Abstract
Background
Magnesium deficiency has been shown to accelerate atherosclerosis. We hypothesized that dietary magnesium intake at a young age is associated with future atherosclerotic lesions and cardiovascular risk in a large, nationally representative cohort of U.S. adults.
Methods
We included U.S. adults aged 20 to 34 years old from the National Health and Nutrition Examination Survey (NHANES) 2007 to 2018, a population-based cross-sectional study. Dietary magnesium intake was assessed using 24-hour diet recalls. Atherosclerotic lesions in the young adult population were predicted by the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) score that was based on age, sex, smoking status, lipids, blood pressure, and obesity. Information on cardiovascular disease (CVD) risk factors including hypertension, non-HDL-C dyslipidemia, and diabetes was also collected. We used multivariable logistic analysis models to test the association between magnesium intake levels and the PDAY score and CVD risk factors, respectively, after adjusting for several potential confounding factors.
Results
7,244 eligible participants were included in the analysis. The magnesium intake level was classified into three categories based on the tertile distribution in the population (i.e., ≤ 224, 225–340, and ≥ 341 mg/day). Compared with the lowest tertile, the multivariable-adjusted odds ratio (OR) and 95% confidence interval (95% CI) for the PDAY score were 0.83 (95% CI, 0.72 to 0.96) and 0.60 (95% CI, 0.49 to 0.74) in the second and the third tertiles of magnesium intake, respectively (P value for trend < 0.001), and there was a negative dose-response relationship (test for trend P value < 0.001). In addition, the highest dietary magnesium intake was significantly inverse associated with non-HDL-C dyslipidemia compared with the lowest magnesium intake (OR = 0.65; 95% CI, 0.46 to 0.91).
Conclusions
Dietary magnesium intake is inversely associated with the risk of future cardiovascular events assessed by the PDAY score and non-HDL-C dyslipidemia in young adulthood years.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20785-2.
Keywords: Magnesium, Cardiovascular diseases, PDAY risk score, Cross-sectional study
Introduction
Cardiovascular diseases (CVD) such as atherosclerosis and heart failure are considered one of the most important causes of death in the aging population. Although serious CVD events primarily affect the elderly, the disease processes commonly develop in youth [1]. The linkage between cardiovascular risk factors exposure earlier in life and future cardiovascular events has been confirmed by a wide range of observational studies [2–4]. To predict the atherosclerotic lesions in the coronary arteries and abdominal aorta of young individuals, the Pathobiological Determinants of Atherosclerosis (PDAY) risk score using traditional CVD risk factors was developed in 2005 [5]. The PDAY risk score was first applied to individuals aged 15 to 34 to predict coronary artery calcium (CAC) and abdominal aortic calcification (AAC), assessed 15 years later by CT scan in the Coronary Artery Risk Development in Young Adulthood (CARDIA) study. Strong associations between the PDAY score and carotid intima-media thickness were later confirmed in the Cardiovascular Risk in Young Finns Study [6]. A recent study suggests that the PDAY risk score can be applied to young adults under the age of 40 to predict ASCVD events, which makes early-life risk assessment for atherosclerosis likelihood a reality [7].
Magnesium, the fourth most abundant mineral in the human body [8], has been shown to provide protective cardiovascular effects by improving glucose homeostasis [9], reducing oxidative stress and inflammation [10], enhancing endothelium-dependent vasodilation [11], improving lipid metabolism [12], and inhibiting platelet function [13]. Magnesium deficiency has been shown to accelerate atherosclerosis by promoting platelet activity and endothelial dysfunction and increasing the production of pro-inflammatory cytokines and neuropeptides [14]. A growing number of studies have investigated the relationship between magnesium intake and cardiovascular outcomes. However, to the best of our knowledge, the association between dietary magnesium intake and predicted atherosclerotic lesions has not been adequately assessed in young adults.
To fill this knowledge gap, we performed a population-based cross-sectional study to explore the relationship between dietary magnesium intake and the PDAY risk score and common cardiovascular risk factors including hypertension, hyperlipidemia, and diabetes in young adults at 20 to 34 years of age from the National Health and Nutrition Examination Survey (NHANES).
Methods
Data source and study population
The NHANES are ongoing repeated cross‑sectional surveys conducted by the National Center for Health Statistics (NCHS) at the Centers for Disease Control and Prevention (CDC). These studies are based on a representative sample of the noninstitutionalized US civilian population that is selected using a multistage, stratified sampling design [15]. To examine the association between magnesium intake and the PDAY score, we used data of 6 cycles from NHANES 2007–2008 to NHANES 2017–2018. Subjects aged 20 to 34 years old were included in the present study. Those with any missing data regarding daily magnesium intake, PDAY score components, and any other variables analyzed were excluded. NHANES was approved by the research ethics review board of the NCHS, and all participants provided written informed consent.
Assessment of daily magnesium intake
Daily magnesium intake was defined as levels of total magnesium intake (foods plus supplements). NHANES collects 24-hour dietary recall data using the Automated Multiple Pass Method (AMPM), a structured interview where participants report all foods and beverages consumed over the previous 24 h. The first recall is conducted in person, while the second occurs 3–10 days later via telephone. The magnesium content of foods was estimated using the U.S. Department of Agriculture Food and Nutrient Database for Dietary Studies (FNDDS), which matches reported foods to standardized nutrient values. Additionally, NHANES gathers information on dietary supplements through a supplement questionnaire that records the type, dose, and frequency of use. During an in-house interview, participants are asked whether they used any supplements in the past 30 days.
Assessment of outcome
The primary outcome is the PDAY score, a prediction of atherosclerotic lesions in the young and middle-aged adult population [5]. Each one-point increase in score is associated with the amount of increase in one year of vascular aging. We calculated the PDAY score using the following measures on CVD risk factors: age with four categories, sex with two categories, non-high-density lipoprotein cholesterol (Non-HDL-C) with five categories, HDL-C with three categories, smoking with two categories, blood pressure with two categories, obesity with two categories, and hyperglycemia with two categories. The detailed algorithm to calculate the PDAY score is provided in Supplemental Table 1.
Secondary outcomes are individual CVD risk factors including obesity, hypertension, non-HDL-C dyslipidemia, and diabetes. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2. Hypertension was defined as if participants reported having ever been told by a physician that they had hypertension or systolic BP ≥ 130 mmHg or diastolic BP ≥ 80 mmHg according to American Heart Association/American College of Cardiology guidelines [16]. Non-HDL-C dyslipidemia was defined as non-HDL-C ≥ 190 mg/dl [17]. Diabetes was defined as when the answer to the question “Doctor told you have diabetes?” was “yes” or the glycohemoglobin level of the participants was 6.5% or above.
Assessment of covariates
Demographic characteristics and lifestyle factors, including race, education, income, drinking status, and physical activity were identified from the NHANES. Participants were classified as physically active if they had at least 150 min of moderate to vigorous physical activity per week and were classified as physically inactive otherwise [18]. Dietary intake factors included total energy, saturated fat, dietary fiber, zinc, vitamin B6, and vitamin D. Comorbidities were defined by self-reported diagnosis with the question “Has a doctor or other health professional ever told you that you had (name of the disease)?”, which included liver disease, stomach or intestinal disease, and kidney disease (variables listed in Table 1).
Table 1.
Characteristics of participants by magnesium intake levels, in National Health and Nutrition Examination Survey (NHANES) 2007 to 2018
| Characteristics | Tertiles of dietary magnesium (mg/day) | P value | ||
|---|---|---|---|---|
| Tertile1 (≤ 224) | Tertile2 (225–340) | Tertile3 (≥ 341) | ||
| Number | 2,389 | 2,386 | 2,469 | |
| Mg, mean (SE), mg | 162.5 (0.6) | 279.5 (0.4) | 497.4 (0.7) | |
| Age, mean (SE), years | 26.3 (0.1) | 26.8 (0.1) | 27.5 (0.1) | < 0.001 |
| Men, % | 37.7 | 47.4 | 63.3 | < 0.001 |
| BMI, mean (SE), kg/m2 | 28.8 (0.2) | 28.3 (0.2) | 27.4 (0.2) | < 0.001 |
| Race, % | < 0.001 | |||
| Non-Hispanic white | 65.7 | 69.9 | 74.9 | |
| Non-Hispanic black | 17.6 | 11.9 | 8.7 | |
| Hispanic | 7.8 | 8.4 | 7.3 | |
| Other | 8.9 | 9.8 | 9.1 | |
| Education, % | < 0.001 | |||
| Less than high school | 17.5 | 12.2 | 11.4 | |
| High school graduate/GED | 30.5 | 22.1 | 18.9 | |
| Some college or above | 52.0 | 65.7 | 69.7 | |
| Family income-poverty ratio, % | < 0.001 | |||
| <1.30 | 37.3 | 26.9 | 25.0 | |
| 1.30–2.99 | 33.7 | 30.2 | 31.0 | |
| 3.00–4.99 | 17.4 | 24.6 | 21.5 | |
| ≥ 5.00 | 11.6 | 18.3 | 22.5 | |
| Drinking, % | < 0.001 | |||
| Non-drinker | 24.5 | 19.5 | 14.7 | |
| Drinker | 75.5 | 80.5 | 85.3 | |
| Smoking, % | 0.002 | |||
| Non-smoker | 59.6 | 65.0 | 61.6 | |
| Smoker | 40.4 | 35.0 | 38.4 | |
| Physical activity, % | < 0.001 | |||
| Active | 31.7 | 40.2 | 53.1 | |
| Inactive | 68.3 | 59.8 | 46.9 | |
| Dietary intake, mean (SE) | ||||
| Total energy, kcal | 3090.2 (12.6) | 2226.7 (17.6) | 1576.1 (16.0) | < 0.001 |
| Saturated fat, g | 37.9 (0.6) | 28.7 (0.4) | 19.3 (0.3) | < 0.001 |
| Dietary fiber, g | 8.8 (0.1) | 15.2 (0.1) | 25.2 (0.3) | < 0.001 |
| Zinc, mg | 7.3 (0.1) | 11.4 (0.1) | 16.8 (0.2) | < 0.001 |
| Vitamin B6, mg | 1.4 (0.0) | 2.1 (0.0) | 3.3 (0.1) | < 0.001 |
| Vitamin D, µg | 2.3 (0.1) | 4.1 (0.1) | 6.8 (0.2) | < 0.001 |
| Comorbid conditions, % | ||||
| Liver disease | 1.3 | 1.1 | 1.1 | 0.041 |
| Stomach or intestinal disease | 8.8 | 7.5 | 6.3 | 0.027 |
| Kidney disease | 1.6 | 1.3 | 1.2 | 0.023 |
BMI, body mass index; SE, standard error; Mg, magnesium
Statistical analysis
Population characteristics were compared among three groups with differing magnesium intake levels. Any covariate that significantly differed among groups was considered a potential confounding factor. Continuous data are presented as means (standard error) and categorical data are presented as percentages. The magnesium intake level was classified into three categories based on the tertile distribution in the population (i.e., ≤ 224, 225–340, and ≥ 341 mg/day). PDAY score was classified according to quartile distribution [7]. We examined the association of magnesium intake with PDAY score using multivariable ordinal logistic regression adjusting for the potential confounders. Additionally, we assessed the relationship between magnesium intake and the eight individual components of the PDAY score. For secondary outcomes, we utilized multivariable binary logistic regression models to explore the association between magnesium intake and several major CVD risk factors. Odds ratios (OR) and related 95% confidence intervals (95%CI) of each outcome among different categories of magnesium intake were estimated by the following models: the initial univariate model estimated the crude OR (model 1); model 2 included race, education, family income-poverty ratio, drinking, smoking, and physical activity as covariates; model 3 included model 2 and dietary composition (total energy, saturated fat, dietary fiber, zinc, vitamin B6 and vitamin D); model 4 included model 3 and comorbid conditions (liver disease, stomach or intestinal disease and kidney disease). The dose-response relationship between levels of magnesium intake and the PDAY score was evaluated by restricted cubic splines regression with two knots defined by the tertile distribution of magnesium intake [19, 20].
We performed statistical analyses using PROC Survey in SAS 9.4 (SAS Institute, Cary, North Carolina, USA) to estimate variance after incorporating the weights for the sample population in NHANES. All P values were 2-sided and P < 0.05 was considered significant for all tests.
Results
In total, 7,244 participants aged 20 to 34 years were included in our analysis. Table 1 shows the characteristics of participants by the three categories of magnesium intake level. Compared with a low intake of magnesium, higher magnesium intakes were more likely to be male (63.3% vs. 37.7%) and non-Hispanic white (74.9% vs. 65.7%), have higher levels of education (69.7% vs. 52.0% completing college or above) and family income (22.5% vs. 11.6% with a family income-poverty ratio ≥ 5%), drinker (85.3% vs. 75.5%), and be physically active (53.1% vs. 31.7%). They also had lower intakes of energy (1576.1 kcal vs. 3090.2 kcal) and saturated fat (19.3 g vs. 37.9 g), but higher intakes of dietary fiber (25.2 g vs. 8.8 g), zinc (16.8 mg vs. 7.3 mg), vitamin B6 (3.3 mg vs. 1.4 mg), and vitamin D (6.8 µg vs. 2.3 µg). Additionally, individuals with higher magnesium intake had a lower prevalence of comorbid conditions at baseline.
The association between dietary magnesium intake and the PDAY score is shown in Table 2. Compared with the lowest tertile, the crude OR and 95% CI was 0.82 (95% CI: 0.72 to 0.93), 0.61 (95% CI: 0.53 to 0.71) from the second to the highest dietary magnesium tertile, respectively (P value for trend < 0.001). After adjusting for race, education, family income, drinking, and physical activity (model 2), the association between dietary magnesium intake and PDAY score remained negative (P value for trend < 0.001). With further adjustment of intake of total energy, saturated fat, dietary fiber, zinc, vitamin B6, and vitamin D based on model 2, the OR for PDAY score was lower than for the lowest tertile in the second (OR = 0.83; 95% CI, 0.72 to 0.96) and third (OR = 0.59; 95% CI, 0.49 to 0.74) tertiles of dietary magnesium intake (P value for trend < 0.001). The OR was only slightly attenuated when comorbid conditions were included simultaneously in a multivariate model (OR = 0.83; 95% CI, 0.72 to 0.96 in the 2nd tertile; OR = 0.60; 95% CI, 0.49 to 0.74 in the 3rd tertile, P value for trend < 0.001). Similarly, we observed consistent findings when assessing the association between magnesium intake and the eight components of the PDAY score (Table 2).
Table 2.
Associations of magnesium intake levels with the PDAY score and eight components of the PDAY score
| Tertiles of magnesium intake (mg/day) | P value for trend | |||
|---|---|---|---|---|
| Tertile1 (≤ 224) | Tertile2 (225–340) | Tertile3 (≥ 341) | ||
| Number | 2,389 | 2,386 | 2,469 | |
| PDAY score, mean (SE) | 13.9 (0.2) | 12.6 (0.2) | 12.2 (0.1) | |
| Model 1 OR (95%CI) | 1.00 (reference) | 0.82 (0.72 to 0.93) | 0.61 (0.53 to 0.71) | < 0.001 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.83 (0.72 to 0.94) | 0.59 (0.51 to 0.69) | < 0.001 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.83 (0.72 to 0.96) | 0.59 (0.49 to 0.74) | < 0.001 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.83 (0.72 to 0.96) | 0.60 (0.49 to 0.74) | < 0.001 |
| Eight components of the PDAY score | ||||
| Age | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.74 (0.64 to 0.85) | 0.56 (0.49 to 0.64) | < 0.001 |
| Model 2 OR (95%CI)* | 1.00 (reference) | 0.73 (0.64 to 0.84) | 0.55 (0.48 to 0.63) | < 0.001 |
| Sex# | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.67 (0.57 to 0.77) | 0.34 (0.29 to 0.39) | < 0.001 |
| Model 2 OR (95%CI)* | 1.00 (reference) | 0.66 (0.57 to 0.77) | 0.33 (0.28 to 0.39) | < 0.001 |
| Non-HDL cholesterol | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.96 (0.83 to 1.12) | 0.95 (0.82 to 1.10) | 0.101 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.86 (0.73 to 1.02) | 0.85 (0.73 to 0.99) | 0.062 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.91 (0.75 to 1.09) | 0.86 (0.74 to 1.00) | 0.019 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.91 (0.75 to 1.09) | 0.86 (0.73 to 1.00) | 0.020 |
| HDL cholesterol | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 1.14 (0.97 to 1.36) | 1.27 (1.06 to 1.52) | 0.029 |
| Model 2 OR (95%CI) | 1.00 (reference) | 1.38 (1.13 to 1.69) | 1.40 (1.14 to 1.70) | 0.002 |
| Model 3 OR (95%CI) | 1.00 (reference) | 1.47 (1.19 to 1.83) | 1.67 (1.27 to 2.21) | 0.001 |
| Model 4 OR (95%CI) | 1.00 (reference) | 1.48 (1.19 to 1.84) | 1.68 (1.26 to 2.23) | 0.001 |
| Smoking | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.92 (0.78 to 1.08) | 0.78 (0.68 to 0.91) | 0.003 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.84 (0.69 to 1.02) | 0.77 (0.65 to 0.92) | 0.014 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.85 (0.68 to 1.08) | 0.78 (0.65 to 0.94) | 0.024 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.85 (0.68 to 1.07) | 0.78 (0.65 to 0.94) | 0.023 |
| Blood pressure | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.96 (0.79 to 1.17) | 0.85 (0.73 to 0.99) | 0.104 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.92 (0.75 to 1.13) | 0.75 (0.63 to 0.88) | 0.018 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.97 (0.77 to 1.21) | 0.83 (0.65 to 1.06) | 0.601 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.97 (0.78 to 1.21) | 0.83 (0.65 to 1.06) | 0.753 |
| Obesity | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.85 (0.72 to 0.98) | 0.73 (0.59 to 0.88) | 0.013 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.88 (0.74 to 1.03) | 0.87 (0.70 to 1.07) | 0.082 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.92 (0.78 to 1.09) | 1.00 (0.78 to 1.28) | 0.735 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.92 (0.78 to 1.09) | 0.99 (0.78 to 1.27) | 0.793 |
| Hyperglycemia | ||||
| Model 1 OR (95%CI) | 1.00 (reference) | 0.93 (0.43 to 1.97) | 0.85 (0.40 to 1.80) | 0.356 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.91 (0.42 to 2.00) | 0.83 (0.38 to 1.80) | 0.289 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.78 (0.24 to 2.54) | 0.77 (0.36 to 1.66) | 0.789 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.79 (0.25 to 2.53) | 0.77 (0.36 to 1.65) | 0.790 |
n, number; OR, odds ratio; CI, confidence interval, PDAY, pathobiological determinants of atherosclerosis in youth
Model 1 was crude OR
Model 2 included race, education, family income-poverty ratio, drinking, physical activity
Model 3 added dietary intake (total energy, saturated fat, dietary fiber, zinc, vitamin B6 and vitamin D) on the basis of model 2
Model 4 added comorbid conditions (liver disease, stomach or intestinal disease and kidney disease) on the basis of model 3
*Model included race and sex for age, race and age for sex
#Results are presented as female vs. male
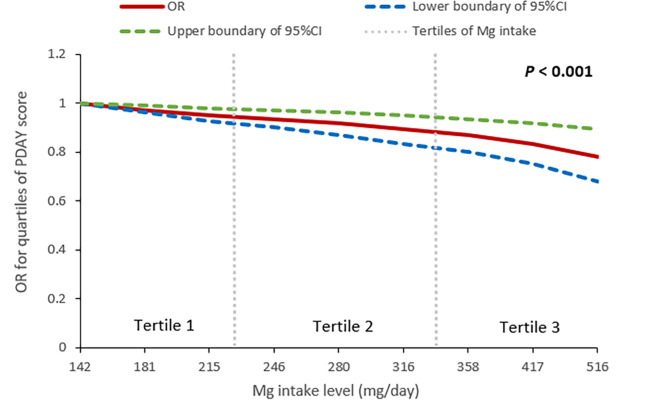
As shown in Fig. 1, magnesium intake, even within the normal range, was inversely associated with the OR for PDAY score in a dose-response-relationship manner (P value for trend < 0.001). Specifically, each 100 mg/day increase in magnesium intake was linked to an average reduction of 0.7 points in the PDAY score (95% CI: -0.9 to -0.5). In addition, when examining the associations of magnesium intake levels with CVD risk factors, after adjusting for potential factors, the highest dietary magnesium intake was significantly inverse associated with non-HDL-C dyslipidemia compared with the lowest magnesium intake (OR = 0.65; 95% CI, 0.46 to 0.91), but not observed the associated with obesity, hypertension, and diabetes (Table 3).
Fig. 1.
Dose-response relationship between magnesium (Mg) intake and the odds ratio (OR) for quartiles of PDAY score. PDAY, Pathobiological Determinants of Atherosclerosis in Youth; CI, confidence interval
Table 3.
Associations of magnesium intake levels with CVD risk factors
| Tertiles of magnesium intake (mg/day) | P value for trend | |||
|---|---|---|---|---|
| Tertile1 (≤ 224) | Tertile2 (225–340) | Tertile3 (≥ 341) | ||
| Obesity, % | 37.1 | 33.1 | 28.7 | |
| Model 1 OR (95%CI) | 1.00 (reference) | 0.85 (0.72 to 0.98) | 0.73 (0.59 to 0.88) | 0.013 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.88 (0.74 to 1.03) | 0.87 (0.70 to 1.07) | 0.082 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.92 (0.78 to 1.09) | 1.00 (0.78 to 1.28) | 0.735 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.92 (0.78 to 1.09) | 0.99 (0.78 to 1.27) | 0.793 |
| Hypertension, % | 22.3 | 21.7 | 21.5 | |
| Model 1 OR (95%CI) | 1.00 (reference) | 0.96 (0.79 to 1.17) | 0.85 (0.73 to 0.99) | 0.104 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.92 (0.75 to 1.13) | 0.75 (0.63 to 0.88) | 0.018 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.97 (0.77 to 1.21) | 0.83 (0.65 to 1.06) | 0.601 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.97 (0.78 to 1.21) | 0.83 (0.65 to 1.06) | 0.753 |
| Non-HDL-C dyslipidemia, % | 15.1 | 13.6 | 13.1 | |
| Model 1 OR (95%CI) | 1.00 (reference) | 0.98 (0.75 to 1.27) | 0.78 (0.63 to 0.96) | 0.037 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.96 (0.73 to 1.27) | 0.72 (0.57 to 0.91) | 0.010 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.92 (0.70 to 1.23) | 0.65 (0.47 to 0.90) | 0.025 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.92 (0.69 to 1.23) | 0.65 (0.46 to 0.90) | 0.024 |
| Diabetes, % | 2.4 | 2.1 | 2.0 | |
| Model 1 OR (95%CI) | 1.00 (reference) | 0.75 (0.45 to 1.24) | 0.66 (0.43 to 1.02) | 0.189 |
| Model 2 OR (95%CI) | 1.00 (reference) | 0.69 (0.40 to 1.20) | 0.49 (0.31 to 0.79) | 0.024 |
| Model 3 OR (95%CI) | 1.00 (reference) | 0.75 (0.42 to 1.34) | 0.61 (0.30 to 1.23) | 0.502 |
| Model 4 OR (95%CI) | 1.00 (reference) | 0.73 (0.41 to 1.30) | 0.60 (0.30 to 1.21) | 0.756 |
n, number; OR, odds ratio; CI, confidence interval
Model 1 was crude OR
Model 2 included race, education, family income-poverty ratio, drinking, physical activity
Model 3 added dietary intake (total energy, saturated fat, dietary fiber, zinc, vitamin B6 and vitamin D) on the basis of model 2
Model 4 added comorbid conditions (liver disease, stomach or intestinal disease and kidney disease) on the basis of model 3
Discussion
In this population-based cross-sectional study, we found dietary magnesium intake was significantly associated with future cardiovascular risk as predicted by the PDAY risk score and non-HDL-C dyslipidemia in young adults at 20 to 34 years of age. However, a modestly but not significantly inverse association was seen between total magnesium intakes and other CVD risk factors including diabetes, hypertension, and obesity. On the other hand, a dose-response analysis revealed that even within the normal range, magnesium intake was inversely associated with the OR for PDAY score. To our knowledge, this is the first study to report associations of dietary magnesium with CVD outcomes by predicting atherosclerotic lesions at a young age.
Comparison with previous studies
Several studies have explored the relationship between dietary magnesium intake and atherosclerotic lesions [21–23]. Notably, these studies have primarily focused on middle-aged or older populations, with the mean age of participants ranging from 50 to 60 years. As a result, their findings may not be directly applicable to younger adults. While numerous studies have explored the association between magnesium intake and cardiovascular disease (CVD) risk factors, younger populations remain underrepresented. For example, a meta-analysis of 40 observational studies found that most participants were over the age of 30 [24]. In contrast, our study targeted adults aged 20 to 34 years, offering novel insights into the potential role of magnesium intake in early atherosclerosis prevention among younger individuals.
Findings on the association between dietary magnesium intake accessed using food-frequency-questionnaire (FFQ) and atherosclerotic lesions have been inconsistent [21–23]. A population-based cross-sectional analysis from southern Germany (KORA-MRI) reported a modest association between higher dietary magnesium intake and reduced carotid plaque burden, assessed by MRI [21]. A sub-study of The Framingham Heart Study in 2014 reported a significant association between dietary magnesium intake and lower coronary artery calcium (CAC) levels, assessed by CT [22]. However, another study found no significant association between dietary magnesium intake and CAC levels [23]. A meta-analysis of cohort studies published in 2016 reported that increased dietary magnesium intake (accessed using FFQ, 24-h recall, or other) was associated with a reduced risk of stroke, heart failure, diabetes, but not significantly linked to incident coronary artery disease or total CVD events [24]. A more recent meta-analysis supported an inverse dose-response relationship between dietary magnesium intake accessed using FFQ and the risk of hypertension [25]. In our study, we observed a modest but non-significant inverse association between dietary magnesium intake and these CVD risk factors. This discrepancy may be due to differences in the age of study populations. Furthermore, several studies have suggested that dietary magnesium using 24-h dietary recall [26] or FFQ [27] may improve lipid profiles by reducing low-density lipoprotein cholesterol and triglyceride levels and increasing high-density lipoprotein cholesterol. Our findings on the association of dietary magnesium intake with these CVD risk factors are consistent with these studies.
Possible explanations
Atherosclerosis is the main risk factor for CVD [28], A few studies have suggested biological mechanisms through which magnesium intake may prevent or reverse plaque formation and atherogenesis in vascular beds. Magnesium may be acting as a calcium antagonist [29], which may directly inhibit crystal precipitation and hydroxyapatite [30–32]. Additionally, the deficiency of magnesium has been shown to accelerate atherosclerosis by increasing the production of pro-inflammatory neuropeptides and cytokines and promoting platelet activity and endothelial dysfunction. Observational studies have shown inverse association between serum magnesium and carotid intima-medial thickness, presence of atherosclerotic plaque, and progression of atherosclerosis [33, 34].
Several other mechanisms have also been postulated. First, magnesium is proven to play a crucial role in modulating vascular smooth muscle tone, which may enhance vascular vasodilation and myocardial contractility [35]. Second, magnesium supplementation may decrease lipid accumulation by increasing lipid oxidation and reducing lipid synthesis, which could contribute to the apparent protective role of magnesium intake on vascular function in humans [36]. Last, low magnesium may induce altered cellular glucose transport, reduced insulin secretion, defective post-receptor insulin signaling and/or altered insulin–insulin receptor interactions [37] and thus aggravate the processes related to Insulin resistance, important risk factor for CVD [38].
Strength and limitations
Several characteristics of our study are worth noting. First, it is based on NHANES data, which provided a large nationally representative sample of the US adult population, increasing the generalizability of our study. Second, we used the PDAY risk scores as our primary outcome, which could predict atherosclerotic lesions and future cardiovascular disease up to 20–25 years later, to link dietary magnesium intake to the risk of future cardiovascular disease at a young age. Third, this study was the first to show a negative association between dietary magnesium intake and the PDAY score, and the results were independent of some major confounders. The reliability of the study findings was therefore greatly improved. Several limitations should also be acknowledged. First, due to the inherent nature of a cross-sectional study, a causal relationship between dietary magnesium intake and outcomes cannot be determined.
Therefore, further prospective studies should be carried out to further investigate the identified association. Second, magnesium intake was assessed using 24-hour dietary recalls, which may not accurately reflect long-term intake. Moreover, self-reported recalls are susceptible to both random and systematic errors, increasing the potential for recall bias. Third, despite the 24-hour dietary recall interview being a validated method to assess dietary magnesium intake, a thorough literature search revealed no studies comparing the 24-hour dietary recall with more direct measures of magnesium intake, such as 24-hour urinary magnesium excretion, to further validate its accuracy. However, the NHANES data did not include 24-hour urinary magnesium excretion, which limits our ability to fully assess the relationship between magnesium intake and future cardiovascular risk. Forth, although numerous studies have confirmed the PDAY score’s value in predicting atherosclerotic lesions and cardiovascular disease in young populations, the score was not measured directly but estimated from various data sources and prediction models. Additionally, although we adjusted for many potential confounders, unmeasured confounders may still exist. The possibility of residual confounding due to unmeasured or poorly measured factors cannot be excluded. Despite these weaknesses, since no previous studies have explored the relationship between dietary magnesium intake and the PDAY score in young adults, this study still has significant value.
Clinical implications
The present findings have potential clinical implications. Prevention of CVD starting early in life could be more effective among the high-risk population [39]. A previous study has shown that elevated cholesterol in youth and young adulthood is a predictor of future cardiovascular disease, independent of older adult levels [40]. Furthermore, regressing atherosclerosis earlier in the life course is the most efficient way to prevent future cardiovascular disease [41]. Our study suggests that adequate consumption of magnesium at a young age may be an effective measure to prevent future cardiovascular events.
Conclusions
In conclusion, the present study has shown a significant inverse association between dietary magnesium intake and future cardiovascular risk assessed by the PDAY score and non-HDL-C dyslipidemia in young adulthood years. Our findings support adequate magnesium intake at a young age as being beneficial in reducing cardiovascular events decades years later.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Abbreviations
- NHANES
the National Health and Nutrition Examination Survey.
- PDAY
Pathobiological Determinants of Atherosclerosis in Youth.
- CVD
cardiovascular disease.
- CAC
coronary artery calcium.
- AAC
abdominal aortic calcification.
- CARDIA
Coronary Artery Risk Development in Young Adulthood.
- ASCVD
arteriosclerotic cardiovascular disease.
- NCHS
the National Center for Health Statistics.
- CDC
the Centers for Disease Control and Prevention.
- HDL
high-density lipoprotein.
- non-HDL-C
non-high-density lipoprotein cholesterol.
- AMPM
Automated Multiple Pass Method.
- FNDDS
Department of Agriculture Food and Nutrient Database for Dietary Studies.
- FFQ
Food-frequency-questionnaire.
Author contributions
CF and LL are the corresponding authors. CF, LL and QS had full access to the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: QS and CF. Acquisition and interpretation of data: QS, LS, HL and HT. Statistical analysis: QS, BY, HZ and CF. Drafting of the manuscript: QS, LS, BY and HT. Critical revision of the manuscript for important intellectual content: HL, HZ, CF and LL. Study supervision: CF and LL. All authors have read, provided critical feedback on intellectual content and approved the final manuscript.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (LL:2021JC0003). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets used and/or analysed during the current study are available in the National Health and Nutrition Examination Survey (NHANES). (https://www.cdc.gov/nchs/nhanes/index.html)
Declarations
Ethics approval and consent to participate
The NCHS Research Ethics Review Committee approved the NHANES study protocol, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Transparency
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chengming Fan, Email: fanchengming@csu.edu.cn.
Liming Liu, Email: liulimingjia@csu.edu.cn.
References
- 1.Santos-Gallego CG, Picatoste B, Badimon JJ. Pathophysiology of acute coronary syndrome. Curr Atheroscler Rep. 2014;16(4):401. [DOI] [PubMed] [Google Scholar]
- 2.D’Agostino RB, Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–53. [DOI] [PubMed] [Google Scholar]
- 3.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, et al. Can Antihypertensive Treatment restore the risk of Cardiovascular Disease to Ideal levels? The coronary artery Risk Development in Young adults (CARDIA) study and the multi-ethnic study of atherosclerosis (MESA). J Am Heart Assoc. 2015;4(9):e002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegman A, Gidding SS, Watts GF, Chapman MJ, Ginsberg HN, Cuchel M, Ose L, Averna M, Boileau C, Boren J, et al. Familial hypercholesterolaemia in children and adolescents: gaining decades of life by optimizing detection and treatment. Eur Heart J. 2015;36(36):2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahan CA, Gidding SS, Fayad ZA, Zieske AW, Malcom GT, Tracy RE, Strong JP, McGill HC Jr. Risk scores predict atherosclerotic lesions in young people. Arch Intern Med. 2005;165(8):883–90. [DOI] [PubMed] [Google Scholar]
- 6.McMahan CA, Gidding SS, Viikari JS, Juonala M, Kahonen M, Hutri-Kahonen N, Jokinen E, Taittonen L, Pietikainen M, McGill HC Jr., et al. Association of Pathobiologic Determinants of Atherosclerosis in Youth risk score and 15-year change in risk score with carotid artery intima-media thickness in young adults (from the Cardiovascular Risk in Young finns Study). Am J Cardiol. 2007;100(7):1124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gidding SS, Colangelo LA, Nwabuo CC, Lewis CE, Jacobs DR, Schreiner PJ, Lima JAC, Allen NB. PDAY risk score predicts cardiovascular events in young adults: the CARDIA study. Eur Heart J. 2022;43(30):2892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tangvoraphonkchai K, Davenport A. Magnesium and Cardiovascular Disease. Adv Chronic Kidney Dis. 2018;25(3):251–60. [DOI] [PubMed] [Google Scholar]
- 9.Nadler JL, Buchanan T, Natarajan R, Antonipillai I, Bergman R, Rude R. Magnesium deficiency produces insulin resistance and increased thromboxane synthesis. Hypertension. 1993;21(6 Pt 2):1024–9. [DOI] [PubMed] [Google Scholar]
- 10.Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24(1–3):107–36. [DOI] [PubMed] [Google Scholar]
- 11.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102(19):2353–8. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Luo S, Zou B, Xie J, Li J, Zeng Y. Magnesium supplementation stimulates autophagy to reduce lipid Accumulation in Hepatocytes via the AMPK/mTOR pathway. Biol Trace Elem Res 2022. [DOI] [PubMed]
- 13.Nadler JL, Malayan S, Luong H, Shaw S, Natarajan RD, Rude RK. Intracellular free magnesium deficiency plays a key role in increased platelet reactivity in type II diabetes mellitus. Diabetes Care. 1992;15(7):835–41. [DOI] [PubMed] [Google Scholar]
- 14.Maier JA, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction: implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689(1):13–21. [DOI] [PubMed] [Google Scholar]
- 15.National health and nutrition. examination survey [https://wwwn.cdc.gov/nchs/nhanes/tutorials/module1.aspx]
- 16.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71(6):1269–1324. [DOI] [PubMed]
- 17.Wu F, Jacobs DR Jr., Daniels SR, Kahonen M, Woo JG, Sinaiko AR, Viikari JSA, Bazzano LA, Steinberger J, Urbina EM, et al. Non-high-density Lipoprotein Cholesterol Levels from Childhood to Adulthood and Cardiovascular Disease events. JAMA. 2024;331(21):1834–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2008 Physical Activity Guidelines for Americans. [https://health.gov/paguidelines/2008/summary.aspx]
- 19.Hunt JR. Teratogenicity of high vitamin A intake. N Engl J Med. 1996;334(18):1197. [DOI] [PubMed] [Google Scholar]
- 20.Schottker B, Herder C, Rothenbacher D, Perna L, Muller H, Brenner H. Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: a competing risk analysis in a large population-based cohort of older adults. Eur J Epidemiol. 2013;28(3):267–75. [DOI] [PubMed] [Google Scholar]
- 21.Shugaa Addin N, Schlett CL, Bamberg F, Thorand B, Linseisen J, Seissler J, Peters A, Rospleszcz S. Subclinical Cardiovascular Disease Markers in Relation to Serum and Dietary Magnesium in Individuals from the General Population: The KORA-MRI Study. Nutrients 2022, 14(23). [DOI] [PMC free article] [PubMed]
- 22.Hruby A, O’Donnell CJ, Jacques PF, Meigs JB, Hoffmann U, McKeown NM. Magnesium intake is inversely associated with coronary artery calcification: the Framingham Heart Study. JACC Cardiovasc Imaging. 2014;7(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Jenny NS, Jiang R, Lima JA, Symanski E, Jacobs DR Jr., Nettleton JA. Dietary micronutrient intakes are associated with markers of inflammation but not with markers of subclinical atherosclerosis. J Nutr. 2011;141(8):1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X, Wang K, Han D, He X, Wei J, Zhao L, Imam MU, Ping Z, Li Y, Xu Y, et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. BMC Med. 2016;14(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han H, Fang X, Wei X, Liu Y, Jin Z, Chen Q, Fan Z, Aaseth J, Hiyoshi A, He J, et al. Dose-response relationship between dietary magnesium intake, serum magnesium concentration and risk of hypertension: a systematic review and meta-analysis of prospective cohort studies. Nutr J. 2017;16(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh RB, Rastogi SS, Mani UV, Seth J, Devi L. Does dietary magnesium modulate blood lipids? Biol Trace Elem Res. 1991;30(1):59–64. [DOI] [PubMed] [Google Scholar]
- 27.Geiger H, Wanner C. Magnesium in disease. Clin Kidney J. 2012;5(Suppl 1):i25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jebari-Benslaiman S, Galicia-Garcia U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, Benito-Vicente A, Martin C. Pathophysiology of atherosclerosis. Int J Mol Sci 2022, 23(6). [DOI] [PMC free article] [PubMed]
- 29.Iseri LT, French JH. Magnesium: Nature’s physiologic calcium blocker. Am Heart J. 1984;108(1):188–93. [DOI] [PubMed] [Google Scholar]
- 30.Peters F, Epple M. Simulating arterial wall calcification in vitro: biomimetic crystallization of calcium phosphates under controlled conditions. Z Kardiol. 2001;90(Suppl 3):81–5. [DOI] [PubMed] [Google Scholar]
- 31.Laurencin D, Almora-Barrios N, de Leeuw NH, Gervais C, Bonhomme C, Mauri F, Chrzanowski W, Knowles JC, Newport RJ, Wong A, et al. Magnesium incorporation into hydroxyapatite. Biomaterials. 2011;32(7):1826–37. [DOI] [PubMed] [Google Scholar]
- 32.Massy ZA, Drueke TB. Magnesium and outcomes in patients with chronic kidney disease: focus on vascular calcification, atherosclerosis and survival. Clin Kidney J. 2012;5(Suppl 1):i52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto T, Hara A, Ohkubo T, Kikuya M, Shintani Y, Metoki H, Inoue R, Asayama K, Kanno A, Nakashita M, et al. Serum magnesium, ambulatory blood pressure, and carotid artery alteration: the Ohasama study. Am J Hypertens. 2010;23(12):1292–8. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Folsom AR, Melnick SL, Eckfeldt JH, Sharrett AR, Nabulsi AA, Hutchinson RG, Metcalf PA. Associations of serum and dietary magnesium with cardiovascular disease, hypertension, diabetes, insulin, and carotid arterial wall thickness: the ARIC study. Atherosclerosis risk in communities Study. J Clin Epidemiol. 1995;48(7):927–40. [DOI] [PubMed] [Google Scholar]
- 35.Kolte D, Vijayaraghavan K, Khera S, Sica DA, Frishman WH. Role of magnesium in cardiovascular diseases. Cardiol Rev. 2014;22(4):182–92. [DOI] [PubMed] [Google Scholar]
- 36.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2 + modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408(2):191–4. [DOI] [PubMed] [Google Scholar]
- 37.Pham PC, Pham PM, Pham SV, Miller JM, Pham PT. Hypomagnesemia in patients with type 2 diabetes. Clin J Am Soc Nephrol. 2007;2(2):366–73. [DOI] [PubMed] [Google Scholar]
- 38.Rosique-Esteban N, Guasch-Ferre M, Hernandez-Alonso P, Salas-Salvado J. Dietary Magnesium and Cardiovascular Disease: a review with emphasis in Epidemiological studies. Nutrients 2018, 10(2). [DOI] [PMC free article] [PubMed]
- 39.Robinson JG, Williams KJ, Gidding S, Boren J, Tabas I, Fisher EA, Packard C, Pencina M, Fayad ZA, Mani V, et al. Eradicating the Burden of Atherosclerotic Cardiovascular Disease by lowering apolipoprotein B lipoproteins earlier in Life. J Am Heart Assoc. 2018;7(20):e009778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Vittinghoff E, Pletcher MJ, Allen NB, Zeki Al Hazzouri A, Yaffe K, Balte PP, Alonso A, Newman AB, Ives DG, et al. Associations of blood pressure and cholesterol levels during Young Adulthood with later Cardiovascular events. J Am Coll Cardiol. 2019;74(3):330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gidding SS, Robinson J. It is now Time to Focus on Risk before Age 40. J Am Coll Cardiol. 2019;74(3):342–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available in the National Health and Nutrition Examination Survey (NHANES). (https://www.cdc.gov/nchs/nhanes/index.html)