Abstract
We investigated the changes of the inner-membrane components and the electron-transfer activities of bovine heart submitochondrial particles induced by the lipid peroxidation supported by NADPH in the presence of ADP-Fe3+. Most of the polyunsaturated fatty acids were lost as a result of the peroxidation, and phospholipids were changed to polar species. Ubiquinone was also modified to polar substances as the peroxidation proceeded. Sodium dodecyl sulphate/polyacrylamide-gel electrophoresis showed the disappearance of 27000-Mr and 30000-Mr proteins and the appearance of highly polymerized substances. Flavins and cytochromes were not diminished, but the respiratory activity was lost. The reactions of NADH oxidase and NADH-cytochrome c reductase were most sensitive to the peroxidation, followed by those of succinate oxidase and succinate-cytochrome c reductase. Succinate dehydrogenase and duroquinol-cytochrome c reductase were inactivated by more extensive peroxidation, but cytochrome c oxidase was only partially inactivated. NADH-ferricyanide reductase was not inactivated. The pattern of the inactivation indicated that the lipid peroxidation affected the electron transport intensively between NADH dehydrogenase and ubiquinone, and moderately at the succinate dehydrogenase step and between ubiquinone and cytochrome c.
Full text
PDF


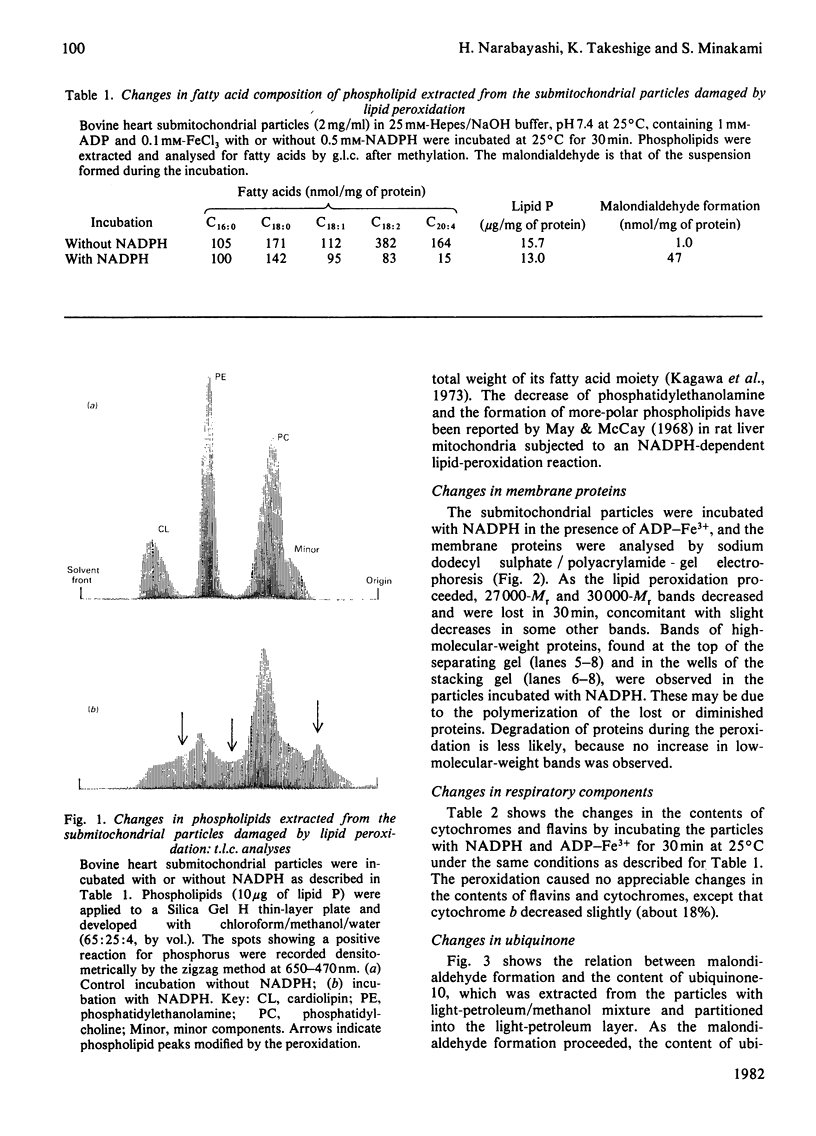
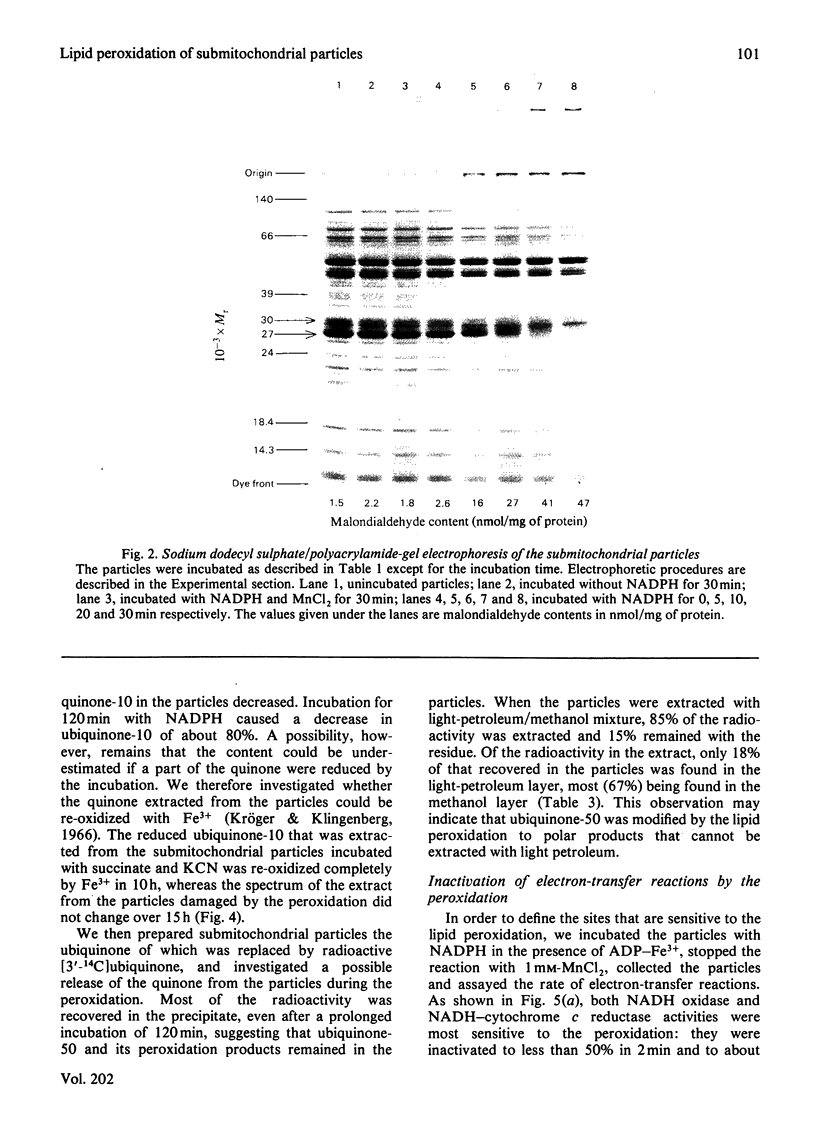




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrell B. A., Kearney E. B., Singer T. P. Mammalian succinate dehydrogenase. Methods Enzymol. 1978;53:466–483. doi: 10.1016/s0076-6879(78)53050-4. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y. Succinate dehydrogenase. I. Purification, molecular properties, and substructure. Biochemistry. 1971 Jun 22;10(13):2509–2516. doi: 10.1021/bi00789a014. [DOI] [PubMed] [Google Scholar]
- Ernster L., Glaser E., Norling B. Extraction and reincorporation of ubiquinone in submitochondrial particles. Methods Enzymol. 1978;53:573–579. doi: 10.1016/s0076-6879(78)53058-9. [DOI] [PubMed] [Google Scholar]
- FLEISCHER S., KLOUWEN H., BRIERLEY G. Studies of the electron transfer system. 38. Lipid composition of purified enzyme preparations derived from beef heart mitochondria. J Biol Chem. 1961 Nov;236:2936–2941. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fry M., Green D. E. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981 Feb 25;256(4):1874–1880. [PubMed] [Google Scholar]
- Hatefi Y., Stiggall D. L. Preparation and properties of succinate: ubiquinone oxidoreductase (complex II). Methods Enzymol. 1978;53:21–27. doi: 10.1016/s0076-6879(78)53008-5. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Krebs J. J., Hauser H., Carafoli E. Asymmetric distribution of phospholipids in the inner membrane of beef heart mitochondria. J Biol Chem. 1979 Jun 25;254(12):5308–5316. [PubMed] [Google Scholar]
- Kröger A., Klingenberg M. On the role of ubiquinone in mitochondria. II. Redox reactions of ubiquinone under the control of oxidative phosphorylation. Biochem Z. 1966 Jun 7;344(4):317–336. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MINAKAMI S., SCHINDLER F. J., ESTABROOK R. W. HYDROGEN TRANSFER BETWEEN REDUCED DIPHOSPHOPYRIDINE NUCLEOTIDE DEHYDROGENASE AND THE RESPIRATORY CHAIN. I. EFFECT OF SULFHYDRYL INHIBITORS AND PHOSPHOLIPASE. J Biol Chem. 1964 Jun;239:2042–2048. [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- McKnight R. C., Hunter F. E., Jr Mitochondrial membrane ghosts produced by lipid peroxidation induced by ferrous ion. II. Composition and enzymatic activity. J Biol Chem. 1966 Jun 25;241(12):2757–2765. [PubMed] [Google Scholar]
- Morimoto H., Imada I., Goto G. Photooxydation von Ubichinon-7. Justus Liebigs Ann Chem. 1970 May;735:65–71. doi: 10.1002/jlac.19707350111. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Gellerfors P. Characterization and resolution of complex III from beef heart mitochondria. Methods Enzymol. 1978;53:80–91. doi: 10.1016/s0076-6879(78)53016-4. [DOI] [PubMed] [Google Scholar]
- PUMPHREY A. M., REDFEARN E. R. A method for determining the concentration of ubiquinone in mitochondrial preparations. Biochem J. 1960 Jul;76:61–64. doi: 10.1042/bj0760061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer P. M., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. VI. Structural changes in mitochondria associated with inactivation of electron transport activity. J Biol Chem. 1972 Nov 10;247(21):6763–6769. [PubMed] [Google Scholar]
- RATHBONE L., MARONEY P. M. PREPARATION OF PHOSPHATIDYLSERINE. Nature. 1963 Nov 30;200:887–888. doi: 10.1038/200887a0. [DOI] [PubMed] [Google Scholar]
- Ragan C. I. The structure and subunit composition of the particulate NADH-ubiquinone reductase of bovine heart mitochondria. Biochem J. 1976 Feb 15;154(2):295–305. doi: 10.1042/bj1540295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi R., Takeshige K., Minakami S. NADH- and NADPH-dependent lipid peroxidation in bovine heart submitochondrial particles. Dependence on the rate of electron flow in the respiratory chain and an antioxidant role of ubiquinol. Biochem J. 1980 Dec 15;192(3):853–860. doi: 10.1042/bj1920853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshige K., Takayanagi R., Minakami S. Lipid peroxidation and the reduction of ADP-Fe3+ chelate by NADH-ubiquinone reductase preparation from bovine heart mitochondria. Biochem J. 1980 Dec 15;192(3):861–866. doi: 10.1042/bj1920861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y. P., Folkers K. Coenzyme Q and analogs for coenzymic activity. Methods Enzymol. 1978;53:591–599. doi: 10.1016/s0076-6879(78)53060-7. [DOI] [PubMed] [Google Scholar]
- Werbin H., Lakchaura B. D., Jagger J. Near-ultraviolet modification of Escherichia coli B ubiquinone in vivo and in vitro. Photochem Photobiol. 1974 May;19(5):321–328. doi: 10.1111/j.1751-1097.1974.tb06519.x. [DOI] [PubMed] [Google Scholar]
- YONETANI T., RAY G. S. STUDIES ON CYTOCHROME OXIDASE. VI. KINETICS OF THE AEROBIC OXIDATION OF FERROCYTOCHROME C BY CYTOCHROME OXIDASE. J Biol Chem. 1965 Aug;240:3392–3398. [PubMed] [Google Scholar]
- Yu L., Yu C. A., King T. E. The indispensability of phospholipid and ubiquinone in mitochondrial electron transfer from succinate to cytochrome c. J Biol Chem. 1978 Apr 25;253(8):2657–2663. [PubMed] [Google Scholar]



