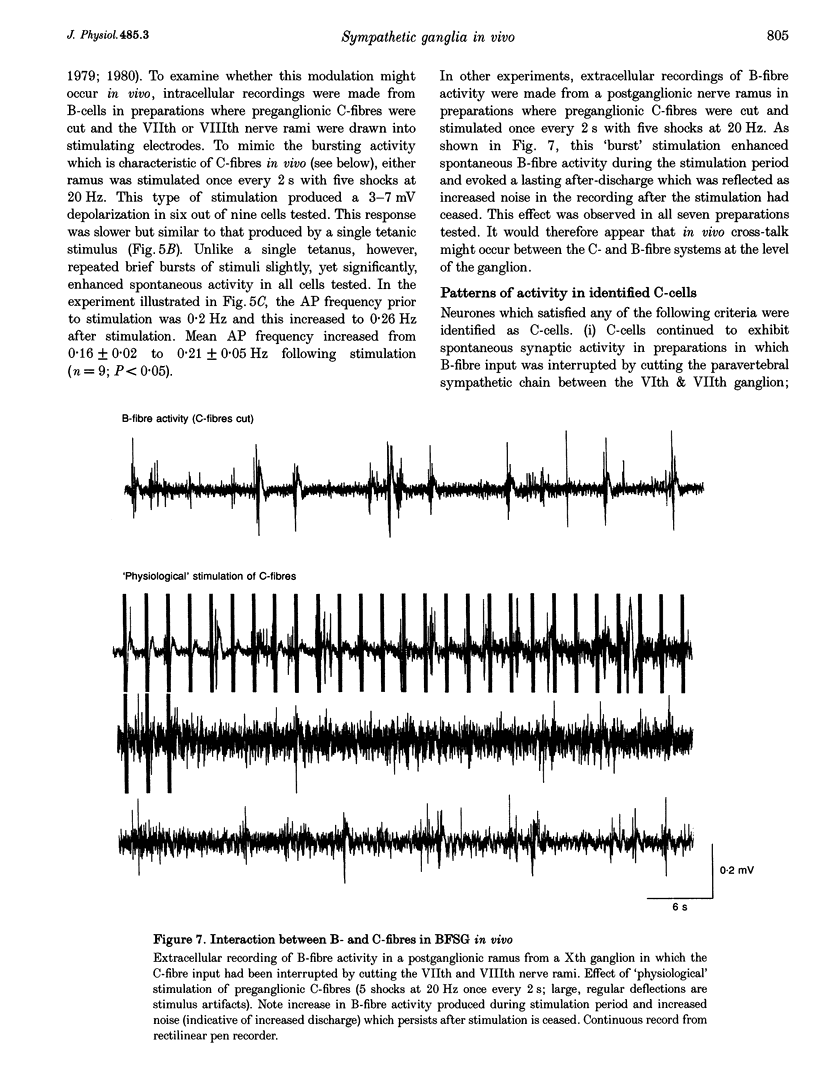
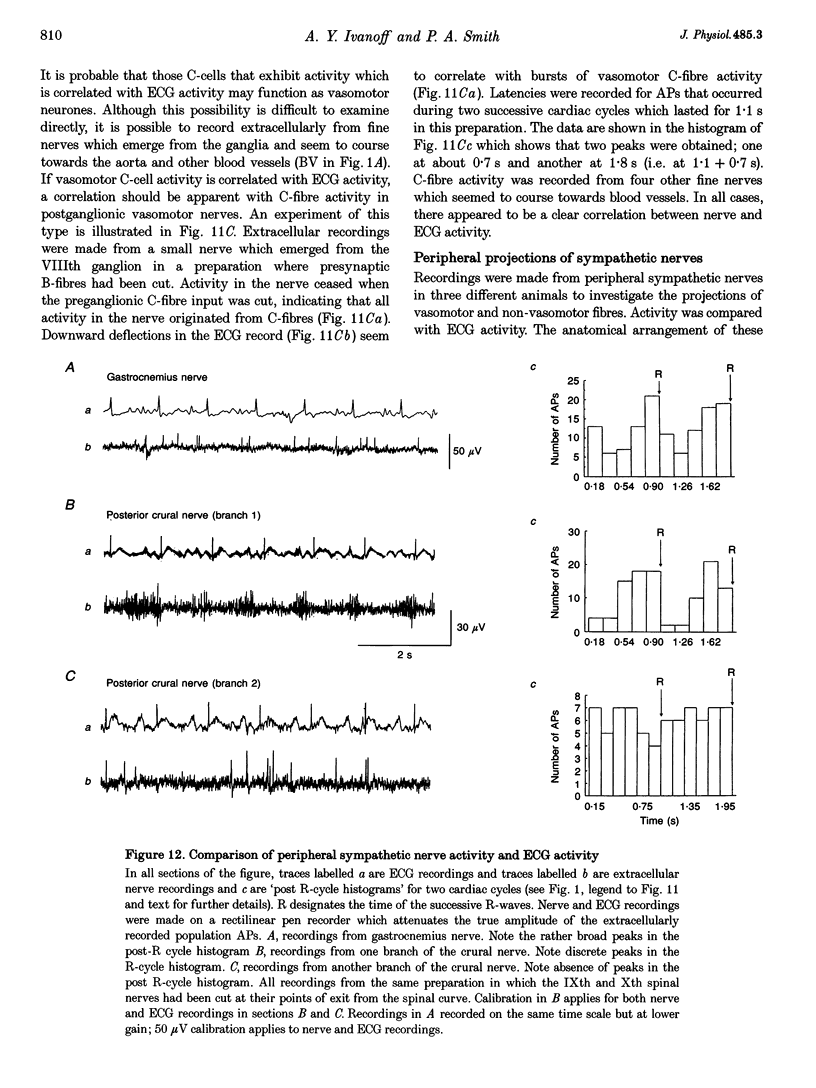
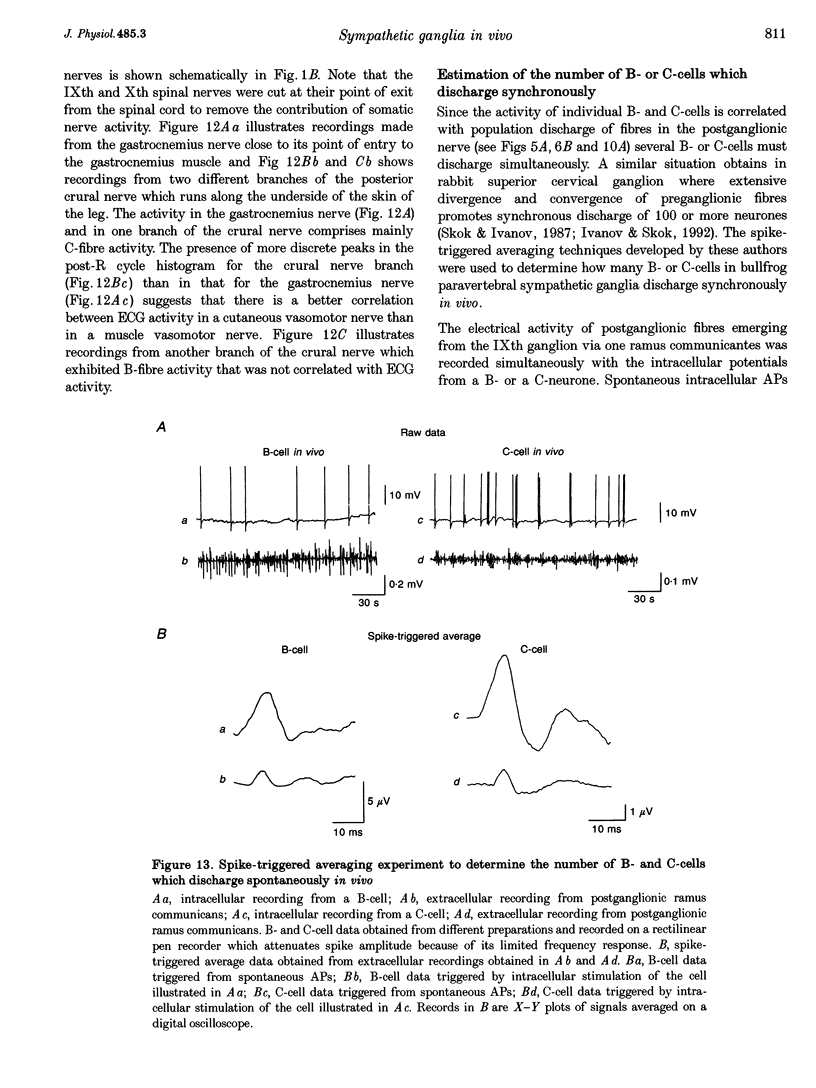




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Jones S. W., Pennefather P., Brown D. A., Koch C., Lancaster B. Slow synaptic transmission in frog sympathetic ganglia. J Exp Biol. 1986 Sep;124:259–285. doi: 10.1242/jeb.124.1.259. [DOI] [PubMed] [Google Scholar]
- Bałuk P. Scanning electron microscopic studies of bullfrog sympathetic neurons exposed by enzymatic removal of connective tissue elements and satellite cells. J Neurocytol. 1986 Feb;15(1):85–95. doi: 10.1007/BF02057907. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. A reclassification of B and C neurones in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J Physiol. 1983 Jan;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma S. Functional differentiation in sB and sC neurons of toad sympathetic ganglia. Jpn J Physiol. 1970 Jun 15;20(3):281–295. doi: 10.2170/jjphysiol.20.281. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Dodd J. Monosynaptic muscarinic activation of K+ conductance underlies the slow inhibitory postsynaptic potential in sympathetic ganglia. Nature. 1981 Aug 13;292(5824):625–627. doi: 10.1038/292625a0. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Fatherazi S., Stofer W. D. Differential projections of B and C sympathetic axons in peripheral nerves of the bullfrog. J Comp Neurol. 1988 Dec 22;278(4):570–580. doi: 10.1002/cne.902780408. [DOI] [PubMed] [Google Scholar]
- Horn J. P., Stofer W. D. Spinal origins of preganglionic B and C neurons that innervate paravertebral sympathetic ganglia nine and ten of the bullfrog. J Comp Neurol. 1988 Feb 1;268(1):71–83. doi: 10.1002/cne.902680108. [DOI] [PubMed] [Google Scholar]
- Ivanov AYa Pattern of ongoing activity in rat superior cervical ganglion neurons projecting to a specific target. J Auton Nerv Syst. 1991 Jan;32(1):77–79. doi: 10.1016/0165-1838(91)90238-x. [DOI] [PubMed] [Google Scholar]
- Ivanov AYa, Skok V. I. Neuronal mechanisms responsible for ongoing activity of rabbit superior cervical ganglion neurons. J Auton Nerv Syst. 1992 Nov;41(1-2):61–66. doi: 10.1016/0165-1838(92)90127-3. [DOI] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Kuffler S. W. Further evidence for peptidergic transmission in sympathetic ganglia. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5008–5012. doi: 10.1073/pnas.77.8.5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Adams P. R., Brownstein M. J., Rivier J. E. Teleost luteinizing hormone-releasing hormone: action on bullfrog sympathetic ganglia is consistent with role as neurotransmitter. J Neurosci. 1984 Feb;4(2):420–429. doi: 10.1523/JNEUROSCI.04-02-00420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985 Sep;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., McLachlan E. M. Characteristics of function-specific pathways in the sympathetic nervous system. Trends Neurosci. 1992 Dec;15(12):475–481. doi: 10.1016/0166-2236(92)90092-m. [DOI] [PubMed] [Google Scholar]
- Jänig W. Pre- and postganglionic vasoconstrictor neurons: differentiation, types, and discharge properties. Annu Rev Physiol. 1988;50:525–539. doi: 10.1146/annurev.ph.50.030188.002521. [DOI] [PubMed] [Google Scholar]
- Kuba K., Koketsu K. Synaptic events in sympathetic ganglia. Prog Neurobiol. 1978;11(2):77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R. Modulation of cutaneous mechanoreceptors by sympathetic stimulation. J Physiol. 1956 Apr 27;132(1):40–60. doi: 10.1113/jphysiol.1956.sp005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L., Sjöberg E., Skoglund C. R. Conductance recording of ionic outflow from frog skin glands during nerve stimulation. Acta Physiol Scand. 1975 Jan;93(1):67–76. doi: 10.1111/j.1748-1716.1975.tb05791.x. [DOI] [PubMed] [Google Scholar]
- Libet B., Chichibu S., Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J Neurophysiol. 1968 May;31(3):383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- Luebke J. I., Wright L. L. Characterization of superior cervical ganglion neurons that project to the submandibular glands, the eyes, and the pineal gland in rats. Brain Res. 1992 Aug 28;589(1):1–14. doi: 10.1016/0006-8993(92)91155-8. [DOI] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- Schulman J. A., Weight F. F. Synaptic transmission: long-lasting potentiation by a postsynaptic mechanism. Science. 1976 Dec 24;194(4272):1437–1439. doi: 10.1126/science.188131. [DOI] [PubMed] [Google Scholar]
- Smith P. A. Amphibian sympathetic ganglia: an owner's and operator's manual. Prog Neurobiol. 1994 Jul-Aug;43(4-5):439–510. doi: 10.1016/0301-0082(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Weight F. F. The pathway for the slow inhibitory postsynaptic potential in bullfrog sympathetic ganglia. J Neurophysiol. 1986 Sep;56(3):823–834. doi: 10.1152/jn.1986.56.3.823. [DOI] [PubMed] [Google Scholar]
- Spray D. C. Characteristics, specificity, and efferent control of frog cutaneous cold receptors. J Physiol. 1974 Feb;237(1):15–38. doi: 10.1113/jphysiol.1974.sp010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stofer W. D., Fatherazi S., Horn J. P. Neuropeptide Y mimics a non-adrenergic component of sympathetic vasoconstriction in the bullfrog. J Auton Nerv Syst. 1990 Nov;31(2):141–151. doi: 10.1016/0165-1838(90)90071-p. [DOI] [PubMed] [Google Scholar]
- Tosaka T., Chichibu S., Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968 May;31(3):396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Acetylcholine and slow synaptic inhibition in frog sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):225–228. doi: 10.1016/0006-8993(73)90506-4. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Padjen A. Slow synaptic inhibition: evidence for synaptic inactivation of sodium conductance in sympathetic ganglion cells. Brain Res. 1973 May 30;55(1):219–224. doi: 10.1016/0006-8993(73)90505-2. [DOI] [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- Weitsen H. A., Weight F. F. Synaptic innervation of sympathetic ganglion cells in the bullfrog. Brain Res. 1977 Jun 10;128(2):197–211. doi: 10.1016/0006-8993(77)90988-x. [DOI] [PubMed] [Google Scholar]