Abstract
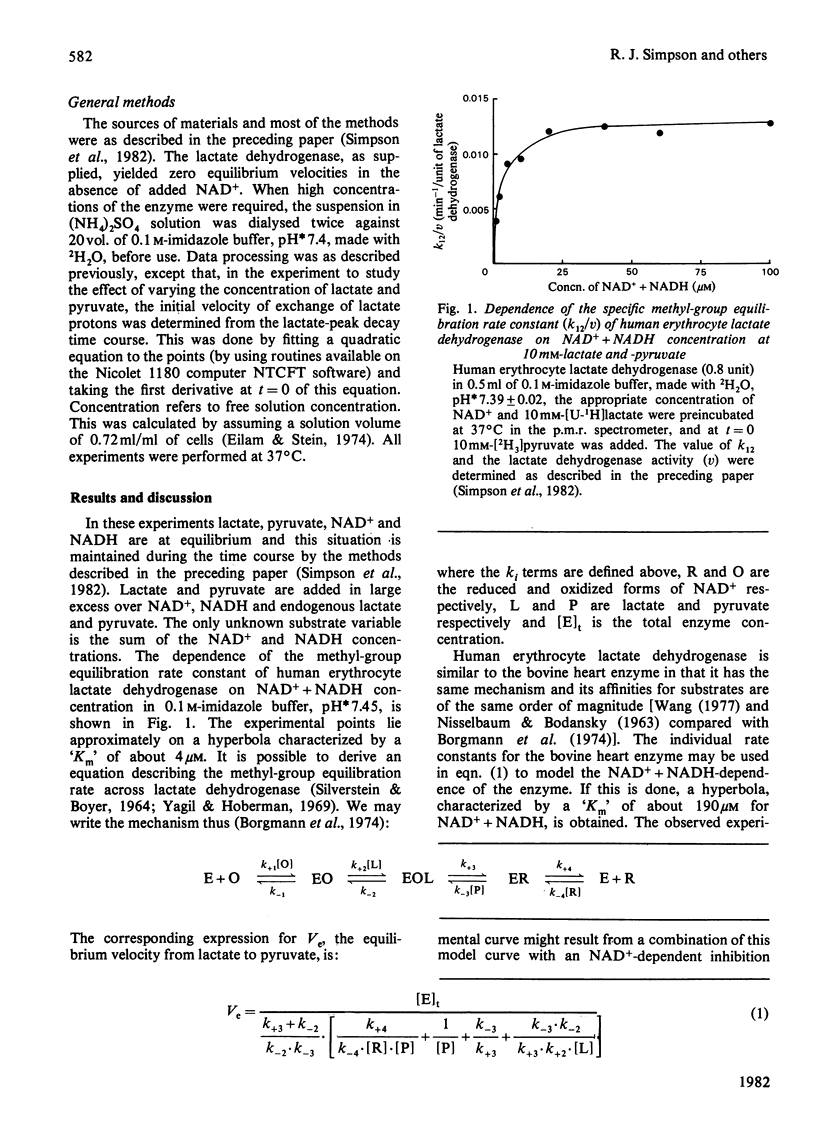
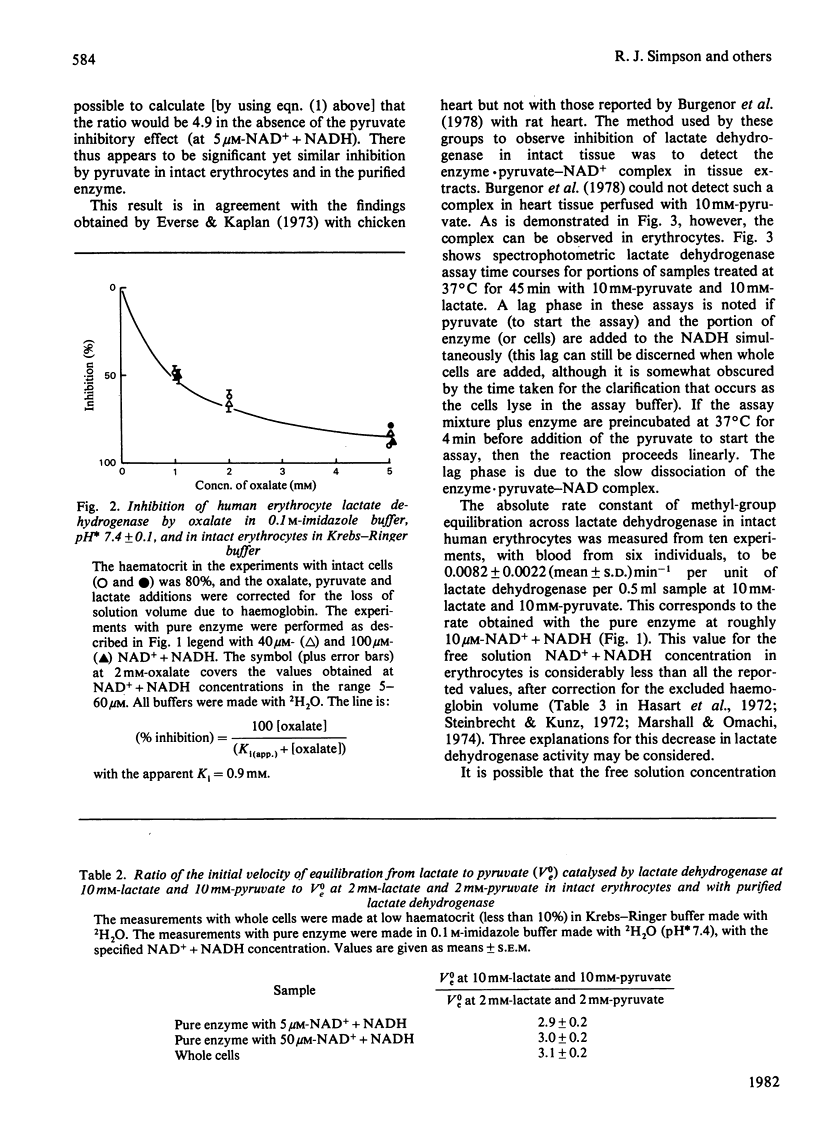
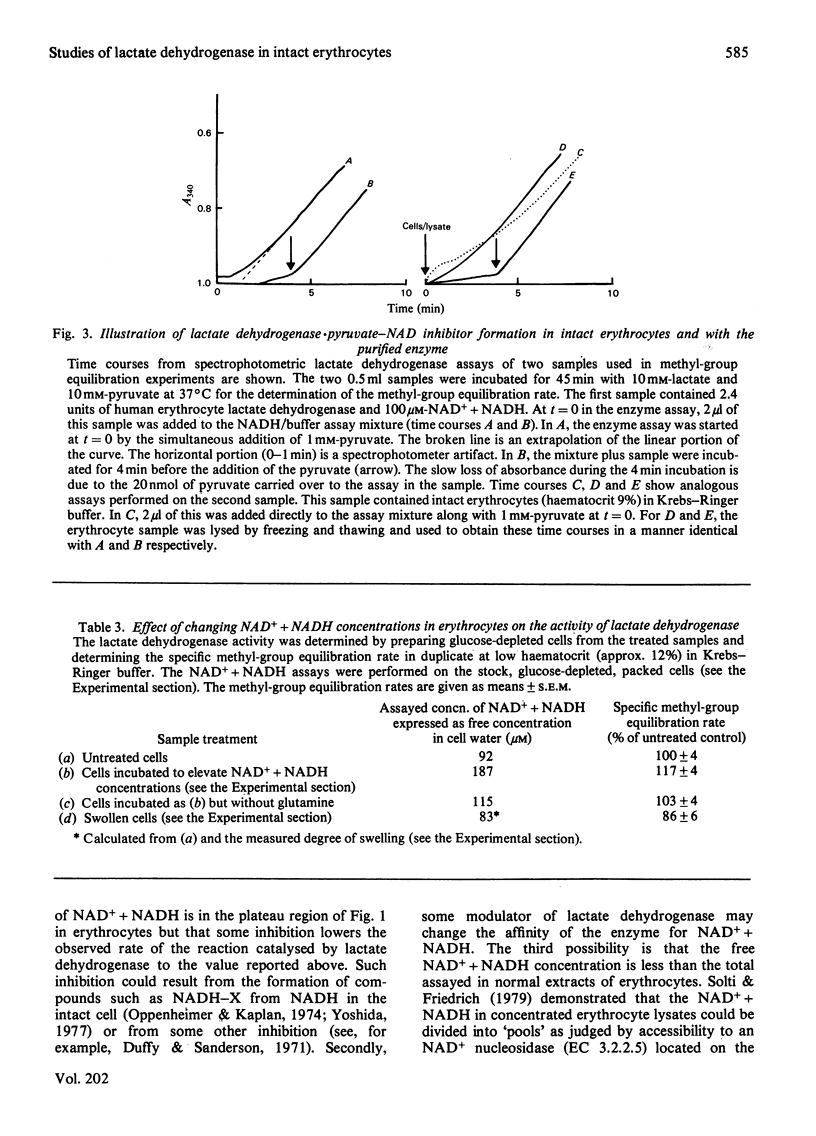
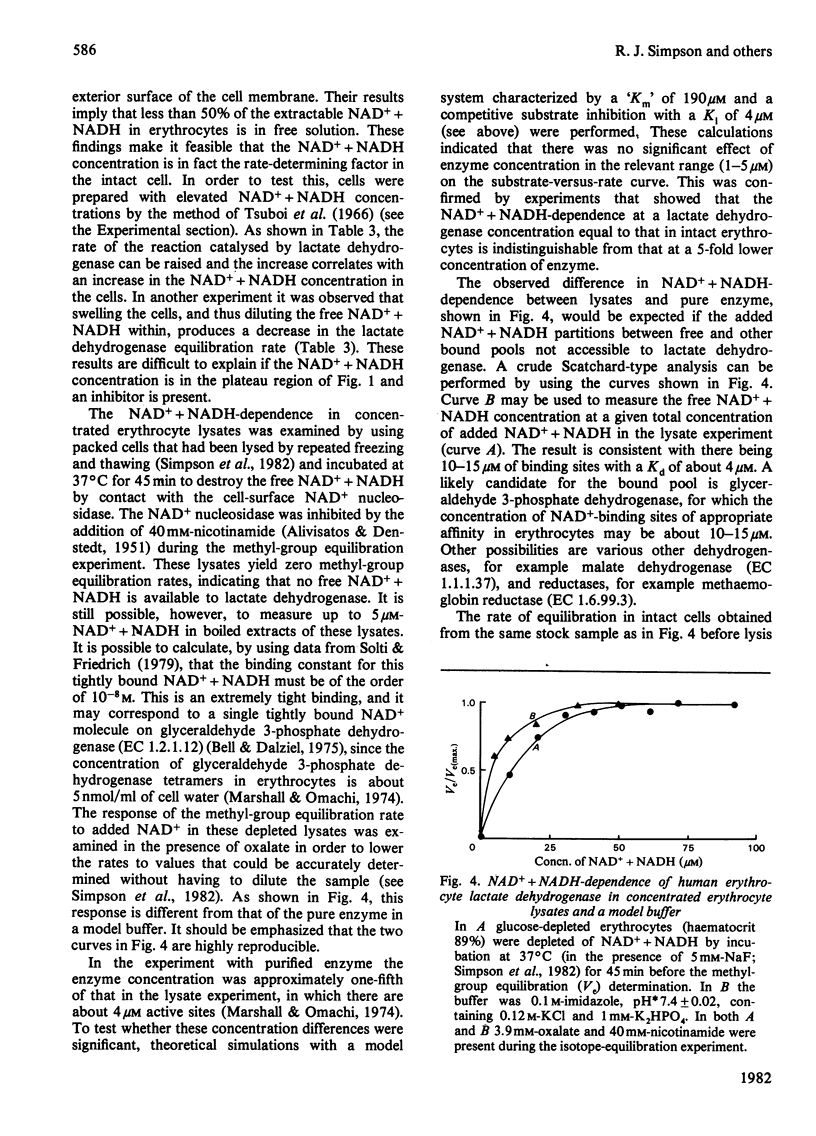
Lactate dehydrogenase in intact erythrocytes was studied by observing isotope exchange between lactate and pyruvate by p.m.r. The inhibition of the enzyme in intact cells by both oxalate and pyruvate was found to be similar to that of the purified enzyme. The activity of the enzyme in intact cells indicates that the free solution NAD+ + NADH concentration in erythrocytes is about 10 microM whereas the total extractable NAD+ + NADH is about 80 nmol/ml of cell water.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. R. Effects of halides on reduced nicotinamide adenine dinucleotide binding properties and catalytic activity of beef heart lactate dehydrogenase. Biochemistry. 1981 Feb 3;20(3):464–467. doi: 10.1021/bi00506a003. [DOI] [PubMed] [Google Scholar]
- Bell J. E., Dalziel K. Studies of coenzyme binding to rabbit muscle glyceraldehyde-3-phoshate dehydrogenase. Biochim Biophys Acta. 1975 Jun 24;391(2):249–258. doi: 10.1016/0005-2744(75)90248-x. [DOI] [PubMed] [Google Scholar]
- Borgmann U., Moon T. W., Laidler K. J. Molecular kinetics of beef heart lactate dehydrogenase. Biochemistry. 1974 Dec 3;13(25):5152–5158. doi: 10.1021/bi00722a016. [DOI] [PubMed] [Google Scholar]
- Brindle K. M., Brown F. F., Campbell I. D., Grathwohl C., Kuchel P. W. Application of spin-echo nuclear magnetic resonance to whole-cell systems. Membrane transport. Biochem J. 1979 Apr 15;180(1):37–44. doi: 10.1042/bj1800037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown F. F., Campbell I. D., Kuchel P. W., Rabenstein D. C. Human erythrocyte metabolism studies by 1H spin echo NMR. FEBS Lett. 1977 Oct 1;82(1):12–16. doi: 10.1016/0014-5793(77)80875-2. [DOI] [PubMed] [Google Scholar]
- Burgner J. W., 2nd, Ainslie G. R., Jr, Cleland W. W., Ray W. J., Jr Bimodal substrate inhibition of lactate dehydrogenase. Factors affecting the enzyme in vivo. Biochemistry. 1978 May 2;17(9):1646–1653. doi: 10.1021/bi00602a011. [DOI] [PubMed] [Google Scholar]
- Duffy P., Sanderson J. C. Studies on the control of lactate dehydrogenase activity in mammalian cells. II. Demonstration of rapid continuous variations in lactate dehydrogenase activity in humans erythrocytes and their relation to a biological activator and an inhibitor of lactate dehydrogenase. Biochim Biophys Acta. 1971 Sep 21;244(3):613–617. doi: 10.1016/0304-4165(71)90078-x. [DOI] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- Halestrap A. P. Transport of pyruvate nad lactate into human erythrocytes. Evidence for the involvement of the chloride carrier and a chloride-independent carrier. Biochem J. 1976 May 15;156(2):193–207. doi: 10.1042/bj1560193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard A. R., Sprandel U., Chalmers R. A. Organic-acid transport in resealed haemoglobin-containing human erythrocyte 'ghosts'. Biochem J. 1980 Sep 15;190(3):653–658. doi: 10.1042/bj1900653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., SCHULZ D. W., ROCK M. K. The measurement of pyridine nucleotides by enzymatic cycling. J Biol Chem. 1961 Oct;236:2746–2755. [PubMed] [Google Scholar]
- Latner A. L., Siddiqui S. A., Skillen A. W. Pyruvate inhibition of lactate dehydrogenase activity in human tissue extracts. Science. 1966 Oct 28;154(3748):527–529. [PubMed] [Google Scholar]
- Marshall W. E., Omachi A. Measured and calculated NAD+-NADH ratios in human erythrocytes. Biochim Biophys Acta. 1974 Jun 20;354(1):1–10. doi: 10.1016/0304-4165(74)90046-4. [DOI] [PubMed] [Google Scholar]
- NOVOA W. B., WINER A. D., GLAID A. J., SCHWERT G. W. Lactic dehydrogenase. V. Inhibition by oxamate and by oxalate. J Biol Chem. 1959 May;234(5):1143–1148. [PubMed] [Google Scholar]
- Nisselbaum J. S., Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem. 1969 Feb;27(2):212–217. doi: 10.1016/0003-2697(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Kaplan N. O. Glyceraldehyde-3-phosphate dehydrogenase catalyzed hydration of the 5-6 double bond of reduced beta-nicotinamide adenine dinucleotide (betaNADH). Formation of beta-6-hydroxy-1,4,5,6-tetrahydronicotinamide adenine dinucleotide. Biochemistry. 1974 Nov 5;13(23):4685–4694. doi: 10.1021/bi00720a002. [DOI] [PubMed] [Google Scholar]
- Ottaway J. H., Mowbray J. The role of compartmentation in the control of glycolysis. Curr Top Cell Regul. 1977;12:107–208. doi: 10.1016/b978-0-12-152812-6.50010-x. [DOI] [PubMed] [Google Scholar]
- Rivedal E., Sanner T. Role of ions in the regulation of porcine lactate dehydrogenase. Biochim Biophys Acta. 1979 Mar 16;567(1):60–65. doi: 10.1016/0005-2744(79)90172-4. [DOI] [PubMed] [Google Scholar]
- SILVERSTEIN E., BOYER P. D. EQUILIBRIUM REACTION RATES AND THE MECHANISMS OF BOVINE HEART AND RABBIT MUSCLE LACTATE DEHYDROGENASES. J Biol Chem. 1964 Nov;239:3901–3907. [PubMed] [Google Scholar]
- Simpson R. J., Brindle K. M., Brown F. F., Campbell I. D., Foxall D. L. A p.m.r. isotope-exchange method for studying the kinetic properties of dehydrogenases in intact cells. Biochem J. 1982 Mar 15;202(3):573–579. doi: 10.1042/bj2020573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solti M., Friedrich P. The 'enzyme-probe' method for characterizing metabolite pools. The use of NAD-glycohydrolase in human erythrocyte sonicate as a model system. Eur J Biochem. 1979 Apr;95(3):551–559. doi: 10.1111/j.1432-1033.1979.tb12996.x. [DOI] [PubMed] [Google Scholar]
- Steinbrecht I., Kunz W. Bestimmung der oxydierten und reduzierten Pyridinnukleotide in Menschen- und Kaninchenblut mit Hilfe der polarographischen Cycling-Technik. Acta Biol Med Ger. 1972;29(4):495–507. [PubMed] [Google Scholar]
- Trommer W. E., Huth H., Wenzel H. R. The nature of the substrate inhibition in lactate dehydrogenases as studied by a spin-labeled derivative of NAD. Biochim Biophys Acta. 1979 Mar 16;567(1):49–59. doi: 10.1016/0005-2744(79)90171-2. [DOI] [PubMed] [Google Scholar]
- Tsuboi K. K., Allan J. F., Fukunaga K. Cofactors and erythrocyte glycolytic capacity. J Biol Chem. 1966 Apr 10;241(7):1616–1620. [PubMed] [Google Scholar]
- WINER A. D., SCHWERT G. W. Lactic dehydrogenase. IV. The influence of pH on the kinetics of the reaction. J Biol Chem. 1958 Apr;231(2):1065–1083. [PubMed] [Google Scholar]
- Wang C. S. Inhibition of human erythrocyte lactate dehydrogenase by high concentrations of pyruvate. Evidence for the competitive substrate inhibition. Eur J Biochem. 1977 Sep;78(2):569–574. doi: 10.1111/j.1432-1033.1977.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Yagil G., Hoberman H. D. Rate of isotope exchange in enzyme-catalyzed reactions. Biochemistry. 1969 Jan;8(1):352–360. doi: 10.1021/bi00829a049. [DOI] [PubMed] [Google Scholar]
- Yoshida A. Glucose-6-phosphate dehydrogenase abnormality and hemolysis. Acta Biol Med Ger. 1977;36(5-6):689–701. [PubMed] [Google Scholar]


