Abstract
1. The initial rapid phase of ATP hydrolysis by bovine heart submitochondrial particles or by soluble F1-ATPase is insensitive to anion activation (sulphite) or inhibition (azide). 2. The second slow phase of ATP hydrolysis is hyperbolically inhibited by azide (Ki approximately 10(-5) M); the inosine triphosphatase activity of submitochondrial particles or F1-ATPase is insensitive to azide or sulphite. 3. The rate of interconversion between rapid azide-insensitive and slow azide-sensitive phases of ATP hydrolysis does not depend on azide concentration, but strongly depends on ATP concentration. 4. Sulphite prevents the interconversion of the rapid initial phase of the reaction into the slower second phase, and also prevents and slowly reverses the inhibition by azide. 5. The presence of sulphite in the mixture when ADP reacts with ATPase of submitochondrial particles changes the pattern of the following activation process. 6. Azide blocks the activation of ATP-inhibited ATPase of submitochondrial particles by phosphoenolpyruvate and pyruvate kinase. 7. The results obtained suggest that the inhibiting effect of azide on mitochondrial ATPase is due to stabilization of inactive E*.ADP complex formed during ATP hydrolysis; the activation of ATPase by sulphite is also realized through the equilibrium between intermediate active E.ADP complex and inactive E*.ADP complex.
Full text
PDF
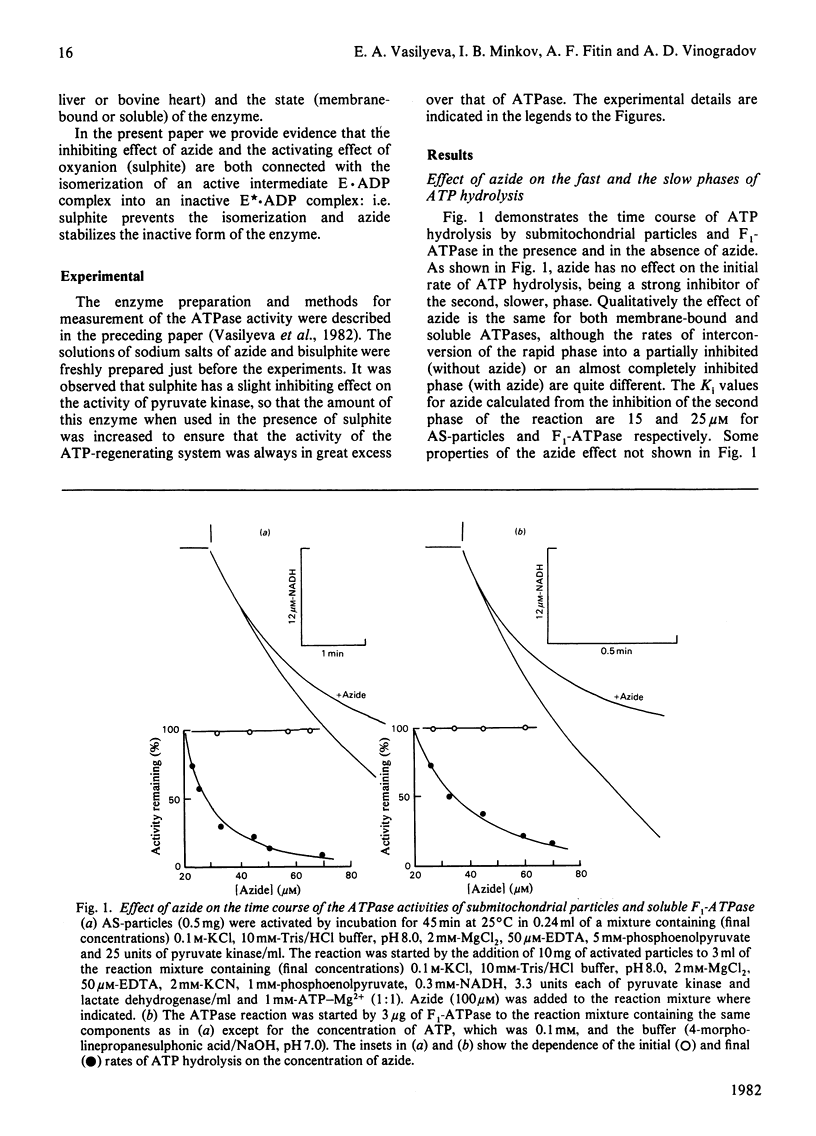
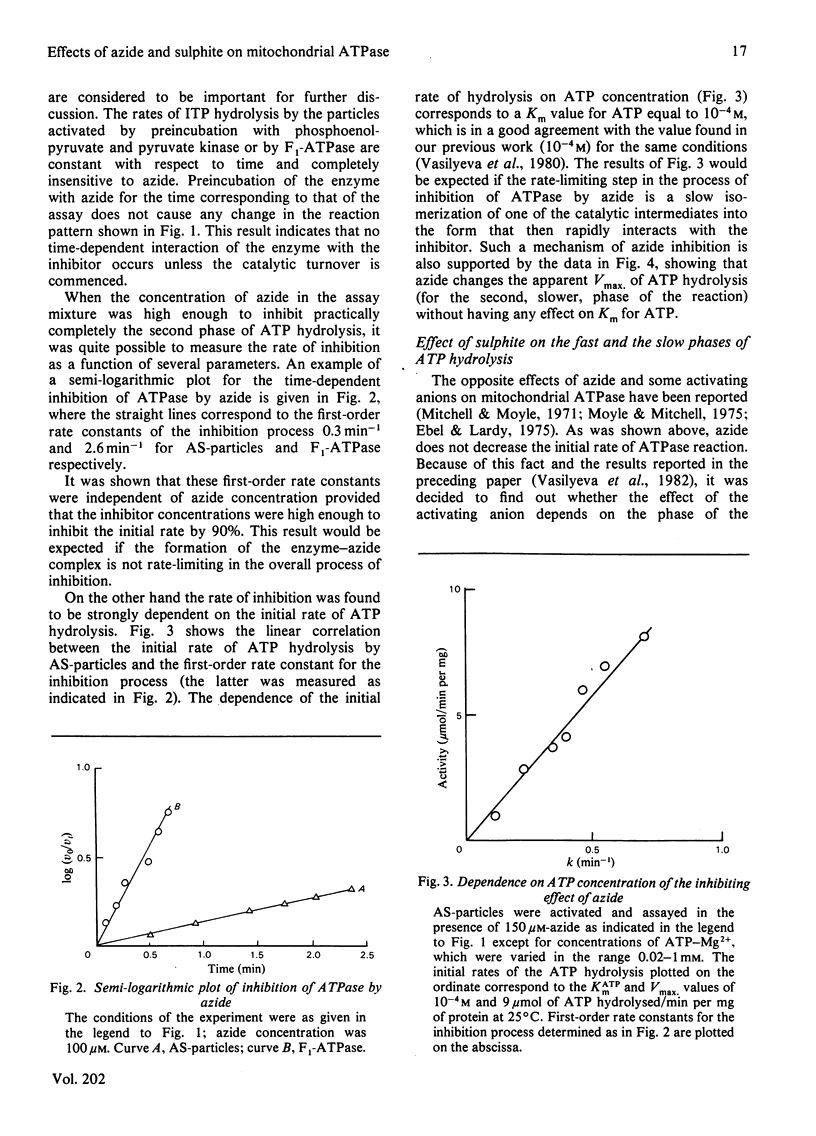
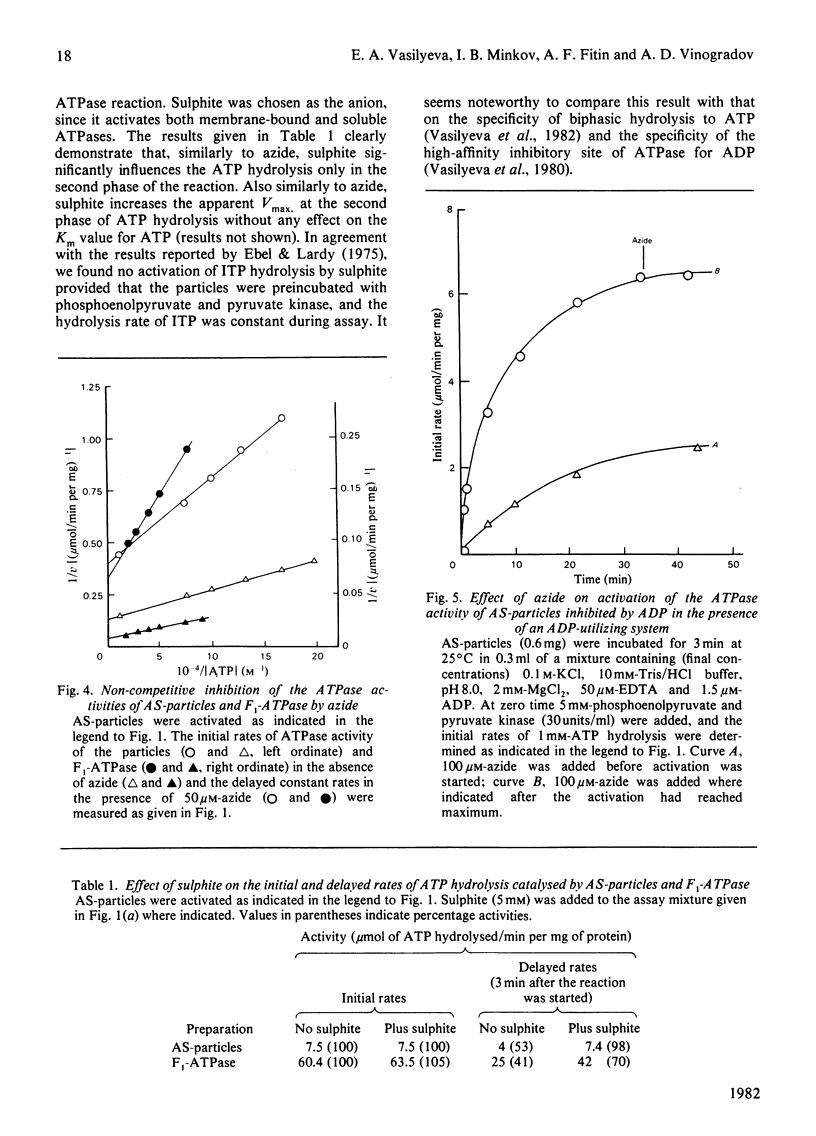
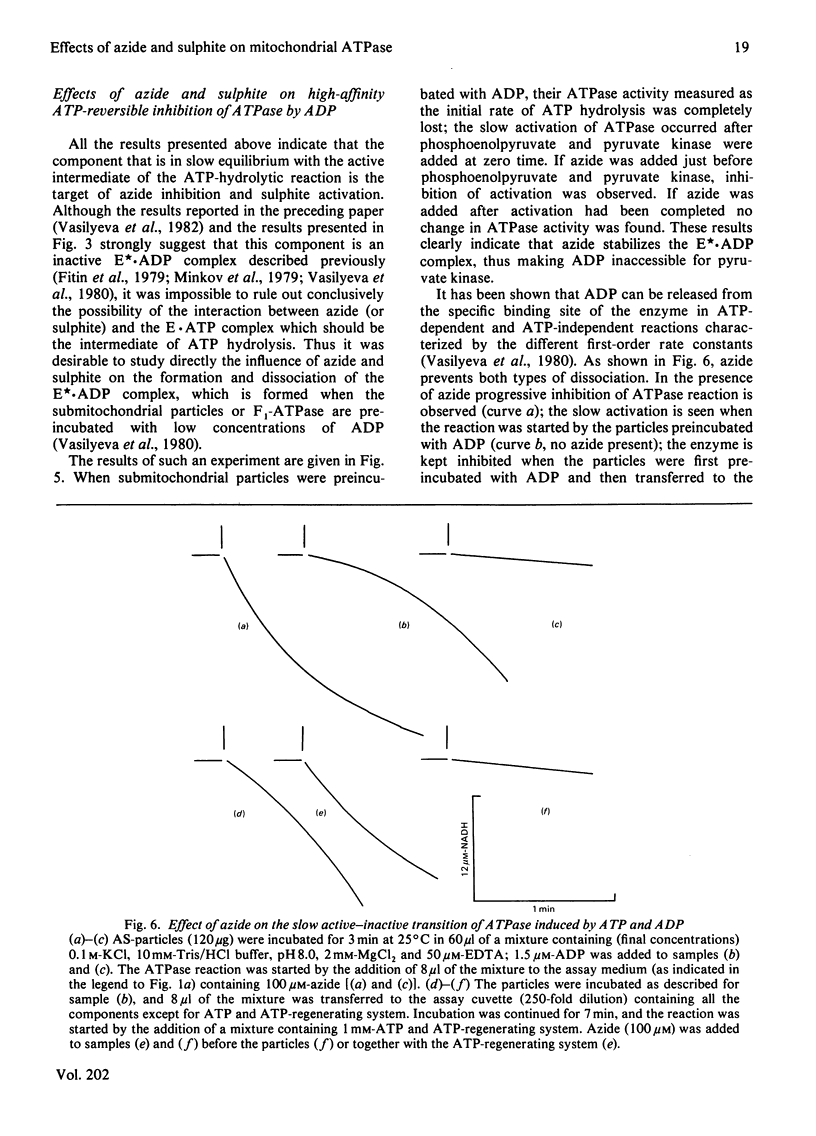



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABDOU I. A., REINHARDT W. O., TARVER H. Plasma protein. III. The equilibrium between blood and lymph protein. J Biol Chem. 1952 Jan;194(1):15–23. [PubMed] [Google Scholar]
- Adolfsen R., Moudrianakis E. N. Control of complex metal ion equilibria in biochemical reaction systems. Intrinsic and apparent stability constants of metal-adenine nucleotide complexes. J Biol Chem. 1978 Jun 25;253(12):4378–4379. [PubMed] [Google Scholar]
- Burns D. D., Midgley M. Localization and possible role of an adenosine triphosphatase in Chlorobium thiosulfatophilum. Eur J Biochem. 1976 Aug 16;67(2):323–333. doi: 10.1111/j.1432-1033.1976.tb10695.x. [DOI] [PubMed] [Google Scholar]
- COOPER C., LEHNINGER A. L. Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. IV. Adenosinetriphosphatase activity. J Biol Chem. 1957 Jan;224(1):547–560. [PubMed] [Google Scholar]
- COOPER C. The effect of dinitrophenol on magnesium-activated adenosinetriphosphatase. Biochim Biophys Acta. 1958 Dec;30(3):484–491. doi: 10.1016/0006-3002(58)90093-3. [DOI] [PubMed] [Google Scholar]
- Cereijo-Santaló R. The stimulation of the 2,4-dinitrophenol-activated ATPase by anions. Can J Biochem. 1968 Sep;46(9):1161–1163. doi: 10.1139/o68-172. [DOI] [PubMed] [Google Scholar]
- Christiansen R. O., Loyter A., Racker E. Effect of anions on oxidative phosphorylation in submitochondrial particles. Biochim Biophys Acta. 1969 May;180(1):207–210. doi: 10.1016/0005-2728(69)90212-6. [DOI] [PubMed] [Google Scholar]
- Ebel R. E., Lardy H. A. Stimulation of rat liver mitochondrial adenosine triphosphatase by anions. J Biol Chem. 1975 Jan 10;250(1):191–196. [PubMed] [Google Scholar]
- Fitin A. F., Vasilyeva E. A., Vinogradov A. D. An inhibitory high affinity binding site for ADP in the oligomycin-sensitive ATPase of beef heart submitochondrial particles. Biochem Biophys Res Commun. 1979 Jan 30;86(2):434–439. doi: 10.1016/0006-291x(79)90884-2. [DOI] [PubMed] [Google Scholar]
- Garrett N. E., Penefsky H. S. Interaction of adenine nucleotides with multiple binding sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1975 Sep 10;250(17):6640–6647. [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L., Galante Y., Hanstein W. G. Mitochondrial ATP-Pi exchange complex. Biochem Biophys Res Commun. 1974 Nov 6;61(1):313–321. doi: 10.1016/0006-291x(74)90568-3. [DOI] [PubMed] [Google Scholar]
- Hilborn D. A., Hammes G. G. Equilibrium binding of nucleotides to beef heart mitochondrial adenosine triphosphatase. Biochemistry. 1973 Feb 27;12(5):983–990. doi: 10.1021/bi00729a030. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Mitchell R. A. Arsenate and phosphate as modifiers of adenosine triphosphate driven energy-linked reduction. Kinetic study of the effects of modifiers on inhibition by adenosine diphosphate. Biochemistry. 1972 Jun 6;11(12):2278–2283. doi: 10.1021/bi00762a011. [DOI] [PubMed] [Google Scholar]
- Katz S., Crissman J. K., Roberson L. C. Volume effects produced by protein-ion interactions. The binding of bovine plasma albumin with thiocyanate and trichloroacetate ions. J Biol Chem. 1974 Dec 25;249(24):7892–7895. [PubMed] [Google Scholar]
- Knight B. L., Skala J. P. The separation, properties, and ethanol treatment of phosphoprotein phosphatases from brown adipose tissue of young rats. J Biol Chem. 1979 Feb 25;254(4):1319–1325. [PubMed] [Google Scholar]
- Kobayashi H., Maeda M., Anraku Y. Membrane-bound adenosine triphosphatase of Escherichia coli. III. Effects of sodium azide on the enzyme functions. J Biochem. 1977 Apr;81(4):1071–1077. doi: 10.1093/oxfordjournals.jbchem.a131530. [DOI] [PubMed] [Google Scholar]
- Kozlov I. A., Milgrom Y. M. The non-catalytic nucleotide-binding site of mitochondrial ATPase is localised on the alpha-subunit(s) of factor F1. Eur J Biochem. 1980 May;106(2):457–462. doi: 10.1111/j.1432-1033.1980.tb04592.x. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- Lambeth D. O., Lardy H. A. Purification and properties of rat-liver-mitochondrial adenosine triphosphatase. Eur J Biochem. 1971 Oct 14;22(3):355–363. doi: 10.1111/j.1432-1033.1971.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Leimgruber R. M., Senior A. E. Removal of "tightly bound" nucleotides from soluble mitochondrial adenosine triphosphatase (F1). J Biol Chem. 1976 Nov 25;251(22):7103–7109. [PubMed] [Google Scholar]
- MEYERHOF O., OHLMEYER P. Purification of adenosinetriphosphatase of yeast. J Biol Chem. 1952 Mar;195(1):11–17. [PubMed] [Google Scholar]
- MYERS D. K., SLATER E. C. The enzymic hydrolysis of adenosine triphosphate by liver mitochondria. 2. Effect of inhibitors and added cofactors. Biochem J. 1957 Dec;67(4):572–578. doi: 10.1042/bj0670572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkov I. B., Fitin A. F., Vasilyeva E. A., Vinogradov A. D. Mg2+-induced ADP-dependent inhibition of the ATPase activity of beef heart mitochondrial coupling factor F1. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1300–1306. doi: 10.1016/0006-291x(79)92150-8. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Hill R. D., Boyer P. D. Mechanistic implications of Mg++, adenine nucleotide, and inhibitor effects on energy-linked reactions of submitochondrial particles. J Biol Chem. 1967 Apr 25;242(8):1793–1801. [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Active/inactive state transitions of mitochondrial ATPase molecules influenced by Mg2+, anions and aurovertin. FEBS Lett. 1975 Aug 1;56(1):55–61. doi: 10.1016/0014-5793(75)80110-4. [DOI] [PubMed] [Google Scholar]
- Ohta S., Tsubo M., Oshima T., Yoshida M., Kagawa Y. Nucleotide binding to isolated alpha and beta subunits of proton translocating adenosine triphosphatase studied with circular dichroism. J Biochem. 1980 Jun;87(6):1609–1617. doi: 10.1093/oxfordjournals.jbchem.a132904. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria: separate sites involved in ATP hydrolysis and in the reversible, high affinity binding of ADP. Biochem Biophys Res Commun. 1975 May 19;64(2):610–616. doi: 10.1016/0006-291x(75)90365-4. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Differential effects of adenylyl imidodiphosphate on adenosine triphosphate synthesis and the partial reactions of oxidative phosphorylation. J Biol Chem. 1974 Jun 10;249(11):3579–3585. [PubMed] [Google Scholar]
- ROBERTSON H. E., BOYER P. D. The effect of azide on phosphorylation accompanying electron transport and glycolysis. J Biol Chem. 1955 May;214(1):295–305. [PubMed] [Google Scholar]
- Soper J. W., Pedersen P. L. Adenosine triphosphatase of rat liver mitochondria: detergent solubilization of an oligomycin- and dicyclohexylcarbodiimide-sensitive form of the enzyme. Biochemistry. 1976 Jun 15;15(12):2682–2690. doi: 10.1021/bi00657a031. [DOI] [PubMed] [Google Scholar]
- Stockdale M., Selwyn M. J. Influence of ring substituents on the action of phenols on some dehydrogenases, phospholinases and the soluble ATPase from mitochondria. Eur J Biochem. 1971 Aug 16;21(3):416–423. doi: 10.1111/j.1432-1033.1971.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Vasilyeva E. A., Fitin A. F., Minkov I. B., Vinogradov A. D. Kinetics of interaction of adenosine diphosphate and adenosine triphosphate with adenosine triphosphatase of bovine heart submitochondrial particles. Biochem J. 1980 Jun 15;188(3):807–815. doi: 10.1042/bj1880807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilyeva E. A., Minkov I. B., Fitin A. F., Vinogradov A. D. Kinetic mechanism of mitochondrial adenosine triphosphatase. ADP-specific inhibition as revealed by the steady-state kinetics. Biochem J. 1982 Jan 15;202(1):9–14. doi: 10.1042/bj2020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielders J. P., Slater E. C., Muller J. L. Binding of ADP to beef-heart mitochondrial ATPase (F1). Biochim Biophys Acta. 1980 Feb 8;589(2):231–240. doi: 10.1016/0005-2728(80)90040-7. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Okamoto H., Sone N., Hirata H., Kagawa Y. Reconstitution of thermostable ATPase capable of energy coupling from its purified subunits. Proc Natl Acad Sci U S A. 1977 Mar;74(3):936–940. doi: 10.1073/pnas.74.3.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Sone N., Hirata H., Kagawa Y. Reconstitution of adenosine triphosphatase of thermophilic bacterium from purified individual subunits. J Biol Chem. 1977 May 25;252(10):3480–3485. [PubMed] [Google Scholar]


